Abstract
When treating patients with unresectable stage III non-small-cell lung cancer (NSCLC), those with a good performance status and disease measured within a radical treatment volume should be considered for definitive concurrent chemoradiotherapy (cCRT). This guidance is based on key scientific rationale from two large Phase 3 randomised studies and meta-analyses demonstrating the superiority of cCRT over sequential (sCRT). However, the efficacy of cCRT comes at the cost of increased acute toxicity versus sequential treatment. Currently, there are several documented approaches that are addressing this drawback, which this paper outlines. At the point of diagnosis, a multidisciplinary team (MDT) approach can enable accurate assessment of patients, to determine the optimal treatment strategy to minimise risks. In addition, reviewing the Advisory Committee on Radiation Oncology Practice (ACROP) guidelines can provide clinical oncologists with additional recommendations for outlining target volume and organ-at-risk delineation for standard clinical scenarios in definitive cCRT (and adjuvant radiotherapy). Furthermore, modern advances in radiotherapy treatment planning software and treatment delivery mean that radiation oncologists can safely treat substantially larger lung tumours with higher radiotherapy doses, with greater accuracy, whilst minimising the radiotherapy dose to the surrounding healthy tissues. The combination of these advances in cCRT may assist in creating comprehensive strategies to allow patients to receive potentially curative benefits from treatments such as immunotherapy, as well as minimising treatment-related risks.
Similar content being viewed by others
Background
When treating patients with locally advanced, stage III, non-small-cell lung cancer (NSCLC), those with a good performance status (PS, defined as an Eastern Cooperative Oncology Group [ECOG] PS 0–1) and disease encompassable within a radical treatment volume should be considered for definitive concurrent chemoradiotherapy (cCRT).1,2,3,4,5,6 This guidance is based on the results of several preclinical studies documenting beneficial interactions between radiation and chemotherapy, as well as two large Phase 3 randomised studies and two meta-analyses (in patients who predominantly had stage III NSCLC), which demonstrated the superiority of cCRT over sequential chemoradiotherapy (sCRT, chemotherapy followed by full-dose radiotherapy, with sequential defined as chemotherapy followed by radiotherapy).7,8,9,10,11,12 Currently in the United Kingdom (UK), 45% of stage III NSCLC patients with curative radiation doses (n = 716) are treated with cCRT, compared with 55% (n = 391) who receive sCRT.13 For comparison, an observational study of national registries demonstrated that in Belgium and The Netherlands, 35 and 55% of patients received cCRT, respectively.14
Several preclinical studies have outlined the synergistic benefits between radiation and chemotherapy drugs, such as cisplatin, carboplatin and cisplatin plus etoposide.12 These early studies outlined the radiosensitising properties of chemotherapy drugs, such as cisplatin, as well as how the close temporal administration of the two can enhance antitumour efficacy, but with high toxicity costs. Although several mechanisms of action have been proposed following in vitro and in vivo studies, platinum-radiation interactions are complex and not fully comprehended at this time. One possibility proposed is reduced recovery from radiation-induced, potentially lethal or sublethal damage, when cisplatin is present.12 In addition, cells can arrest in the second growth phase following radiotherapy, and are shown to be hypersensitive to the cytotoxic effect of etoposide.11 Early clinical studies of CRT in NSCLC examined whether a sequential approach to treatment delivery was useful to maximise both locoregional and micrometastatic disease control, whilst minimising the risks of cumulative toxicity. Despite modest improvements with sCRT, two large Phase 3 randomised studies demonstrated superiority of cCRT over sCRT.8,9
Review of key studies comparing concurrent and sequential chemoradiotherapy
In the Radiation Therapy Oncology Group trial (RTOG 9410), 610 patients with unresectable stage III NSCLC were randomised to one of three arms: two cycles of cisplatin plus vinblastine with either concurrent or sequential radiotherapy (60 Gy in 30 fractions) or two cycles of cisplatin plus oral etoposide with concurrent radiotherapy delivered twice daily (69.6 Gy in 20 fractions delivered at 1.2 Gy per fraction). Patients who received cCRT with cisplatin plus vinblastine demonstrated improved overall survival (OS) compared with those who received sequential treatment (median OS, 17.0 vs. 14.6 months; HR 0.81; 95% CI 0.66–0.996) at the cost of increased rates of acute grade ≥3 non-haematologic toxicity. Late toxicity rates were similar overall for all arms of the study.8
In a second Phase 3 randomised study, 320 patients were randomly assigned to cisplatin, mitomycin and vindesine with a concurrent, split course of thoracic radiotherapy (2 Gy/fraction given 14 times for 3 weeks and then followed by a rest period of 10 days) or to the same chemotherapy regimen followed by a single course of radiotherapy (56 Gy in 28 fractions of 2 Gy each). In both arms of the study, the radiotherapy planning techniques, dose constraints and treatment delivery were the same (either delivered using a linear accelerator [≥4 MeV] or a cobalt-60 machine). The cCRT arm was associated with an improved response rate (84% vs. 66%), median OS (16.5 vs. 13.3 months) and 2- and 5-year survival rates (34.6% vs. 27.4% and 15.8% vs. 8.9%, respectively) compared with the sCRT arm. Treatment-related toxicity included myelosuppression, which occurred more frequently in patients in the concurrent arm compared to the sequential arm (p = 0.0001). There was no significant difference in the incidence of other toxicities, including oesophagitis, between the two treatment arms.9
A meta-analysis published in 2010 demonstrated the superiority of cCRT over radiotherapy alone or sCRT.7 The meta-analysis included 19 randomised studies (with over 2,700 patients) and reported a significantly reduced overall risk of death (HR 0.71; 95% CI 0.64–0.80) and improved progression-free survival (PFS) at any site (HR 0.69; 95% CI 0.58–0.81) for those receiving cCRT compared with radiotherapy alone.7,15 These improvements were at the cost of increased toxicity with higher rates of acute oesophagitis, neutropenia and anaemia in patients receiving cCRT over sCRT.7 The meta-analysis also analysed six trials (1024 patients) comparing cCRT versus sCRT. A significant benefit was shown in OS (HR 0.74; 95% CI 0.62–0.89) for cCRT. This survival improvement equated to a 10% absolute survival benefit at 2 years for cCRT.7 Again, this was at the cost of toxicity with increased rates of severe oesophagitis (relative risk [RR] 4.96; 95% CI 2.17–11.37) and a non-significant increase in treatment-related deaths (4% vs. 2%) reported in the cCRT arm versus the sCRT arm (RR 2.02; 95% CI 0.90–4.52), respectively.7
A subsequent meta-analysis (1205 patients) by Auperin et al. analysed randomised trials directly comparing cCRT versus sCRT and demonstrated the superiority of cCRT, which was shown to improve OS in patients with locally advanced NSCLC (HR 0.84; 95% CI 0.74–0.95; p = 0.004).10 This improvement in OS was at the cost of increased acute toxicity, particularly oesophagitis; cCRT increased acute oesophagitis (grade 3–4) from 4 to 18% with a RR of 4.9 (95% CI 3.1–7.8; p < 0.001).10 Two further randomised Phase 3 studies that compared sCRT versus cCRT in patients with unresectable NSCLC failed to show a statistically significant difference in OS between arms.16,17 The first of these studies was not sufficiently powered and was closed early; however, radical radiotherapy (66 Gy), given concurrently with daily low-dose cisplatin, or after two courses of gemcitabine plus cisplatin, was well tolerated with 2-year OS rates of 34 and 39%, and 3-year OS rates of 22 and 34%, respectively, for sCRT versus cCRT.16 In the cCRT arm, oesophagitis occurred in nine patients (14%) at grade 3 and in two patients (3%) at grade 4, while in the sCRT arm, it occurred in four patients (5%) at grade 3 and no patients at grade 4. Acute haematological toxicity was more common in the sCRT arm compared with the cCRT arm.16 The second randomised study failed to show a statistically significant improvement in OS; however, it did reveal clinically important differences in the median 2-, 3- and 4-year OS rates with a trend in favour of cCRT, suggesting that this is the optimal strategy for patients with locally advanced NSCLC.17 The culmination of these studies led to cCRT to be considered the standard of care for patients with unresectable stage III NSCLC.
Determining treatable areas for radiotherapy
Determining the optimal treatment plan for a patient with NSCLC requires an accurate assessment of their overall fitness, medical comorbidities, cardiorespiratory reserve, genomic background, tumour stage and mutation status.18 Once this assessment is completed, it is then possible to develop the optimal treatment strategy for the patient. When selecting stage III NSCLC patients for CRT, it is important to consider that certain aspects of therapy remain controversial. Hence, a multidisciplinary team (MDT) approach that includes expert opinions from thoracic surgeons, clinical and medical oncologists, radiologists, nuclear medicine physicians and pathologists18 is necessary to ensure that all patients are offered optimal treatments based on their surgical operability, performance status, stage and extent of disease.
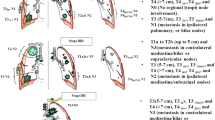
When pathological mediastinal lymph node (N2) disease is evident at the time of diagnosis, a combined-modality approach is normally recommended if the patient is deemed radically treatable. Due to the presence of nodal disease, such patients are considered high risk for both local and distant recurrence, and surgical resection as the sole treatment modality is considered inadequate. Consequently, the most common approach to managing patients with confirmed N2 nodal involvement is cCRT, using a platinum doublet-based chemotherapy combined with a radical dose of radiotherapy. Within this group of N2 patients, there is a highly selected subset of patients who may be considered for surgery following preoperative chemotherapy and/or CRT, with existing guidelines suggesting that such patients should have minimal N2 disease.2 It is currently debated whether surgery should be considered for the subset of N2 patients who require pneumonectomy (those with extensive mediastinal N2 infiltration), due to the high mortality rates associated with the procedure.19 For patients with N3 or T4 surgically unresectable tumours, the standard approach to management is cCRT.3 Hence, radiotherapy in the context of cCRT plays a major role in the radical treatment of NSCLC patients with locally advanced disease. Guidelines have been developed by the ACROP committee, on behalf of the European Society for Radiotherapy and Oncology (ESTRO) for the treatment of locally advanced NSCLC.20 These guidelines provide recommendations for target volume (TV) and organ-at-risk (OAR) delineation for standard clinical scenarios with definitive CRT (and adjuvant radiotherapy) for locally advanced NSCLC, and also give a comprehensive guide on how to plan a patient’s radical radiotherapy from pre-treatment imaging to planning computed tomography (CT) acquisition and optimal gross tumour volume (GTV), clinical target volume (CTV), planning target volume (PTV) and OAR definitions.20
Treatment statistics and toxicity profiles for concurrent versus sequential chemoradiotherapy
As discussed, cCRT leads to improvements in efficacy, at the cost of increased acute toxicity, compared with sCRT.7,8,9,10,15 Following the publication of the meta-analyses described above, Koning et al. conducted a systematic literature review to evaluate the toxicity of cCRT for locally advanced NSCLC;21 13 studies in patients with stage III NSCLC were identified, which featured mono- or polychemotherapy schedules as single dose, double dose, triple high-dose or daily cisplatin-containing chemotherapy.21 From these studies, acute (grade ≥ 3) oesophagitis was observed in up to 18% of the patients, and high-dose cisplatin regimens resulted in more frequent haematologic toxicity, nausea and vomiting (at least four trials with ≥16%) compared with other chemotherapy regimens.21 The review concluded that the toxicity profile was more favourable with low-dose chemotherapy schedules, and cCRT with daily cisplatin monochemotherapy resulted in more favourable acute and late toxicity compared with cCRT with single high-dose chemotherapy, doublets or triplets.21
A further retrospective study from The Netherlands of 154 patients with inoperable stage III NSCLC receiving cCRT also demonstrated good efficacy when low-dose chemotherapy was delivered concurrently with conformal, intensity-modulated radiation therapy (IMRT), or volumetric-modulated arc therapy (VMAT). Patients who received 66 Gy (24 fractions of 2.75 Gy) with low-dose daily cisplatin (6 mg/m2) had a 5-year survival rate of 40%.22 In addition, Arrieta et al. also reported that the use of induction gemcitabine plus carboplatin followed by cCRT utilising gemcitabine led to unacceptably high rates of pulmonary toxicity (39.1% of patients had grade 3–5 toxicity), despite improved response rates.23 Based on all these findings, platinum doublet chemotherapy remains the standard of care when delivering cCRT, but there is no clear evidence to support one regimen over another.
The UK Phase 2 SOCCAR trial, which compared sequential versus concurrent chemotherapy and radical hypofractionated radiotherapy in 130 patients with unresectable stage III NSCLC and good performance status (ECOG PS 0–1), also confirmed that it was feasible to deliver accelerated hypofractionated radiotherapy with chemotherapy to these patients. Patients recruited to the trial received concurrent cisplatin and vinorelbine chemotherapy with a minimum standard of conformally planned radiotherapy (4D-CT radiotherapy planning was used by one participating centre, otherwise IMRT was not routinely used). The incidence of at least one serious adverse event (AE) was similar in both arms. Rates of grade 3–5 AEs were 32% in the cCRT arm and 41% in the sCRT arm, with oesophagitis reported in 8.8% and 8.5% of patients and pneumonitis in 3.1% and 5.2% of patients, respectively.24 The conclusion of the SOCCAR trial was that the encouraging 2-year survival rates (50% in the concurrent arm) suggest that a 4-week hypofractionated regimen of radiotherapy should be compared with conventionally fractionated radiotherapy in an adequately powered randomised controlled Phase 2 trial.24
Comparison of United Kingdom lung cancer guidelines with European Union standards
Within the UK, there is no shortage of guidance on the optimal management of stage III NSCLC patients as both national (e.g. National Institute for Health and Care Excellence [NICE] and The Royal College of Radiologists and British Thoracic Society) and regional (e.g. London Cancer Alliance) guidelines exist.1,4,6,18,25 All provide clear and consistent guidance on the management of patients with locally advanced NSCLC, including the use of cCRT. A summary of UK national guidance is provided in Table 1, with comparison to the European Society for Medical Oncology (ESMO) locally advanced NSCLC guidelines.2,6 These comparisons reveal strong correlation on the optimal management of locally advanced NSCLC patients, and it is reassuring that cCRT is also considered the treatment of choice in patients evaluated as having unresectable stage IIIA and IIIB disease based on published ESMO consensus guidance.2,3 Comparison of ESMO guidance,2,3 with that from NICE,5 The Royal College of Radiologists25 and the British Thoracic Oncology Group4 reveals no significant variations in standards, and therefore highlights the consensus agreement that exists on the optimal management of these patients across the European Union (EU).
Optimal chemotherapy regimens and radiotherapy dose fractionation for concurrent chemoradiotherapy
The choice of chemotherapy can additionally be important to the outcome of cCRT. According to the ESMO guidelines “in the absence of contraindications, the optimal chemotherapy to be combined with radiation in stage III NSCLC should be based on cisplatin”.2 Current evidence is inconclusive on use of cisplatin as a single agent, but it may be combined with etoposide, vinorelbine or other vinca alkaloids, based on most comparative clinical trials of cCRT and sCRT when treatment has curative intent.2,10 If a patient has specific comorbidities, other treatment options that can be considered include carboplatin-based cCRT. In terms of regimen delivery, ESMO guidelines recommend that “in the stage III disease chemoradiotherapy strategy, two to four cycles of concomitant chemotherapy should be delivered”.2
In terms of radiotherapy, multiple studies have examined the use of alternative dose-fractionation schedules to improve outcomes for NSCLC patients. Approaches have included hyperfractionation (two or three fractions per day with a lower dose per fraction than the standard 2 Gy), accelerated fractionation (the overall duration of treatment is shortened, but the fraction size and total dose is kept the same), hypofractionation (where a larger daily fraction size is used than the standard 2 Gy), dose escalation (where the total dose is increased) or combinations of these different approaches.24,26,27,28,29,30,31,32,33
One of the earliest studies to assess the effect of total radiation dose on outcomes in patients with NSCLC was the RTOG 7301 study that examined the effects of dose escalation on tumour control by randomising 365 stage III NSCLC patients to one of four treatment arms, with local tumour response rates being significantly improved with the highest dose (60 Gy in 30 daily fractions, over 5 days a week for 6 weeks; 56% response rate), although survival was similar between arms.33 This study was the first to establish 60 Gy in 30 daily fractions, over 5 days a week for 6 weeks, as the standard radiotherapy dose-fractionation schedule for NSCLC.30,33
The Phase 3 RTOG 0617 trial examined whether high-dose (74 Gy in 37 daily fractions) radiotherapy offers an advantage over standard-dose (60 Gy in 30 daily fractions) radiotherapy, whether the method of treatment delivery (IMRT vs. 3D-conformal radiotherapy) improves patient outcomes and whether the addition of cetuximab to carboplatin and paclitaxel has any beneficial effects.30 Between November 2007 and November 2011, 544 stage III NSCLC patients from the United States and Canada were enrolled in the open-label 2×2 factorial trial and were randomised 1:1:1:1 to one of two systemic regimens (weekly carboplatin [AUC 2] plus paclitaxel [45 mg/m2] with or without cetuximab) and to either standard-dose (60 Gy) or high-dose radiotherapy (74 Gy), delivered using either 3D-conformal radiotherapy techniques or by IMRT. Two weeks after their chemoradiation finished, all enrolled patients received two 21-day cycles of consolidation chemotherapy with full doses of carboplatin (AUC 6) and paclitaxel (200 mg/m2).30 Unexpectedly, the high-dose (74-Gy) arm was associated with significantly shorter OS and an increased risk of death compared with the standard-dose radiotherapy arm (median OS, 20 vs. 29 months; HR 1.38; 95% CI 1.09–1.76).30 Both the radiation and cetuximab comparisons crossed prespecified futility boundaries. The reasons for why the RTOG 0617 trial failed to show a benefit for radiotherapy dose escalation seem to be multifactorial. The escalated radiotherapy dose in the experimental arm (74 Gy) resulted in less patients completing their planned treatment compared with the control arm (64% vs. 70%, respectively), higher rates of treatment planning non-compliance in the 74-Gy arm (26% vs. 17%) and higher doses of radiotherapy to the heart in the 74-Gy arm.30 Bradley et al. also noted that fewer patients in the high-dose arm completed consolidation chemotherapy and hypothesised that 74 Gy given over 7.5 weeks allowed increased tumour repopulation to occur.
Despite these shortcomings, the RTOG 0617 trial was the first Phase 3 trial to permit IMRT in NSCLC and demonstrated that IMRT improved outcomes compared with 3D-conformal radiotherapy. IMRT showed similar survival and locoregional control rates to 3D-conformal radiotherapy, but lower rates of grade ≥ 3 pneumonitis and lower radiation doses to the heart.30,34 Movsas et al. also reported improved quality of life in patients on RTOG 0617 at 3- and 12 months following IMRT compared with 3D-conformal radiotherapy planning at the plenary session of the 2013 American Society for Radiation Oncology (ASTRO) Annual Meeting.35 The 60-Gy standard therapy arm in the RTOG 0617 trial also achieved a 28.7-month median survival that is a positive improvement when compared with previously reported stage III NSCLC studies; however, it should be noted that 90% of enrolled participants had undergone positron emission tomography (PET) staging prior to treatment, which may have contributed towards this finding. Overall, although the RTOG 0617 study failed to show a benefit from dose escalation, it has highlighted the important impact cardiac radiotherapy doses can have on patient outcomes and the subsequent risk of death.30
Despite the results of the RTOG 0617 trial, the issue of radiation dose escalation continues to be controversial due to the study factorial design, such as how patients were selected and the inclusion of regimens with mixed efficacies and toxicities, such as carboplatin, paclitaxel and cetuximab. Important ongoing studies, such as RTOG 1106/ACRIN 6697, a randomised Phase 2 study comparing standard 60-Gy radiation therapy with 80-Gy high-dose radiation therapy using adaptive radiation therapy techniques, and ADSCaN, a randomised Phase 2 study of accelerated, dose-escalated, sequential chemoradiotherapy in stage III NSCLC, could help determine the feasibility, treatment toxicity and survival associated with alternative radiotherapy dosing.36,37
Advances in radiotherapy techniques for NSCLC
For NSCLC, new radiotherapy techniques have evolved allowing higher radiation doses in tumour- positive areas while avoiding high doses in surrounding tissues. The replacement of conventional treatment simulation with CT simulation has been associated with a survival advantage,38 as has cone beam CT (CBCT) for image guidance.39 Stereotactic ablative radiotherapy (SABR) utilises small margins for positional uncertainty, facilitated by 4D-CT, multiple conformal or intensity- modulated beams or arcs and volumetric image guidance.40 IMRT has been created as a highly conformal form of radiotherapy, owing to modern advances in radiotherapy treatment planning software (TPS) and treatment delivery. The integration of onboard CT scanner technology into radiotherapy treatment machines has also enabled clinicians to target tumours more accurately, and has led to the creation of image-guided radiotherapy (IGRT).41 By utilising both IMRT and IGRT, it is now possible to treat substantially larger lung tumours with higher radiotherapy doses safely, with greater accuracy, and whilst minimising the radiotherapy dose to the surrounding normal tissues.41,42,43 These evolving technologies could be combined with targeted agents to further enhance systemic therapy regimens, reducing the risk of distant metastases. Incorporating these potential advances with recent developments in disease staging, diagnostic imaging and molecular profiling could create comprehensive investigational strategies to improve outcomes in future stage III NSCLC clinical trials.44,45,46,47,48,49
Adjuvant immunotherapy after concurrent chemoradiotherapy in stage III NSCLC
The synergistic effect between radiation and immune-checkpoint modulations has been demonstrated in multiple preclinical studies,50,51,52,53,54,55,56,57 and more recently in the clinical setting following the publication of the Phase 3 PACIFIC trial results.49 The use of immune-checkpoint antagonists, specifically anti-PD-1 and anti-PD-L1 therapeutics, has resulted in improved OS in patients with metastatic lung cancer, and has transformed the therapeutic landscape in the first-58 and second-line treatment settings.59 More recently, the anti-PD-L1 agent durvalumab (Imfinzi®▼; AstraZeneca UK Limited) has also been shown to benefit patients with stage III NSCLC, whose tumours express PD-L1 in ≥ 1% of tumour cells, when administered following cCRT.49 The use of immunotherapy after platinum-based CRT seems to offer a therapeutic synergism, which up until now has only been hypothesised.
A rationale for combining immunotherapy with radiation was outlined in a recent editorial by Yip and colleagues.60 They cited Gajewski et al.61 who recognised that patients with non-immunogenic tumours are unlikely to respond to immunotherapy alone due to both factors intrinsic to the tumour itself, such as its mutational burden,62 neoantigen heterogeneity63 and tumour microenvironment,64 and those related to the patient, including human leukocyte antigen (HLA) type, germ-line polymorphisms in immune cell receptors and gut microbiota impact on the immunogenicity of the tumour.64 Yip and colleagues acknowledged that by using such knowledge, Smyth and colleagues could provide a framework to discuss how to best tailor combination therapies to the tumour microenvironment by stratifying them into four types based on the presence or absence of tumour- infiltrating lymphocytes (TILs) and their PD-L1 expression status,65 as shown in Table 2. Strategies to promote immunogenic death, which in turn activate the innate immune system to prime T cells, may help to convert the immunogenically “cold” tumours found in Type 2 and 4 microenvironments (Table 2) into tumours with a more “inflamed phenotype”, thereby improving their response to checkpoint modulation.61 This may be achieved by combining immune-checkpoint modulators with an oncolytic virus,66 chemotherapy (KEYNOTE-021, IMpower131 studies)67,68 or radiotherapy.69
In terms of combining immune-checkpoint inhibitors with radiotherapy, there have been several mechanisms proposed regarding the interaction between radiation and the tumour microenvironment. The first mechanism, already known to play an important role in inducing tumour immunogenicity,70 is through the release of tumour antigens and molecules collectively known as the “damage associated molecular pattern” (DAMPs),71,72 which can activate CD8+ cytotoxic T cells via the major histocompatibility complex (MHC) class I loading pathway. A second pathway that could be activated is the “STING” pathway, which upregulates the expression of type 1 interferon,73 MHC class 1 molecules and the generation of novel peptides.74,75 Other possible mechanisms of radiation-induced tumour microenvironment stimulation that could improve T-cell recruitment may include the generation of appropriate chemokines and increased blood flow.76,77,78,79
Conclusions
At the point of diagnosis, a multidisciplinary team (MDT) approach can enable accurate assessment of patients with unresectable stage III NSCLC, to determine the optimal treatment strategy to minimise risks and toxicity. In addition, reviewing the Advisory Committee on Radiation Oncology Practice (ACROP) guidelines can provide clinical oncologists with additional recommendations for outlining target volume and organ-at-risk delineation for standard clinical scenarios in definitive cCRT (and adjuvant radiotherapy). Modern advances in radiotherapy treatment planning software and treatment delivery mean that radiation oncologists can now treat substantially larger unresectable tumour and nodal volumes with higher radiotherapy doses and greater accuracy, whilst minimising unwanted radiotherapy doses to the surrounding normal healthy tissues. Furthermore, the combination of advances in both cCRT and immune drug therapy has led to an era of emerging treatments for unresectable stage III NSCLC. We can now routinely offer a more efficacious, evidence-based, radical intent treatment strategy to our stage III NSCLC patients.
References
Maconachie, R., Mercer, T., Navani, N., McVeigh, G. et al. Lung cancer: diagnosis and management: summary of updated NICE guidance. BMJ 365, l1514 (2019).
Eberhardt, W. E. E., Ruysscher, D. D., Weder, W., Le Pechoux, C., De Leyn, P., Hoffmann, H. et al. 2nd ESMO Consensus conference in lung cancer: locally advanced stage III non-small-cell lung cancer. Ann. Oncol. 26, 1573–1588 (2015).
Postmus, P. E., Kerr, K. M., Oudkerk, M., Senan, S., Waller, D. A., Vansteenkiste, J. et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 28, iv1–iv21 (2017).
Lim, E., Baldwin, D., Beckles, M., Duffy, J., Entwisle, J., Faivre-Finn, C. et al. Guidelines on the radical management of patients with lung cancer. Thorax 65, iii1–iii27 (2010).
Baldwin, D. R., White, B., Schmidt-Hansen, M., Champion, A. R. et al. Diagnosis and treatment of lung cancer: summary of updated NICE guidance. BMJ 342, d2110 (2011).
National Institute for Health and Care Excellence. Lung cancer: diagnosis and management. https://www.nice.org.uk/guidance/NG122. (last accessed December 2019).
O'Rourke, N., i Figuls, M. R., Bernadó, N. F. & Macbeth, F. Concurrent chemoradiotherapy in non‐small cell lung cancer. Cochrane Database Syst. Rev. CD002140 (2010).
Curran, W. J., Paulus, R., Langer, C. J., Komaki, R., Lee, J. S., Hauser, S. et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J. Natl Cancer Inst. 103, 1452–1460 (2011).
Furuse, K., Fukuoka, M., Kawahara, M., Nishikawa, H., Takada, Y., Kudoh, S. et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J. Clin. Oncol. 17, 2692–2699 (1999).
Aupérin, A., Le Pechoux, C., Rolland, E., Curran, W. J., Furuse, K., Fournel, P. et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J. Clin. Oncol. 28, 2181–2190 (2010).
Giocanti, N., Hennequin, C., Balosso, J., Mahler, M. & Favaudon, V. DNA Repair and Cell Cycle Interactions in Radiation Sensitization by the Topoisomerase II Poison Etoposide. Cancer Res. 53, 2105–2111 (1993).
Reboul, F. L. Radiotherapy and chemotherapy in locally advanced non-small cell lung cancer: preclinical and early clinical data. Hematol. Oncol. Clin. North Am. 18, 41–53 (2004).
Royal College of Physicians. NLCA Annual Report 2018. https://www.rcplondon.ac.uk/projects/outputs/nlca-annual-report-2018 (2019).
Walraven, I., Damhuis, R. A., Ten Berge, M. G., Roskamp, M., van Eycken, L., de Ruysscher, D. et al. Treatment variation of sequential versus concurrent chemoradiotherapy in stage III non-small cell lung cancer patients in the Netherlands and Belgium. Clin. Oncol. (R. Coll. Radiol.) 29, e177–e185 (2017).
O’Rourke, N. & Macbeth, F. Is concurrent chemoradiation the standard of care for locally advanced non-small cell lung cancer? A review of guidelines and evidence. Clin. Oncol. R. Coll. Radiol. 22, 347–355 (2010).
Belderbos, J., Uitterhoeve, L., van Zandwijk, N., Belderbos, H., Rodrigus, P. et al. Randomised trial of sequential versus concurrent chemo-radiotherapy in patients with inoperable non-small cell lung cancer (EORTC 08972-22973). Eur. J. Cancer 43, 114–121 (1990).
Fournel, P., Robinet, G., Thomas, P., Souquet, P. J., Lena, H., Vergnenegre, A. et al. Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer: Groupe Lyon-Saint-Etienne d’Oncologie Thoracique-Groupe Français de Pneumo-Cancérologie NPC 95-01 Study. J. Clin. Oncol. 23, 5910–5917 (2005).
London Cancer Alliance. LCA Lung Cancer Clinical Guidelines. 2013, 1–94. Available at: http://www.londoncanceralliance.nhs.uk/media/62369/Lung%20Cancer%20Clinical%20Guidelines%20041213%20FINAL%20REV.pdf (last accessed Dec 2019)
Fountain, S. W. Guidelines on the selection of patients with lung cancer for surgery. Thorax 56, 89–108 (2001).
Nestle, U., Ruzsscher, D. D., Ricardi, U., Geets, X., Belderbos, J., Pottgen, C. et al. ESTRO ACROP guidelines for target volume definition in the treatment of locally advanced non-small cell lung cancer. Radiother. Oncol. 127, 1–5 (2018).
Koning, C. C., Wouterse, S. J., Daams, J. G., Uitterhoeve, L. L., van den Heuvel, M. M. & Belderbos, J. S. Toxicity of concurrent radiochemotherapy for locally advanced non–small-cell lung cancer: a systematic review of the literature. Clin. Lung Cancer 14, 481–487 (2013).
Dieleman, E. M. T., Uitterhoeve, A. L. J., van Hoek, M. W., van Os, R. M., Wiersma, J., Koolen, M. G. J. et al. Concurrent daily cisplatin and high-dose radiation therapy in patients with stage III non-small cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 102, 543–551 (2018).
Arrieta, O., Gallardo-Rincon, D., Villarreal-Garza, C. V., Michel, R. M., Astorga-Ramos, A. M. & Martinez-Barrera, L. High frequency of radiation pneumonitis in patients with locally advanced non-small cell lung cancer treated with concurrent radiotherapy and gemcitabine after induction with gemcitabine and carboplatin. J. Thorac. Oncol. 4, 845–852 (2009).
Maguire, J., Khan, I., Mcmenemin, R., O’Rouke, N., McNee, S., Kelly, V. et al. SOCCAR: a randomised phase ii trial comparing sequential versus concurrent chemotherapy and radical hypofractionated radiotherapy in patients with inoperable stage III non-small cell lung cancer and good performance status. Eur. J. Cancer 50, 2939–2949 (1990).
The Royal College of Radiologists. Radiotherapy dose fractionation, 2nd edn, 1–135, 2016. Available at: https://www.rcr.ac.uk/system/files/publication/field_publication_files/bfco163_dose_fractionation_2nd_ed_march2017.pdf. (last accessed Dec 2019).
Mauguen, A., Le Pechouz, C., Saunders, M. I., Schild, S. E., Turrisi, A. T., Baumann, M. et al. Hyperfractionated or accelerated radiotherapy in lung cancer: an individual patient data meta-analysis. J. Clin. Oncol. 30, 2788–2797 (2012).
Saunders, M., Dische, S., Barrett, A., Harvey, A., Gibson, D. & Parmar, M. Continuous hyperfractionated accelerated radiotherapy (CHART) versus conventional radiotherapy in non-small-cell lung cancer: a randomised multicentre trial. CHART Steering Committee. Lancet 350, 161–165 (1997).
Hatton, M., Nankivell, M., Lyn, E., Chir, M. B. B., Falk, S., Pugh, C. et al. Induction chemotherapy and continuous hyperfractionated accelerated radiotherapy (CHART) for patients with locally advanced inoperable non–small-cell lung cancer: The MRC INCH randomized trial. Int. J. Radiat. Oncol. 81, 712–718 (2011).
Brower, J. V., Amini, A., Chen, S., Hullett, C. R., Kimple, F. J., Wojcieszynski, A. P. et al. Improved survival with dose-escalated radiotherapy in stage III non-small-cell lung cancer: analysis of the National Cancer Database. Ann. Oncol. 27, 1887–1894 (2016).
Bradley, J. D., Paulus, R., Komaki, R., Masters, G., Blumenschein, G., Schild, S. et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 16, 187–199 (2015).
Hatton, M. Q. F., Hill, R., Fenwick, J. D., Morgan, S. A., Wilson, P. C., Atherton, P. J. et al. Continuous hyperfractionated accelerated radiotherapy—escalated dose (CHART-ED): a phase I study. Radiother. Oncol. 118, 471–477 (2016).
Landau, D. B., Hughes, L., Baker, A., Bates, A. T., Bayne, M. C., Counsell, N. et al. IDEAL-CRT: a phase 1/2 trial of isotoxic dose-escalated radiation therapy and concurrent chemotherapy in patients with stage II/III non-small cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 95, 1367–1377 (2016).
Perez, C. A., Stanley, K., Rubin, P., Kramer, S., Brady, L., Perez-Tamayo, R. et al. A prospective randomized study of various irradiation doses and fractionation schedules in the treatment of inoperable non-oat-cell carcinoma of the lung. Preliminary report by the Radiation Therapy Oncology Group. Cancer 45, 2744–2753 (1980).
Chun, S. G., Hu, C., Choy, H., Komaki, R. U., Timmerman, R. D., Schild, S. E. et al. Comparison of 3-D conformal and intensity modulated radiation therapy outcomes for locally advanced non-small cell lung cancer in NRG Oncology/RTOG 0617. Int. J. Radiat. Oncol. 93, S1–S2 (2015).
Movsas, B., Hu, C., Sloan, J., Bradley, J. D., Kavadi, V. S., Narayan, S. et al. Quality of life (QOL) analysis of the randomized radiation (RT) dose-escalation NSCLC Trial (RTOG 0617): the rest of the story. Int. J. Radiat. Oncol. 87, S1–S2 (2013).
Cancer Research UK. Clinical Trials Unit Glasgow ADSCaN. http://www.crukctuglasgow.org/eng.php?pid=adscan (last accessed Dec 2019).
NCT01507428. Study of Positron Emission Tomography and Computed Tomography in Guiding Radiation Therapy in Patients With Stage III Non-small Cell Lung Cancer. https://clinicaltrials.gov/ct2/show/NCT01507428 (last accessed Dec 2019).
Chen, A. B., Neville, B. A., Sher, D. J., Chen, K. & Schrag, D. Survival outcomes after radiation therapy for stage III non-small-cell lung cancer after adoption of computed tomography-based simulation. J. Clin. Oncol. 29, 2305–2311 (2011).
Grills, I. S., Hugo, G., Kestin, L. L., Galerani, A. P., Chao, K. K., Wloch, J. et al. Image-guided radiotherapy via daily online cone-beam CT substantially reduces margin requirements for stereotactic lung radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 70, 1045–1056 (2008).
Louie, A. V., Palma, D. A., Dahele, M., Rodrigues, G. B. & Senan, S. Management of early-stage non-small cell lung cancer using stereotactic ablative radiotherapy: controversies, insights, and changing horizons. Radiother. Oncol. 114, 138–147 (2015).
National Cancer Action Team. National Radiotherapy Implementation Group Report. 2012. 1–93, Available at: https://www.sor.org/sites/default/files/document-versions/National%20Radiotherapy%20Implementation%20Group%20Report%20IGRT%20Final.pdf. (last accessed Dec 2019).
Zhang, J., Yu, X. L., Zheng, G. F. & Zhao, F. Intensity-modulated radiotherapy and volumetric-modulated arc therapy have distinct clinical advantages in non-small cell lung cancer treatment. Med. Oncol. 32, 94–94 (2015).
Bezjak, A., Rumble, R. B., Rodrigues, G., Hope, A., Ward, P. et al. Intensity-modulated radiotherapy in the treatment of lung cancer. Clin. Oncol. R. Coll. Radiol. 24, 508–520 (2012).
Bayman, N., Blackhall, F., McCloskey, P., Taylor, P. & Faivre-Finn, C. How can we optimise concurrent chemoradiotherapy for inoperable stage III non-small cell lung cancer? Lung Cancer 83, 117–125 (2014).
Hudson, A., Chan, C., Woolf, D., McWilliam, A., Hiley, C., O’Connor, J. et al. Is heterogeneity in stage 3 non-small cell lung cancer obscuring the potential benefits of dose-escalated concurrent chemo-radiotherapy in clinical trials? Lung Cancer 118, 139–147 (2018).
Kong, F. M., Ten Haken, R. K., Schipper, M., Frey, K. A., Hayman, J., Gross, M. et al. Effect of midtreatment PET/CT-adapted radiation therapy with concurrent chemotherapy in patients with locally advanced non-small-cell lung cancer: a phase 2 clinical trial. JAMA Oncol. 3, 1358–1365 (2017).
Koshy, M., Malik, R., Spiotto, M., Mahmood, U., Rusthoven, C. G. & Sher, D. J. Association between intensity modulated radiotherapy and survival in patients with stage III non-small cell lung cancer treated with chemoradiotherapy. Lung Cancer 108, 222–227 (2017).
Lin, H., Chen, Y., Shi, A., Pandya, K. J., Yu Rong, Yuan, Y. et al. Phase 3 randomized low-dose paclitaxel chemoradiotherapy study for locally advanced non-small cell lung cancer. Front. Oncol. 6, 260 (2016).
Antonia, S. J., Villegas, A., Daniel, D., Vicente, D., Murakami, S., Hui, R. et al. Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. N. Engl. J. Med. 377, 1919–1929 (2017).
Dewan, M. Z., Galloway, A. E., Kawashima, N., Dewynegaert, J. K., Babb, J. S., Formenti, S. C. et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin. Cancer Res. 15, 5379–5388 (2009).
Deng, L., Liang, H., Burnette, B., Beckett, M., Darga, T., Weichselbaum, R. R. & Fu, Y. X. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J. Clin. Invest. 124, 687–695 (2014).
Yoshimoto, Y., Suzuki, Y., Mimura, K., Ando, K., Oike, T., Sato, H. et al. Radiotherapy-induced anti-tumor immunity contributes to the therapeutic efficacy of irradiation and can be augmented by CTLA-4 blockade in a mouse model. PloS ONE 9, e92572 (2014).
Dovedi, S. J., Adlard, A. L., Lipowska-Bhalla, G., McKenna, C., Jones, S., Cheadle, E. J. et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 74, 5458–5468 (2014).
Zeng, J., See, A. P., Phallen, J., Jackson, C. M., Belcaid, Z., Ruzevick, J., Durham, N. et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int. J. Radiat. Oncol. Biol. Phys. 86, 343–349 (2013).
Demaria, S., Kawashima, N., Yang, M. A., Devitt, M. L., Babb, J. S., Allison, J. P. et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin. Cancer Res. 11, 728–734 (2005).
Twyman-Saint Victor, C., Rech, A. J., Maity, A., Rengan, R., Pauken, K. E., Stelekati, E. et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 520, 373–377 (2015).
Yokouchi, H., Yamazaki, K., Chamoto, K., Kikuchi, E., Shinagawa, N., Oizumi, S. et al. Anti-OX40 monoclonal antibody therapy in combination with radiotherapy results in therapeutic antitumor immunity to murine lung cancer. Cancer Sci. 99, 361–367 (2008).
Reck, M., Rodriguez-Abreu, D., Robinson, A. G., Hui, R., Csoszi, T., Fulop, A. et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. 375, 1823–1833 (2016).
Herbst, R. S., Baas, P., Kim, D. W., Felip, E., Perez-Garcia, J. L., Han, J. Y. et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387, 1540–1550 (2016).
Yip, K., Melcher, A., Harrington, K., Illidge, T., Nobes, J., Webster, A. et al. Pembrolizumab in combination with radiotherapy for metastatic melanoma—introducing the PERM Trial. Clin. Oncol. 30, 201–203 (2018).
Gajewski, T. F. The next hurdle in cancer immunotherapy: overcoming the non-t-cell-inflamed tumor microenvironment. Semin. Oncol. 42, 663–671 (2015).
Rizvi, N. A., Hellmann, M. D., Snyder, A., Kvistborg, P., Makarov, V., Havel, J. J. et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348, 124–128 (2015).
McGranahan, N., Furness, A. J. S., Rosenthal, R., Ramskov, S., Lyngaa, R., Saini, S. K. et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 351, 1463–1469 (2016).
Pitt, J. M., Vetizou, M., Daillere, Roberti, M. P., Yamazaki, T., Routy, B. et al. Resistance mechanisms to immune-checkpoint blockade in cancer: tumor-Intrinsic and -Extrinsic Factors. Immunity 44, 1255–1269 (2016).
Smyth, M. J., Ngiow, S. F., Ribas, A. & Teng, M. W. L. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat. Rev. Clin. Oncol. 13, 143–158 (2016).
Long, G. V., Dummer, R., Ribas, A., Puzanov, I., Michielin, O., VanderWalde, A. et al. A Phase I/III, multicenter, open-label trial of talimogene laherparepvec (T-VEC) in combination with pembrolizumab for the treatment of unresected, stage IIIb-IV melanoma (MASTERKEY-265). J. Immunother. Cancer 3, P181 (2015).
Langer, C. J., Gadgeel, S. M., Borghaei, H., Papadimitrakopoulou, V. A., Patnaik, A., Powell, S. F. et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 17, 1497–1508 (2016).
Jotte, R. M., Cappuzzo, F., Vynnychenko, I., Stroyakovsky, D., Abreu, D. R., Hussein, M. A. et al. IMpower131: Primary PFS and safety analysis of a randomized phase III study of atezolizumab + carboplatin + paclitaxel or nab-paclitaxel vs carboplatin + nab-paclitaxel as 1L therapy in advanced squamous NSCLC. J. Clin. Oncol. 36, LBA9000 (2018).
Demaria, S., Coleman, S. N. & Formenti, S. C. Radiotherapy: changing the game in immunotherapy. Trends Cancer 2, 286–294 (2016).
Melero, I., Ainhoa, A., Murillo, O., Dubrot, J., Alfaro, C., Perez-Garcia, J. L. et al. Immunogenic cell death and cross-priming are reaching the clinical immunotherapy arena. Clin. Cancer Res. 12, 2385–2389 (2006).
Golden, E. B. & Apetoh, L. Radiotherapy and immunogenic cell death. Semin. Radiat. Oncol. 25, 11–17 (2015).
McBride, W. H., Chiang, C. S., Olson, J. L., Wang, C. C., Hong, J. H., Pajonk, F. et al. A sense of danger from radiation. Radiat. Res. 162, 1–19 (2004).
Deng, L., Liang, H., Zu, M., Yang, X., Burnette, B., Arina, A. et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity 41, 843–852 (2014).
Sharabi, A. B., Nirschl, C. J., Kochel, C. M., Nirschl, T. R., Francica, B. J., Velarde, E. et al. Stereotactic radiation therapy augments antigen-specific PD-1-mediated antitumor immune responses via cross-presentation of tumor antigen. Cancer Immunol. Res. 3, 345–355 (2015).
Reits, E. A., Hodge, J. W., Herberts, C. A., Groothuis, T. A., Chakraborty, M., Wansley, E. K. et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J. Exp. Med. 203, 1259–1271 (2006).
Matsumura, S. & Demaria, S. Up-regulation of the pro-inflammatory chemokine CXCL16 is a common response of tumor cells to ionizing radiation. Radiat. Res. 173, 418–425 (2010).
Dubinett, S. M., Lee, J. M., Sharma, S. & Mulé, J. J. Chemokines: can effector cells be redirected to the site of the tumor? Cancer J. Sudbury Mass. 16, 325–335 (2010).
Park, H. J., Griffin, R. J., Hui, S., Levitt, S. H. & Song, C. W. Radiation-induced vascular damage in tumors: implications of vascular damage in ablative hypofractionated radiotherapy (SBRT and SRS). Radiat. Res. 177, 311–327 (2012).
Lugade, A. A., Moran, J. P., Gerber, S. A., Rose, R. C., Frelinger, J. G. & Lord, E. M. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J. Immunol. 174, 7516–7523 (2005).
Jalal, S. I., Riggs, H. D., Melnyk, A., Richards, D., Agarwala, A., Neubauer, M. et al. Updated survival and outcomes for older adults with inoperable stage III non-small-cell lung cancer treated with cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel: analysis of a phase III trial from the Hoosier Oncology Group (HOG) and US Oncology. Ann. Oncol. 23, 1730–1738 (2012).
Schild, S. E., Stella, P. J., Geyer, S. M., Bonner, J. A., McGinnis, W. L., Mailliard, J. A. et al. The outcome of combined-modality therapy for stage III non-small-cell lung cancer in the elderly. J. Clin. Oncol. 21, 3201–3206 (2003).
Schild, S. E., Mandrekar, S. J., Jatoi, A., McGinnis, W. L., Stella, P. J., Deming, R. L. et al. The value of combined modality therapy in elderly patients with stage III non-small cell lung cancer (NSCLC). J. Clin. Oncol. 25, 19503–19503 (2007).
Miller, E. D., Fisher, J. L., Haglund, K. E., Grecula, J. C., Zu-Welliver, M. X., Bertino, E. M. et al. The addition of chemotherapy to radiation therapy improves survival in elderly patients with stage III non-small cell lung cancer. J. Thorac. Oncol. 13, 426–435 (2018).
Oxford Centre for Evidence-based Medicine. Levels of Evidence. 2009. Available at: https://www.cebm.net/2009/06/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/. (last accessed Dec 2019).
Acknowledgements
AstraZeneca suggested the topics for the supplement, selected the authors, made honoraria payments to the authors, provided editorial comments and a full technical and medical review of the materials included within this supplement.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent to publish
Not applicable.
Data availability
Not applicable.
Competing interests
J. Conibear has attended advisory boards for, and received speaker fees from, AstraZeneca. The author does not report any competing interests with regard to the contents of this study other than those stated.
Funding information
This promotional supplement has been commissioned and funded by AstraZeneca UK Limited (“AstraZeneca”).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This promotional supplement has been commissioned and funded by AstraZeneca UK Limited (“AstraZeneca”). AstraZeneca suggested the topics for the supplement, selected the authors, made honoraria payments to the authors, provided editorial comments and a full technical and medical review of the materials included within the supplement. Date of preparation: December 2020 / GB-25434
Appendix
Appendix
Prescribing information
IMFINZI® ▼(durvalumab) 50 mg/ml solution for infusion.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Conibear, J., on behalf of AstraZeneca UK Limited. Rationale for concurrent chemoradiotherapy for patients with stage III non-small-cell lung cancer. Br J Cancer 123 (Suppl 1), 10–17 (2020). https://doi.org/10.1038/s41416-020-01070-6
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-020-01070-6
- Springer Nature Limited
This article is cited by
-
Establishment of a prognostic nomogram for elderly patients with limited-stage small cell lung cancer receiving radiotherapy
Scientific Reports (2024)
-
I-125 seeds with chemotherapy for progressive non-small-cell lung cancer after first-line treatment: a meta-analysis
Journal of Cardiothoracic Surgery (2022)
-
Immunotherapy in Small Cell Lung Cancer Treatment: a Promising Headway for Future Perspective
Current Treatment Options in Oncology (2022)
-
PDS5B inhibits cell proliferation, migration, and invasion via upregulation of LATS1 in lung cancer cells
Cell Death Discovery (2021)