Abstract
Introduction The purpose of this study was to test the short-term efficacy of four commercial mouthwashes versus water in reducing SARS-CoV-2 viral load in the oral cavity over clinically relevant time points.
Methods In total, 32 subjects that were proven SARS-CoV-2-positive via polymerase chain reaction (PCR)-based diagnostic test were recruited and randomised into five parallel arms. Cycle threshold (Ct) values were compared in saliva samples between the groups, as well as within the groups at baseline (pre-rinse), zero hours, one hour and two hours post-rinse, using SARS-CoV-2 reverse transcription-PCR analysis.
Results We observed a significant increase in Ct values in saliva samples collected immediately after rinsing with all the four mouthwashes - 0.12% chlorhexidine gluconate, 1.5% hydrogen peroxide, 1% povidone iodine, or Listerine - compared to water. A sustained increase in Ct values for up to two hours was only observed in the Listerine and chlorohexidine gluconate groups. We were not able to sufficiently power this clinical trial, so the results remain notional but encouraging and supportive of findings in other emerging mouthwash studies on COVID-19, warranting additional investigations.
Conclusions Our evidence suggests that in a clinical setting, prophylactic rinses with Listerine or chlorhexidine gluconate can potentially reduce SARS-CoV-2 viral load in the oral cavity for up to two hours. While limited in statistical power due to the difficulty in obtaining this data, we advocate for pre-procedural mouthwashing, like handwashing, as an economical and safe additional precaution to help mitigate the transmission of SARS-CoV-2 from a potentially infected patient to providers.
Key points
-
Prophylactic mouthwash with Listerine or chlorhexidine gluconate may potentially reduce SARS-CoV-2 oral viral load.
-
In our analysis, Listerine and chlorhexidine gluconate mouthwash outperformed hydrogen peroxide and povidone iodine in reducing SARS-CoV-2 oral viral load.
-
Prophylactic mouthwashes, including Listerine or chlorhexidine gluconate, can be effective in reducing oral viral load for up to two hours during oral exams and procedures.
-
We advocate for clinicians to employ mouthwashing with Listerine or chlorhexidine gluconate before oral procedures, like handwashing, to reduce the potential spread of SARS-CoV-2 in the healthcare setting.
Similar content being viewed by others
Introduction
Considering the role of the oral cavity in the pathogenicity and transmission of severe acute respiratory syndrome coronavirus (SARS-CoV-2)1,2 and the routine aerosol-generating nature of procedures in dentistry,3 dental providers and oral surgeons are at particular high risk of COVID-19 transmission.1,4,5,6 During the ongoing COVID-19 pandemic, dentists and other healthcare professionals who work in the oral cavity have implemented the use of mouth rinses to reduce viral loads as recommended by the American Dental Association (ADA). Prophylactic rinses are commonly used before routine procedures like dental fillings and cleanings, or before surgeries requiring intubation.7 By disinfecting the oral cavity through bacteriostatic, bactericidal and virucidal rinses, practitioners seek to mitigate the risk of spreading an infection from a patient's mouth to other parts of their body or to the outside environment.
Therapeutic mouthwashes which contain active ingredients, such as chlorhexidine gluconate (CHX), hydrogen peroxide (H2O2), povidone-iodine (PI), or alcohol-based Listerine, are commonly used in dentistry. Existing data provide evidence for their efficacy in reducing bacterial and viral loads in in vitro cell cultures,8,9,10,11 oral cavities12 and aerosols13,14 generated during procedures. Recent systematic reviews also strongly support the hypothesis that prophylactic rinses can be effective in reducing the oral viral load of SARS-Cov-2.2,4,15 At the time of this study design, there was little empirical data to support pre-procedural rinses to reduce COVID-19 transmission to healthcare professionals or patients.12,16,17 Currently, the ADA states that evidence supports pre-procedural mouth rinses as effective in reducing oral viral load, but this has not been directly proven to reduce in-office spread of viruses and more research is needed.18 It is imperative to know if a reduction in viral loads lasts for a clinically meaningful duration that would provide a safe window to conduct routine procedures.19 While in vitro experiments provide controlled information, we attempted to best mimic the in vivo environments encountered in a healthcare setting during oral procedures. Hence, we conducted a prospective, parallel groups, randomised, double-blind, controlled, clinical trial to compare the efficacy of prophylactic rinses in reducing the salivary load of SARS-CoV-2 for up to two hours post rinsing. We compared the cycle threshold (Ct) values compared in saliva samples between the groups and within the groups at baseline (pre-rinse), zero hours, one hour and two hours post rinse, using SARS-CoV-2 reverse transcription-polymerase chain reaction (RT-PCR) analysis. We aim to identify a safe and economical way of reducing the potential spread of COVID-19 from patient to providers and staff through pre-procedural rinses and furthermore, determine which rinse, if any, is the most effective in doing so.
Methods
Participants
COVID-19 patients, whose nasopharyngeal swabs or saliva sample tested positive for SARS-CoV-2 virus in a PCR-based diagnostic test at the Augusta University (AU) Medical Centre drive-through COVID testing facility, were approached for study participation. The study was opened to recruitment in October 2020. However, due to low subject recruitment rate from the hospitalised population, the protocol was amended to include remote study visits. For the later cohort, subject eligibility was assessed via telephone and subjects consented over a virtual platform using the guidelines provided by AU Institutional Review Board. Once consent was obtained, a study kit was delivered to the subject's location. Study samples were self-collected under the observation of a study team member and stored on ice until collection. Most subjects were recruited remotely between February and June 2021. Patients included in this clinical trial were at least 18 years old, could understand English, were either non-symptomatic or symptomatic but not intubated, and self-reported no allergies to Listerine, PI, or CHX. In addition, patients willing to do remote visits required access to video conference technology. A total of 32 COVID-19 patients consented to the trial. Informed consent was obtained from all subjects, all subjects were able to consent for themselves, and were able to withdraw from the study if desired at any time. The study design was reviewed and approved by the Institutional Review Board of AU (protocol number 1607808) and the Human Subjects Ethics Board of AU and was conducted in accordance with the Helsinki Declaration of 1975.
Table 1 presents the characteristics of the recruited subjects.
Experimental design
Enrolled patients completed a demographic/health assessment form. Eligible subjects were randomly assigned to one of the five treatment groups: A) 0.12% CHX (Peridex); B) 1.5% H2O2; C) PI mouthwash (diluted 1% Betadine); D) Listerine (eucalyptol 0.092%, menthol 0.042%, methyl salicylate 0.060%, thymol 0.064% as active components, with 20% ethanol as inactive agent); or E) water. Concentrations of mouthwashes were determined to either commercially available antimicrobial formulations of 1.5% H2O2, Peridex and Listerine, while 1% PI antimicrobial mouthwash dilution was determined according to published antimicrobial recommendations.20 Visits were conducted by the coordinator and the principal investigator. Participants were assigned to interventions using a block randomisation allocation sequence generated by a biostatistician using SAS 9.4 programme (SAS Institute Inc, Cary, NC, 2016) and a size five block. Solutions were coded, though the colour and taste of the mouthwashes were not masked. Thirty minutes before each saliva sample collection, the patients were asked to rinse their mouths with water to remove food debris and to refrain from eating or drinking to prevent interference in downstream processing of the sample. At the time of planning this study, there were a very limited number of clinical trials reported that examined the effect of mouth rinses in eliminating SARS-CoV-2 virus from oral cavity. Hence, initially, the study was designed with five subjects in each group.21
Subsequently, several clinical trials were published that reported the effect of certain oral mouth rinses (PI, CHX included) in decreasing the salivary load of SARS-CoV-2. These studies were used to a power analysis for sample size calculation. Sample size was calculated based on differences in absolute Ct value for each group, assuming equivalence superiority of the treatment groups (mouth rinses) over the control group (water). The mean Ct value under null hypothesis was set at 27.7 (standard deviation [SD] = 4.8). A difference of nine units between the groups was considered negligible and a difference greater than one unit was considered significant. To achieve 95% power using these assumptions, six patients per group were necessary. Based on these assumptions, a sample size of n = 7 considering dropout for each group was required to achieve 95% power, with a significance level set at 0.05. Because of unknowns regarding COVID-19 in our initial study design and difficulties encountered in both enrolment and retention, we were not able to achieve this power; thus, the results remain promising but speculative, warranting further investigation.
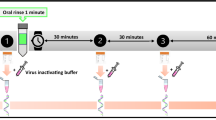
For the study, participants were required to have video conferencing capabilities. A team member video-called each participant before the start of the protocol and reviewed the study details. The team member proctored all procedures via close remote video supervision throughout the study, including the opening of the kit, sample collections, rinsing, and sealing and packaging for pick-up. Four unstimulated saliva samples were collected from each subject by asking them to spit approximately 1 ml of saliva in a saliva collection tube as previously reported.22 A baseline saliva sample (pre-rinse) was collected before rinsing the oral cavity with the assigned mouthwash. The subjects were then asked to rinse their mouth with 5 ml of the assigned mouthwash for two minutes and to discard the rinse. Three post-rinse saliva samples were collected immediately after (post), one-hour post, and two-hours post rinse. To rule out any interference from mouthwash solution in the estimation of viral loads in saliva samples, spiked samples were run as controls before running the test samples (data not shown).
Samples were stored on ice and transported to the SARS-CoV-2 testing facility at AU within 12 hours of sample collection for further processing in accordance with the Centers for Disease Control and Prevention interim guidelines.23 The testing facility was blinded to samples and was not provided with group assignments. The complete protocol can be obtained from the corresponding author upon reasonable request.
SARS-CoV-2 detection method
Viral load was measured through quantitative PCR using Ct values, or the number of PCR cycles required for machine detection, as a readout of viral load. Therefore, lower Ct values were interpreted as higher viral loads and higher Ct values indicated a lower viral load. The assay was performed on a Food and Drug Administration emergency use authorisation kit in a Clinical Laboratory Improvement Amendments facility. Perkin Elmer's coronavirus nucleic acid detection kit (P/N: 2019-nCOV-PCR-AUS) (PerkinElmer Inc, Waltham, MA) was used to detect SARS-CoV-2. The SARS-CoV-2 assay used was based on automated ribonucleic acid (RNA) extraction in the Chemagic 360 instrument (Perkin Elmer) using Chemagic Viral DNA/RNA kit H96 (P/N: CMG-1033-S), followed by TaqMan-based real time-PCR assay. For the detection step, in vitro transcription of SARS-CoV-2 RNA, complementary DNA amplification, and fluorescence detection were completed in a single tube. The assay targeted specific genomic regions of SARS-CoV-2: nucleocapsid (N) gene and ORF1ab gene. The TaqMan probe for the N gene target amplicon was labelled with 6-carboxyfluorescein (6-FAM), while the ORF1ab gene target amplicon was labelled with 6-carboxy-X-rhodamine (6-ROX) fluorescent dyes, respectively, to generate target-specific signals. The assay included an RNA internal control (Bacteriophage MS2) that served as assay control from nucleic acid extraction to fluorescence detection. The internal control probe was labelled with victoria fluorescent (VIC) dye to differentiate its fluorescent signal from SARS-CoV-2 targets. Real time PCR was carried out either in QuantStudio3's or QuantStudio5's PCR instrument (ABI, Thermofisher). Several tubes arrived without sufficient sample to conduct a PCR test and had to be excluded from the analysis. RT-PCR data analysis was carried out QuantStudio Design and Analysis Software.24
Data analysis
Samples for which Ct values were below the detection limit for both N gene and ORF1ab gene at the baseline sample pre-rinse time points were excluded from the data analysis. For statistical analysis, Ct values that were below the detection limit were replaced by 40 in post-rinse samples across the samples.
The absolute Ct values were analysed using a repeated-measures analysis of variance with one within-factor timepoint, with four levels: pre-rinse (pre), immediate post rinse (post), one-hour post-rinse, two-hour post-rinse; and one between-factor mouth rinse, with five levels: 0.12% CHX; 1.5% H2O2; PI mouthwash; Listerine mouthwash; and control. Because of the exploratory nature of this study, there was no adjustment for multiple testing. All statistical tests were two-tailed and were performed at the 0.05 level of significance. All analyses were carried out using SAS 9.4.
Results
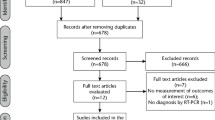
Of the 32 subjects enrolled, one withdrew before the visit, and another was withdrawn due to non-compliance with the protocol after randomisation and completion of the visit. Baseline saliva (pre-rinse) samples for 14 subjects had Ct values below the detection limit for both N and ORF1ab genes, and these subjects were also excluded from further analysis (Fig. 1). In addition, baseline saliva (pre-rinse) samples for two subjects had Ct values below the detection limit for the N gene and were not included in the analysis.
Flow diagram depicting the progress of the study from participant enrolment to data analysis phase. (Note: @ = subject withdrew from the study after consenting and randomisation; * = Ct values below the detection limit for both N gene and ORF1ab gene in the baseline [pre-rinse] sample; # = non-compliance with sample collection protocol)
Figure 2 presents the Ct values for the N gene for individual subjects and group mean at each time point within each group. The mean and standard error (SE) for the N gene within each group are presented in Figure 2f. Compared to control, a significant increase in Ct values was observed immediately after rinsing with each of the mouthwashes tested (p <0.05) (post-timepoint). The Ct values for the Listerine group were higher than the H2O2 and PI groups and reached the statistical significance cut-off (Listerine vs H2O2 [p = 0.0457] and Listerine vs PI [p = 0.0451]). A statistically significant difference in Ct values was also observed between the CHX group and Listerine group compared to the water group, at the post one-hour and post two-hours rinse. As Ct values are inversely proportional to the viral load in the sample, a sustained increase in Ct value for the CHX group and Listerine group suggests their effectiveness in reducing viral load in the oral cavity over the two-hour course we tested compared to water control. In contrast, the H2O2group Ct value was significantly higher at the two-hour time point (p <0.05), but not at the one-hour time point.
Ct values for the N gene in the saliva samples collected at baseline (pre) and immediately after rinsing (post), one hour and two hours. The dotted line represents the Ct values for individual subjects and the solid black line represents the group means along with SE. a) 0.12% chlorhexidine gluconate mouthwash. b) 1.5% H2O2 mouthwash. c) 1% betadine mouthwash. d) Alcohol-based Listerine. e) Water groups. f) The mean and SE for the N gene within each group. As Ct values are inversely proportional to viral load, the y-axis represents Ct values on a reverse scale
Figure 3 presents the Ct values for the ORF1ab gene for individual subjects and group mean at each time point within each group. Similar to the N gene, there were statistically significant differences between those who rinsed with water and those who rinsed with Listerine over the two-hour time course post rinse (p <0.5). For the other three types of mouthwash tested, Ct values were increased in post-rinse samples, but none of the values were statistically significant (Fig. 3f).
Ct values for the ORF1ab gene in the saliva samples collected at baseline (pre) and immediately after rinsing (post), one hour and two hours. The dotted line represents the Ct values for individual subjects and the solid black line represents the group means along with SE. a) 0.12% chlorhexidine gluconate mouthwash. b) 1.5% H2O2 mouthwash. c) 1% betadine mouthwash. d) Alcohol-based Listerine. e) Water groups. f) The mean and SE for the ORF1ab gene within each group. As Ct values are inversely proportional to viral load, the y-axis represents Ct values on a reverse scale
There were no statistically significant differences among the time points within the mouthwash groups for both N and ORF1ab genes. Among those participants who rinsed with CHX, the following timepoint comparisons almost did not reach statistical significance: pre vs post (p = 0.0761) and pre vs one hour (p = 0.0812). Among those who rinsed with H2O2, the following comparisons almost, but did not, reach significance: pre vs post (p = 0.0761) and pre vs one hour (p = 0.0677) for N gene and pre vs post (p = 0.0966) for ORF1ab gene.
No adverse events were reported by any subject during the study.
Discussion
In response to the COVID-19 pandemic, healthcare had to act quickly to adopt interventions to reduce the risk of spreading coronavirus in the workplace. One of the recommendations by dental professional associations was to use pre-procedural mouthwash rinses with 1.5% H2O2or 0.2% PI to curtail the transmission of SARS-CoV-2. Though limited by sample size, this randomised clinical trial (RCT) provides preliminary evidence to support the use of prophylactic mouth rinses with alcohol-based Listerine or 0.12% CHX to reduce SARS-CoV-2 viral load in the oral cavity. In contrast, we have limited evidence to support the use of prophylactic rinses with 1% PI and 1.5% H2O2to reduce SARS-CoV-2. While gargling was not performed in this study, the utility of the mouth rinse might be expanded if patients gargled before intraoral procedures.
SARS-CoV-2 is a lipid-enveloped virus, and hence the agents which interfere with its outer membrane may be effective in inactivating it.25 Alcohol-based mouthwashes have been shown to inactivate enveloped viruses in vitro, such as herpes, influenza and even coronavirus.11,26,27 These studies provide proof-of-concept that ethanol-based mouthwashes can be effective in reducing enveloped viruses and support our findings in the Listerine group. To our knowledge, this is the first report of the effectiveness of alcohol-based mouthwash in reducing the viral load of SARS-CoV-2 in the oral cavity in an RCT,28,29 and is worth pursuing further with a sufficiently powered trial.
CHX is a standard antiseptic recommended for plaque control and management of periodontitis. Despite limited evidence for CHX inactivation of SARS-CoV-2 in in vitro assays,26,27 data from human studies support our findings. Early into the pandemic, Yoon et al. first reported a significant reduction in SARS-CoV-2 viral load in the saliva for up to two hours following gargling with 0.12% CHX. Similar results were observed in three recently reported RCTs, who tested the efficacy of 0.12% CHX in reducing the oral load of SARS-CoV-2 for a up to one hour in a larger cohort.30,31,32 In contrast, another RCT that collected saliva samples 3-6 hours after rinsing the oral cavity with 0.2% CHX solution did not find consistent reductions in Ct values, compared to baseline or control.33 Together with our findings, it suggests that rinsing with CHX has the potential to be effective in reducing the oral viral load over short durations.
A significant increase in Ct values was also observed, immediately after rinsing with 1% PI or 1.5% H2O2. However, in the former group, the increased Ct values were not sustained over longer time intervals, whereas for the latter group, the Ct-values did not show a consistent trend, making it difficult to make meaningful conclusions about the effectiveness of H2O2in decreasing viral load for longer time durations. However, these findings in the H2O2group agree with a recent clinical trial, which showed that pre-rinse with 1.5% H2O2mouthwash resulted in only transiently reduced oral SARS-CoV-2, lasting about 30 minutes, with nearly baseline Ct values at the 60-minute time point.31
Despite the qualitative trends in this trial, there are some shortcomings to discuss. There was great difficulty in obtaining this data set, related to the regulation of working with SARS-CoV-2, the difficulty in obtaining the specimens, and the logistical issues raised by this global pandemic. Subject recruitment was stopped before the planned recruitment target, impeding a statistically significant outcome. SARS-CoV-2 infection rate declined drastically by June 2021 in Augusta, Georgia, and many of the patients enrolled were determined to be false-positive. Consequently, we withdrew 14 subjects from the study, as Ct values were below detection limits in the baseline saliva samples of these subjects. This major withdrawal also resulted in disturbing the randomisation of the subjects and one discrepancy was a significant difference between Listerine and water (p = 0.0363) in the pre-rinse samples for these groups.
Due to difficulties in culturing SARS-CoV-2 and the highly contagious nature of the virus, the viability of the viruses in the saliva samples was not determined. Hence, it cannot be clearly established if the viruses detected based on RT-PCR methodology were still infectious. Debate on whether aerosols or droplets of oral and respiratory secretions are the primary means of COVID-19 spread, but regardless, these fluids contain infectious viral particles that can spread the virus from host to host.34,35 Additionally, saliva has been established as a reliable clinical sample source in order to determine the viral load in the subjects.36 Our data supports qualitative trends that justify the need for additional studies on the use of Listerine or CHX as promising agents to reduce the spread of SARS-CoV-2 when used as pre-procedural rinses.
Conclusion
Considering the evolution of novel strains of SARS-CoV-2, we must find the tools to live with this virus. Despite the limitations, our study does provide clinical evidence in utilising prophylactic mouth rinses with Listerine and CHX to reduce the oral load of the SARS-CoV-2 virus. We promote the use of pre-procedural mouth rinses in a utility similar to handwashing as an additional tool to help reduce the spread of COVID-19 from a potentially infected patient to providers that will be working in or around an unmasked mouth. This simple, affordable, and innocuous technique, which is already in place in many dental settings, may have the potential to reduce transmission of SARS-CoV-2 and create a safer work environment in healthcare.
References
Peng X, Xu X, Li Y, Cheng L, Zhou X, Ren B. Transmission routes of 2019-nCoV and controls in dental practice. Int J Oral Sci 2020; 12: 9.
Herrera D, Serrano J, Roldán S, Sanz M. Is the oral cavity relevant in SARS-CoV-2 pandemic? Clin Oral Investig 2020; 24: 2925-2930.
Harrel S K, Molinari J. Aerosols and splatter in dentistry: a brief review of the literature and infection control implications. J Am Dent Assoc 2004; 135: 429-437.
Silvestre F-J, Martinez-Herrera M, Márquez-Arrico C-F, Silvestre-Rangil J. COVID-19, A new challenge in the dental practice. J Clin Exp Dent 2021; DOI: 10.4317/jced.57362.
Meng L, Hua F, Bian Z. Coronavirus Disease 2019 (COVID-19): Emerging and Future Challenges for Dental and Oral Medicine. J Dent Res 2020; 99: 481-487.
American Dental Association. ADA recommending dentists postpone elective procedures. 2020. Available at https://www.ada.org/publications/ada-news/2020/march/ada-recommending-dentists-postpone-elective-procedures (accessed March 2023).
Gunsolley J C. Clinical efficacy of antimicrobial mouthrinses. J Dent 2010; DOI: 10.1016/S0300-5712(10)70004-X.
Wood A, Payne D. The action of three antiseptics/disinfectants against enveloped and non-enveloped viruses. J Hosp Infect 1998; 38: 283-295.
Bernstein D, Schiff G, Echler G, Prince A, Feller M, Briner W. In vitro virucidal effectiveness of a 0.12%-chlorhexidine gluconate mouthrinse. J Dent Res 1990; 69: 874-876.
Siddharta A, Pfaender S, Vielle N J et al. Virucidal Activity of World Health Organisation-Recommended Formulations Against Enveloped Viruses, Including Zika, Ebola, and Emerging Coronaviruses. J Infect Dis 2017; 215: 902-906.
Dennison D K, Meredith G M, Shillitoe E J, Caffesse R G. The antiviral spectrum of Listerine antiseptic. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1995; 79: 442-448.
Goutham B S, Manchanda K, Sarkar A D, Prakash R, Jha K, Mohammed S. Efficacy of two commercially available Oral Rinses - Chlorohexidine and Listrine on Plaque and Gingivitis - A Comparative Study. J Int Oral Health 2013; 5: 56-61.
Marui V C, Souto M L S, Rovai E S, Romito G A, Chambrone L, Pannuti C M. Efficacy of preprocedural mouthrinses in the reduction of microorganisms in aerosol: A systematic review. J Am Dent Assoc 2019; 150: 1015-1026.
Mohd-Said S, Mohd-Dom T N, Suhaimi N, Rani H, McGrath C. Effectiveness of Pre-procedural Mouth Rinses in Reducing Aerosol Contamination During Periodontal Prophylaxis: A Systematic Review. Front Med (Lausanne) 2021; 8: 600769.
Moosavi M-S, Aminishakib P, Ansari M. Antiviral mouthwashes: possible benefit for COVID-19 with evidence-based approach. J Oral Microbiol 2020; 12: 1794363.
Burton M J, Clarkson J E, Goulao B et al. Use of antimicrobial mouthwashes (gargling) and nasal sprays by healthcare workers to protect them when treating patients with suspected or confirmed COVID-19 infection. Cochrane Database Syst Rev 2020; DOI: 10.1002/14651858.CD013626.pub2.
Burton M J, Clarkson J E, Goulao B et al. Antimicrobial mouthwashes (gargling) and nasal sprays administered to patients with suspected or confirmed COVID-19 infection to improve patient outcomes and to protect healthcare workers treating them. Cochrane Database Syst Rev 2020; DOI: 10.1002/14651858.CD013627.pub2.
American Dental Association. Mouthrinse (Mouthwash). 2021. Available at https://www.ada.org/resources/research/science-and-research-institute/oral-health-topics/mouthrinse-mouthwash (accessed March 2023).
American Dental Association. 2010 Survey of Dental Practice. Oral and Maxillofacial Surgeons in Private Practice. 2012. Available at https://www.ada.org/resources/research/health-policy-institute/dental-practice-research (accessed March 2023).
Eggers M. Infectious Disease Management and Control with Povidone Iodine. Infect Dis Ther 2019; 8: 581-593.
Seneviratne C J, Balan P, Ko K K K et al. Efficacy of commercial mouth-rinses on SARS-CoV-2 viral load in saliva: randomized control trial in Singapore. Infection 2021; 49: 305-311.
Sahajpal N S, Mondal A K, Ananth S et al. Clinical Validation of a Sensitive Test for Saliva Collected in Healthcare and Community Settings with Pooling Utility for Severe Acute Respiratory Syndrome Coronavirus 2 Mass Surveillance. J Mol Diagn 2021; 23: 788-795.
Centers for Disease Control and Prevention. Specimen Collection. 2022. Available at https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html#previous (accessed March 2023).
Sahajpal N S, Mondal A K, Njau A et al. Proposal of RT-PCR-Based Mass Population Screening for Severe Acute Respiratory Syndrome Coronavirus 2 (Coronavirus Disease 2019). J Mol Diagn 2020; 22: 1294-1299.
O'Donnell V B, Thomas D, Stanton R et al. Potential Role of Oral Rinses Targeting the Viral Lipid Envelope in SARS-CoV-2 Infection. Function (Oxf) 2020; DOI: 10.1093/function/zqaa002.
Davies K, Buczkowski H, Welch S R et al. Effective in vitro inactivation of SARS-CoV-2 by commercially available mouthwashes. J Gen Virol 2021; 102: 001578.
Meister T L, Brüggemann Y, Todt D et al. Virucidal Efficacy of Different Oral Rinses Against Severe Acute Respiratory Syndrome Coronavirus 2. J Infect Dis 2020; 222: 1289-1292.
Hsue V B, Itamura K, Wu A W et al. Topical Oral and Intranasal Antiviral Agents for Coronavirus Disease 2019 (COVID-19). Adv Exp Med Biol 2021; 1327: 169-189.
Cavalcante-Leão B L, de Araujo C-M, Basso I-B et al. Is there scientific evidence of the mouthwashes effectiveness in reducing viral load in Covid-19? A systematic review. J Clin Exp Dent 2021; DOI: 10.4317/jced.57406.
Chaudhary P, Melkonyan A, Meethil A et al. Estimating salivary carriage of severe acute respiratory syndrome coronavirus 2 in nonsymptomatic people and efficacy of mouthrinse in reducing viral load: A randomized controlled trial. J Am Dent Assoc 2021; 152: 903-908.
Eduardo F P, Corrêa L, Heller D et al. Salivary SARS-CoV-2 load reduction with mouthwash use: A randomized pilot clinical trial. Heliyon 2021; DOI: 10.1016/j.heliyon.2021.e07346.
Costa D D, Brites C, Vaz S N, de Santana D S, Dos Santos J N, Cury P R. Chlorhexidine mouthwash reduces the salivary viral load of SARS-CoV-2: A randomized clinical trial. Oral Dis 2022; DOI: 10.1111/odi.14086.
Shetty S K, Sharath K, Shenoy S, Sreekumar C, Shetty R N, Biju T. Compare the effcacy of two commercially available mouthrinses in reducing viable bacterial count in dental aerosol produced during ultrasonic scaling when used as a preprocedural rinse. J Contemp Dent Pract 2013; 14: 848-851.
Shamsoddin E. Saliva: a diagnostic option and a transmission route for 2019-nCoV. Evid Based Dent 2020; 21: 68-70.
Johnson I G, Jones R J, Gallagher J E et al. Dental periodontal procedures: a systematic review of contamination (splatter, droplets and aerosol) in relation to COVID-19. BDJ Open 2021; 7: 15.
Nasiri K, Dimitrova A. Comparing saliva and nasopharyngeal swab specimens in the detection of COVID-19: A systematic review and meta-analysis. J Dent Sci 2021; 16: 799-805.
Acknowledgements
The authors would like to thank Dr Elizabeth Floodeen, resident at the Department of Oral and Maxillofacial Surgery, Dental College of Georgia, AU for her assistance in conducting remote study visits.
All data and materials relevant to this publication will be freely available to any scientist wishing to use them for non-commercial purposes, without breaching participant confidentiality. The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Funding
This study was sponsored by an intramural grant from Centre for Excellence in Research, Scholarship, and Innovation (CERSI) at the Dental College of Georgia (DCG). The funding organisations had no role in the study design, data collection and analysis, decision to publish, or preparation of the article.
Author information
Authors and Affiliations
Contributions
Jaspreet Kaur Farmaha: contributed to design, data acquisition and interpretation, and drafted the manuscript; Jeffrey N. James: contributed to conception, design, and critically revised the manuscript; Kyle Frazier: contributed to data acquisition and reviewed the manuscript; Nikhil Shri Sahajpal, Ashis K. Mondal and Ravindra Kolhe: contributed to methodology, sample analysis, data analyses and interpretation, and critically reviewed the manuscript; Doan T Bloomquist: critically revised the manuscript; Stephen W. Looney: contributed to statistical analyses, data interpretation, drafting and reviewed the manuscript; Ryan Bloomquist: contributed to conception, design, data acquisition and interpretation, and critically revised the manuscript. Jaspreet Kaur Farmaha and Jeffrey N. James contributed equally to this work.
Corresponding author
Ethics declarations
The authors declare no conflicts of interest.
All experiments and methods were performed in accordance with relevant guidelines and regulations. The study design was reviewed and approved by the Institutional Review Board of AU (protocol number 1607808). The study received an expedited review based on applicable federal regulations 45 CFR 46 (DHHS). This study was approved by the Human Subjects Ethics Board of AU and was conducted in accordance with the Helsinki Declaration of 1975 as revised in 2013.
Informed consent was obtained from all subjects and all subjects were able to consent for themselves. All subjects consented accordingly, approved of their own participation and were able to withdraw from the study if desired at any time.
This clinical trial was registered at ClinicalTrials.gov. The registration number is NCT04719208.
Rights and permissions
About this article
Cite this article
Farmaha, J., James, J., Frazier, K. et al. Reduction of SARS-CoV-2 salivary viral load with pre-procedural mouth rinses: a randomised, controlled, clinical trial. Br Dent J 234, 593–600 (2023). https://doi.org/10.1038/s41415-023-5741-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41415-023-5741-9
- Springer Nature Limited