Abstract
Ageing as a natural irreversible process inherently results in the functional deterioration of numerous organ systems and tissues, including the skeletal and immune systems. Recent studies have elucidated the intricate bidirectional interactions between these two systems. In this review, we provide a comprehensive synthesis of molecular mechanisms of cell ageing. We further discuss how age-related skeletal changes influence the immune system and the consequent impact of immune system alterations on the skeletal system. Finally, we highlight the clinical implications of these findings and propose potential strategies to promote healthy ageing and reduce pathologic deterioration of both the skeletal and immune systems.
Similar content being viewed by others
Introduction
Ageing is a complex and multifaceted process, resulting in functional decline and heightened vulnerability to diseases across various organ systems and tissues. Notably, the immune and skeletal systems, essential to overall health and vitality throughout one’s lifespan, exhibit pronounced vulnerability in this regard.1 Recent studies reveal a profound interconnection between the two systems, suggesting a finely tuned balance governing bone equilibrium and immune responses.2 Immunoageing, or the gradual weakening of immune functions over time, is characterized by decreased immune cell diversity and responsiveness, along with shifts in cytokine and chemokine profiles. Consequently, this culminates in a compromised immune surveillance mechanism, heightening vulnerability to a range of conditions comprising infections, cancers, and autoimmune disorders.3,4 Concurrently, age-related changes of the skeletal system is associated with reduced bone mass and density, increasing the risk of fractures and osteoporosis.5 Recent research emphasizes the mutual influence between the immune and the skeletal systems. The immune system modulates bone remodeling and regulates bone mass through the production of cytokines, chemokines, and growth factors.6 Inversely, bone-derived molecules such as fibroblast growth factor-23 (FGF-23) and osteopontin influence the development and function of immune cells, thereby contributing to the maintenance of immune homeostasis.7,8 Understanding the intricate interplay between the immune and skeletal systems is of paramount significance, as it paves the way for innovative therapeutic approaches aimed at promoting healthy ageing and countering age-related diseases.
The present review aims to provide a comprehensive overview of the current understanding of the interrelationship between age-related changes in bone and immunity. We summarize the essence of recent findings on this bone-immune nexus, elucidate the mechanisms governing bone and immune balance, and explore the potential clinical implications of these insights.
Age-related changes in bone structure and cell ageing
A comprehensive understanding of the complex interplay between bone structure alterations and cellular ageing is essential to unravel the intricate mechanisms underlying age-related bone deterioration. This section delves into the prominent aspects of age-related changes in bone structure and the implications of cell ageing within this context.
Age-related changes in bone structure
Age-related changes in bone structure encompass reduction of bone mass and microarchitectural alterations, culminating in the decline of skeletal integrity with subsequent compromised mechanical properties.9,10 The decline in bone mineral density (BMD) is a hallmark of skeletal ageing. This reduction predominantly arises from an imbalance between bone formation and bone resorption, with the latter becoming more pronounced as age advances. This nuanced interaction is influenced by factors such as reduced osteoblast activity and an increased release of pro-inflammatory molecules, including tumor necrosis factor-alpha (TNF-α), interleukin-1 (IL-1), and IL-6. These molecular signals collectively enhance the function of osteoclasts, the cells responsible for bone resorption.11,12 Moreover, alterations in bone microarchitecture, including trabecular thinning and increased cortical porosity, further contribute to reduced bone strength with increasing age. These structural disruptions can be attributed to imbalances in bone remodeling, where bone resorption outpaces bone formation, resulting in a negative bone turnover. Ageing induces a myriad of alterations in the structure, composition, and function of bones, resulting in a variety of age-related diseases of the skeletal system, such as osteoporosis, osteoarthritis, and fractures.13 As bone ages, there are notable shifts in its composition, including alterations in the collagen matrix and mineral content. An increase in collagen cross-linking with age results in enhanced bone rigidity and diminished mechanical resilience, factors that amplify bone fragility and increase fracture risk.14 The mechanical properties of bones, such as strength and flexibility, are vital for maintaining overall skeletal health and mobility.15 Ageing leads to a decline in these properties due to a confluence of changes in bone structure, composition, and overall function (Fig. 1). Therefore, understanding the complex signaling pathways and molecular regulators governing these alterations is crucial for devising interventions to counteract age-associated bone deterioration.
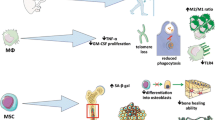
Age-related transformations in bone structure involve a decrease in bone mass and alterations in microarchitecture. The ageing process results in a reduction in the population of MSCs and osteoblasts, while there is an increase in osteoclasts and adipocytes, along with heightened levels of pro-inflammatory cytokines
Age-related changes in bone cell ageing
Cellular ageing is increasingly recognized as a central factor in the ageing process, with a significant impact on bone health. The senescence-associated secretory phenotype (SASP), characterized by the release of proinflammatory cytokines and growth factors, creates a microenvironment able to disrupt bone remodeling and to promote chronic inflammation.
Bone marrow mesenchymal stem cells (BMSCs) ageing
BMSCs are pivotal players in bone homeostasis and regeneration. However, with advancing age, BMSCs undergo ageing, leading to a series of molecular alterations that undermine their regenerative potential. Telomere shortening, a key mechanism underlying cellular ageing, occurs in BMSCs, prompting DNA damage response and subsequent growth arrest.16 In particular, the histone chaperone nucleosome assembly protein 1-like 2 (NAP1L2) activates NF-κB, thereby inducing MSCs ageing and hindering osteogenic differentiation. Mitophagy, which is vital for cellular maintenance, regulates the ageing of BMSCs by selectively removing damaged and dysfunctional mitochondria, thereby maintaining cellular homeostasis and forestalling harmful accumulation of mitochondrial components triggering BMSC ageing. Techniques such as increasing sirtuin 1 and 3 (Sirt1 and Sirt3) expression or inhibiting LRRc17 showcase the potential for modulating BMSC ageing through mitophagy activation.17,18 Concurrently, p16INK4a and p53 pathways are upregulated, further reinforcing the aged status.19 These molecular changes collectively contribute to diminished osteogenic differentiation, decreased self-renewal capacity, and altered secretion profiles.
The clinical consequences of MSCs ageing reverberate across various facets of bone health and regenerative capacity. A decline in osteogenic potential hinders MSCs from developing into effective osteoblasts, leading to compromised bone formation and decreased mineralization, ultimately leading to age-related bone loss and posing further challenges in fracture healing and bone repair.20 Furthermore, the shift in secretory profile toward proinflammatory cytokines and chemokines within the SASP creates a local microenvironment that disrupts the delicate balance between bone formation and resorption. This exacerbates chronic inflammation and disrupts normal bone remodeling processes, underpinning the pathogenesis of conditions such as osteoporosis and age-related bone fractures.21
Therapeutic approaches targeting MSC ageing hold immense potential. Measures focusing on molecular pathways involved in MSCs ageing, such as telomere maintenance, p16INK4a inhibition, and DNA damage response modulation, could provide avenues to rejuvenate MSC functionality.22,23 Clinically, 1,25-dihydroxyvitamin D (1,25(OH)2 D) has been reported to suppress BMSCs ageing, bolstering anti-osteoporosis effects.24 Additionally, pioneering stem cell-based therapies involving the transplantation of exogenous MSCs or their derivatives, and small molecules, such as noncoding RNAs, are gaining traction to counter the detrimental effects of MSCs ageing on bone health.25,26
Osteocyte and osteoprogenitor cell ageing
Osteocytes, the primary cells in bone tissue, govern bone remodeling by detecting mechanical signals and regulating osteoblast and osteoclast functions. Ageing-related changes in osteocytes are featured by a decrease in lacunae that contain osteocytes, loss of directional orientation of lacunae, and the number and length of dendrites that connect through canniculi.27 The removal of osteocytes culminates in the buildup of SASP in the bone marrow, disrupting the process of osteogenesis and accelerating skeletal ageing characterized by osteoporosis.28 Age induces ageing in these vital cells, driven by a multitude of factors (such as diabetes and estrogen), coupled with DNA damage due to oxidative stress and weakened DNA repair systems. This results in cell cycle arrest and SASP activation, diminishing the mechanical sensing functions of osteocytes and increasing the expression of neuropeptide Y (NPY), which curbs bone adaptation.29,30,31
osteoprogenitor cell, the cells responsible for bone formation, also undergo ageing, characterized by decreased telomerase activity, telomere shortening, oxidative accumulation and DNA damage.32 Research on Osx1-Cre; TdRFP mice has revealed a substantial decline in the number of TdRFP-Osx1 cells in the bone marrow, implying osteoprogenitor ageing. This is marked by DNA damage, cell cycle arrest, elevated p53 and p21CIP1 levels, all of which impact bone regeneration and create a proinflammatory microenvironment.33 Disrupted signaling pathways such as the Nrf2/GPX4, ROS/PADI2, mTORC1/Scn1a pathways also contribute to osteoblast ageing.34,35,36 Moreover, altered microRNA profiles (such as miR-29a, miR-19a-3p) in aged osteoblasts weaken bone formation.37,38 The SASP, extracellular vesicles (EVs), and cytokines (e.g., RANKL released by aged osteoblasts lead to disrupted bone structure by exacerbating inflammation as well as promoting osteoclastogenesis and angiogenesis dysfunctions.39
The ageing of osteocyte and osteoprogenitor cell have far-reaching clinical consequences and implications. Aged osteocytes fail to adequately transmit mechanical signals, thereby affecting bone quality and rendering it more susceptible to fractures and delayed fracture healing in the elderly population. Similarly, the decline in osteoprogenitor cell functionality contributes to reduced bone formation, leading to decreased bone mass and compromised bone strength. Novel therapeutic approaches are emerging to counteract ageing of both osteocytes and osteoprogenitor cell, which comprise targeting oxidative stress and DNA damage response pathways, aiming to mitigate the initiation of ageing.40 Additionally, modulating main pathways and microRNA profiles could enhance osteoprogenitor cell functionality. Innovative methods using senolytic drugs, like dasatinib and quercetin, to selectively remove aged cells hold promise in restoring bone remodeling balance.41
Osteoclast ageing
Osteoclasts, derived from hematopoietic progenitor cells, are pivotal for bone remodeling through resorption of old bone tissue. With advancing age, osteoclasts undergo ageing, characterized by molecular alterations driven by factors such as an accumulation of oxidative stress and DNA damage, ultimately resulting in delayed osteoclastogenesis while preserving bone resorption capacity.42 Imbalances, such as a deficiency in the molecule cathepsin K and integrins, can contribute to an excess of osteoclast due to impaired apoptosis and ageing, highlighting the pivotal role of molecules in regulating osteoclast lifespan and bone homeostasis.43
The clinical implications of osteoclast ageing are profound. An imbalance in the number of osteoclasts can disrupt the delicate equilibrium between bone formation and resorption, causing age-related bone density changes and compromised bone remodeling. The delayed osteoclastogenesis also leads to impaired removal of damaged bone tissue, consequently hindering its replacement with new bone tissue. As a result, the bone microarchitecture is altered, rendering bones more susceptible to fractures. Moreover, the altered SASP from aged osteoclasts fosters a proinflammatory microenvironment within the bone, contributing to the chronic low-grade inflammation inherent to ageing.
Strategies targeting osteoclast ageing present promising avenues for intervention. Modulation of oxidative stress and DNA damage response pathways could delay the initiation of ageing. Additionally, therapeutic approaches able to restore signaling pathways and eliminate cytokines are crucial for osteoclast functionality, such as C5AR1, which could improve bone resorption capacity.44 While merely removing aged osteoclasts does not mitigate age-related loss of bone mass,45 novel approaches involving senolytic agents such as zoledronic acid to selectively eliminate SASP hold potential for restoring the balance between bone formation and resorption.46
Bone marrow adipocyte (BMAd) ageing
BMAd cells, once viewed simply as passive energy storers, are now understood to actively influence bone balance and act as critical energy sources for certain cells in the bone marrow. Therefore, it is essential to recognize the impact of ageing on these cells.47 While research on BMAd ageing remains limited, there is growing evidence suggesting that adipocytes within the bone marrow may also undergo ageing. This ageing process is influenced by various factors, including oxidative stress, DNA damage, and chronic inflammation, ultimately leading to a notable shift in their secretory profile with elevated levels of proinflammatory cytokines. Moreover, recent studies have substantiated the notion that aged adipocytes could induce secondary ageing in osteoblasts and vascular cells, thereby compounding the creation of an inflammatory microenvironment that disrupts the delicate equilibrium between bone formation and resorption.48 Consequently, there is an urgent need to delve deeper into the phenomenon of BMAd ageing to better comprehend its implications for bone health and overall skeletal integrity.
Vascular ageing
Vascular ageing, a multifaceted process influenced by factors such as telomere shortening, oxidative stress, and chronic inflammation, brings about profound transformations in the ageing vasculature.49 Notably, endothelial dysfunction, a hallmark of vascular ageing, is characterized by decreased levels of nitric oxide, thereby compromising vasodilation and disrupting the regulation of blood flow within the bone microenvironment. Additionally, the accumulation of collagen and loss of elastin results in arterial stiffening, further exacerbating vascular dysfunction. Previous studies have documented a significant reduction in type H vessels, arteries, and arterioles within the femur of ageing patients with osteoporosis, indicating a substantial decline in the skeletal vascular network, which could impact various physiological aspects and further exacerbate bone ageing.50 The bone microenvironment is impacted to a great extent by vascular ageing since diminished nutrient and oxygen supply, primarily a result of endothelial dysfunction, hinder adequate support for bone cells, ultimately impeding their regenerative potential.51 Consequently, impaired blood flow compromises the delivery of osteogenic precursors and growth factors to sites involved in bone formation, thereby undermining the processes of bone remodeling and repair.
To mitigate the detrimental impact of vascular ageing on bones, novel therapeutic strategies are being developed. These strategies comprise targeting oxidative stress and inflammation pathways to attenuate the initiation of ageing in vascular cells. In aged mice, enhanced HIF and Notch signaling result in the proliferation of type H vessels, subsequently leading to increased formation of trabecular bone.52 Moreover, innovative approaches involving senolytic agents, EVs, designed to eliminate aged cells, offer a means to restore proper vascular function, thus enhancing nutrient and oxygen supply to bone cells and potentially fostering improved bone health.53,54
Hematopoietic stem cells (HSCs) ageing
The ageing HSCs is a complex process with significant implications for immune function and overall health. Inflammation is identified as a key mediator in accelerating HSCs aging and functional decline. During inflammation, HSCs shift from anaerobic glycolysis to oxidative respiration, leading to accumulated ROS stress, triggering DNA damage, or apoptosis.55,56 Inflammatory cytokines such as IFN-γ have a dual role in regulating HSCs proliferation, either stimulating or hindering depending on the context. HSCs ageing results in diminished self-renewal capacity, indicated by an increased number of aged HSCs without a corresponding increase in self-renewal ability.57,58 Besides, HSCs ageing results in reduced differentiation potential, leading to a decrease in the formation of naive T cells and B cells. This may further negatively impact on the immune system, increasing the risk of infection, inflammation, and other relative disorders.59 For instance, exposure to inflammation during early to mid-life stages in mice can result in features typical of hematopoiesis in the elderly, including hemocytopenia, bone marrow cytopenia, and adipocyte accumulation.58 In addition, various factors, both intrinsic and extrinsic, contribute to the decline in HSCs function with age. As HSCs basically reside in the bone marrow niches, ageing of HSCs and the haematopoietic system could be linked to ageing of bones.60 Strategies to intervene in the aging process may have clinical relevance, especially considering the strong association between hematological pathologies and ageing. Mitochondrial metabolism, autophagy, and DNA damage response play vital roles in maintaining HSCs function and are potential targets for intervention.61,62
Chondrocyte ageing
The ageing process of chondrocytes, the unique cell type in articular cartilage, is a multifaceted phenomenon with several contributing factors during cartilage degeneration. Depletion of SIRT6, a member of the sirtuin family implicated in aging-related diseases, in human chondrocytes leads to increased DNA damage and telomere dysfunction, resulting in premature senescence.63 This is evidenced by decreased proliferation, elevated senescence-associated markers, and upregulated mediators for DNA damage-induced ageing. Additionally, mechanical overloading accelerates chondrocyte ageing through the downregulation of FBXW7, a key factor linking mechanical stress to chondrocyte aging.64 The dysregulation of specific genes, such as MMP-1 and MMP-13, further contributes to this process. Moreover, the involvement of various signaling pathways, including IL-15/JAK3/STAT5 and Notch, has been identified in the regulation of chondrocyte ageing.65 Clathrin-mediated endocytosis and activation of Notch signaling have been identified as contributing factors to chondrocyte ageing, while interventions targeting these pathways show promise in alleviating osteoarthritis (OA). Additionally, several non-coding RNAs are identified during chondrocyte ageing, offering potential therapeutic targets for IOA.66,67 Overall, understanding the molecular mechanisms underlying chondrocyte ageing is crucial for developing interventions to mitigate osteoarthritis progression.
Synovial cell ageing
Synovial cell ageing plays a crucial role in the progression of OA, and recent studies have revealed key mechanisms underlying this phenomenon. Aged fibroblast-like synoviocytes (FLSs) exhibit a marked increase during OA progression, accompanied by impaired autophagy leading to the upregulation of SASP. Notably, METTL3-mediated m6A modification negatively regulates autophagy in OA-FLS, specifically decreasing the expression of autophagy-related 7. Targeted METTL3 inhibition reverses FLS ageing and alleviates OA development.68 Additionally, inflammation-mediated tissue priming, involving synovial fibroblasts, contributes to aggravated arthritis, suggesting potential therapeutic interventions to abrogate tissue priming. The ageing synovium undergoes transcriptomic changes, with angiogenesis and fibrosis-associated genes upregulated, and FOXO1 identified as a major regulator of synovial ageing.69 In addition, synovial ageing promotes OA progression through immune inflammation, with identified biomarkers such as MCL1, SIK1, JUND, NFKBIA, and JUN.70 The synovial samples from OA patients showed strong immunoreactivity activity potential with abundant CD68+ macrophage, along with other immune cell infiltrate.71,72 For instance, the decreased omentin-1 level in synovial tissue promotes OA progression through M1 macrophage activation.73 Then, Grieshaber-Bouyer et al. reported that decreased CXCR1+ neutrophil and increased FcγRI, HLA-DR+ neutrophil appeared in the synovial fluid of inflamed joint.74 Therefore, the comprehensive understanding of synovial cell ageing and its implications in OA provides a rich foundation for developing targeted and effective therapeutic strategies.
In summary, age-related changes in the bone microarchitecture, as well as alterations in bone cells (Fig. 2), lead to decreased bone strength and reduced mechanical properties. These changes synergistically contribute to decreased mobility and an increased risk of fractures and morbidity. A better understanding of the underlying mechanisms involved in the ageing process of the skeletal system and cell ageing is essential for the development of new therapeutic strategies to prevent, mitigate, and treat age-related bone diseases.
Aged-related changes on bone cell. During the ageing process, BMSCs experience ageing, which triggers the activation of NF-κB, leading to ROS production, thereby inhibits osteoblast precursor differentiation. This phenomenon is further exacerbated by elevated levels of CCL2 and CCL5, which contribute to the ageing of osteoblasts. Moreover, the decline in the levels of Sirt in aged MSCs promotes adipocyte differentiation. This results in an increase in ROS levels, leading to adipocyte ageing. This, in turn, contributes to secondary ageing in osteoblasts and vascular cells. The cumulative effect of these changes includes increased production of inflammatory cytokines and reduced oxygen availability in the vasculature, resulting in vascular stiffness and a disruption of blood flow within the bone microenvironment. Furthermore, a deficiency in molecular cathepsin K and integrins may lead to ageing in osteoclasts while preserving their bone resorption capacity
Age-related changes in immune system
The immune system is critical for the maintenance of homeostasis and mediation of defense mechanisms against pathogens or neoplastic transformations. It is subject to continuous changes throughout life, with alterations in immune cell populations and function occurring with increasing age. Ageing of the immune system, known as immunoageing, is associated with various quantitative and qualitative changes in immune cell subsets, cytokine production, and immune responses, which all result in increased susceptibility to infections, neoplastic transformations, and autoimmune diseases. This section presents a comprehensive exploration of the intricate age-related alterations in the immune system, encompassing morphological and functional transitions of immune organs and the onset of immune cell ageing. Thoroughly understanding these changes is essential to decode the multifaceted dimensions of immunoageing and its consequent implications on bone health.
Age-related changes in immune organs
The primary lymphoid organs, including the thymus and bone marrow, serve as central hubs for immune cell generation, maturation, and maintenance. With ageing, these critical organs undergo structural and functional changes, impacting the immune system. In childhood, the thymus is highly active, producing diverse naive T cells. However, as individuals age, the thymus shrinks and loses function, resulting in reduced production of naive T cells.75 This limits the diversity of the T cell receptor (TCR) spectrum, making older individuals more susceptible to infections. Thymic decline also reduces the production of regulatory T cells (Tregs), affecting immune tolerance and potentially contributing to autoimmune diseases.76 The bone marrow, responsible for generating various immune cells, experiences age-related alterations. Hematopoietic stem cells (HSCs) in the bone marrow change in composition and differentiation capacity.77 This shift towards myeloid cell production at the expense of lymphoid cells leads to an immune system reliant on innate immunity, impairing responses to pathogens requiring adaptive immunity. Changes in HSC populations can compromise overall immune competence and contribute to hematological malignancies like myelodysplastic syndromes.78
Age-related changes in immune cell ageing
The initiation of immune cell ageing constitutes a central aspect of the ageing immune system. These changes are characterized by a gradual loss of immune cell functionality and the development of a proinflammatory state, both of which have far-reaching clinical ramifications.
T cell ageing
T cells, pivotal components of adaptive immunity, exhibit age-related ageing driven by complex molecular mechanisms.79
CD4+ T cell ageing
The process of CD4+ T cell ageing is characterized by a spectrum of intricate alterations in these immune cells unfolding with advancing age. A hallmark of this transformation includes the progressive accumulation of memory-like T cells, which exhibit alterations in their functionality and phenotype. This process coincides with the loss of expression of critical co-stimulatory molecules, which are essential for robust T cell activation and immune response. Aged CD4+ T cells also exhibit a shift towards a proinflammatory state, contributing to chronic inflammation seen in ageing. This altered inflammatory profile is often coupled with changes in cytokine production patterns, leading to imbalances that can further compromise immune responses. Moreover, aged CD4+ T cells display impaired proliferation and weakened responsiveness to antigenic stimulation, collectively diminishing the immune system’s capacity to combat infections and maintain overall health. These multifaceted changes underscore the complex nature of CD4+ T cell ageing and its impact on immune function. Several molecular mechanisms contribute to CD4+ T cell ageing, including altered gene expression, epigenetic changes, and alterations in various signaling pathways. Factors such as reduced HELIOS expression, aberrant T cell receptor (TCR) signaling, and increased expression of specific genes (e.g., DUSP4) have been implicated in driving ageing in this specific subtype of immune cells.80,81
Clinical consequences of CD4+ T cell ageing include increased vulnerability to infections, decreased responsiveness to vaccines, autoimmune diseases, and a higher risk of age-related and inflammation-associated conditions like cardiovascular disease, neurodegenerative disorders, and cancer.82 Addressing CD4+ T cell ageing is challenging, but potential therapies are emerging. Senolytic drugs aim to eliminate aged cells, potentially restoring immune function.83 Modulating metabolic pathways, targeting mitochondria or glycolysis, may counter metabolic changes in aged CD4+ T cells.84 Additionally, MSC-derived exosomes enriched with miRNA-21 show promise in preserving CD4+ T cell functionality by protecting them from oxidative damage.85 These strategies hold potential for mitigating CD4+ T cell ageing and enhancing immune vitality in ageing individuals. However, further research and clinical trials are needed to validate their effectiveness and safety across diverse ageing populations.
CD8+ T cell ageing
CD8+ T cell ageing, a consequence of ageing, involves reduced proliferative capacity, loss of CD28 expression, altered cytokine production, and increased ageing-associated markers like SA-β-gal. This weakens immune responses, increases infection susceptibility, reduces vaccine effectiveness, and promotes age-related diseases through chronic inflammation.86 The molecular mechanisms driving CD8+ T cell ageing involves a complex interplay of factors. Replicative ageing is caused by telomere shortening, while decreased CD28 expression reduces responsiveness to antigenic stimulation. Chronic exposure to inflammatory cytokines and oxidative stress activates stress response pathways, such as p38 MAP kinase signaling, promoting SASP.87 Secreting pro-inflammatory mediators amplifies inflammation and reinforces ageing, consequently impairing CD8+ T cell function.
Clinical consequences of CD8+ T cell ageing include compromised immunity against infections, reduced vaccine efficacy, and increased risk of age-related conditions like cardiovascular disease and cancer. Dysfunctional CD8+ T cells hinder tumor recognition and elimination, potentially promoting tumor development.88
Addressing CD8+ T cell ageing involves rejuvenating or promoting a more effective CD8+ T cell phenotype by targeting molecular pathways like FOXO1 or p38 MAPK signaling.87,89 Strategies to enhance telomere maintenance and reduce DNA damage can mitigate ageing. Immunotherapies, including immune checkpoint blockade, counter inhibitory signals contributing to CD8+ T cell dysfunction. Boosting the naive CD8+ T cell pool through regenerative medicine enhances immune responsiveness. A comprehensive approach may combine pharmacological, immunological, and regenerative strategies tailored to individuals and their associated diseases.90
Treg cell ageing
Treg cell ageing involves changes in their regulatory function within the immune system, including reduced proliferative capacity, weakened suppressive function, and altered cytokine profiles. These changes lead to chronic low-grade inflammation, impacting immune balance and homeostasis. Molecular mechanisms behind Treg cell ageing include dysregulated signaling pathways like ERK1/2 and p38, altered expression of cell-cycle-regulatory molecules (p16, p21, p53).91 Importantly, the downregulation of specific factors, such as DCAF1 (DDB1- and CUL4-associated factor 1), plays a critical role in Treg cell ageing by allowing increased levels of ROS and compromising Treg function.92 These changes result in Treg cell dysfunction, affecting immune tolerance and inflammation control.
Treg cells play a vital role in immune regulation. When these cells age, their ability to suppress immune reactions diminishes, leading to immune-related problems like autoimmune diseases, chronic inflammation, and allergies. Ageing Treg cells also lose their capacity to combat cancer cells, contributing to tumor growth.93 To address these issues, interventions targeting molecular pathways linked to Treg cell ageing, such as ROS regulation and key regulatory proteins like DJ-1, could be explored.94 Strategies to enhance mitochondrial function and metabolic activity in Treg cells may help mitigate ageing. Immunotherapies that boost Treg cell function through selective expansion or activation could restore immune balance and reduce inflammation. Innovative approaches like adoptive Treg cell therapy offer promise in replenishing Treg cells and rejuvenating the immune system. Tailored strategies for Treg cell ageing in different diseases could provide individualized solutions for immune balance and inflammation control.
B cell ageing
B cell ageing comprises a series of different changes. The production of new B cells decreases, leading to reduced diversity and a shift towards memory B cells. Additionally, B cells exhibit alterations in antibody production, particularly a decrease in high-affinity specific antibodies and an increase in low-affinity autoantibodies.95 The ageing process also affects the longevity and homing of B cells within the body, leading to changes in their distribution in different tissues.The molecular mechanisms driving B cell ageing are multifaceted. Dysregulation of critical transcription factors, such as E2A, Pax-5, and STAT5, can impair B cell development and survival.96 Moreover, the transcription factor Aire, responsible for promoting central T cell tolerance by mediating the presentation of self-antigens, declines in thymic B cells with age, potentially contributing to autoimmune susceptibility.97 Notably, the age-associated decline in spermidine levels can affect eIF5A hypusination, leading to reduced autophagy and compromised B cell function.98
Aged B cells exhibit reduced responsiveness to vaccinations and infections, weakening immune defense, especially against new antigens. They also contribute to autoimmune diseases through increased low-affinity autoantibodies. Age-related changes in B cell function further impact chronic inflammatory diseases.99 Addressing B cell ageing poses therapeutic challenges but offers hope for enhancing immune function in the elderly. Strategies to rejuvenate B cell function include targeting molecular pathways tied to ageing. For example, restoring autophagy through spermidine supplementation shows promise in rejuvenating memory B cell responses.98 Modulating key transcription factors like E47 can enhance B cell immune responses.100 Therefore, understanding the mechanisms behind B cell ageing is vital for developing targeted therapies to combat age-related immune dysfunction.
Macrophage ageing
Macrophage ageing involves significant changes, including reduced phagocytic activity, impaired debris clearance, compromised immunity, and SASP. These alterations have clinical implications, particularly in age-related diseases with tissue dysfunction, chronic inflammation, and weakened immunity.101 The molecular processes behind macrophage ageing are complex, initiated by factors like DNA damage and chronic inflammation, leading to specific signaling pathways activation, cell cycle arrest, increased mTOR activity, and altered autophagy.102 Aged macrophages upregulate CD47 and show changes in cytokine and chemokine expression, influenced by epigenetic modifications.103
Macrophage ageing has diverse clinical consequences, exacerbating age-related conditions like cardiovascular diseases, neurodegenerative disorders, and cancer, causing chronic inflammation, impaired pathogen clearance, reduced tissue repair, and pro-inflammatory molecule secretion. Addressing macrophage ageing holds promise in mitigating these consequences. Senolytic drugs can eliminate aged cells, including macrophages, thereby potentially reducing their detrimental impact. Inhibiting BRD4 and modulating macrophage phenotypes from M1 to M2 states can prevent ageing.104 Rejuvenating aged macrophages through autophagy enhancement or mitochondrial restoration is another option. Targeting SASP with specific cytokine or chemokine inhibitors may reduce chronic inflammation. Promoting tissue repair and regeneration can counter the negative effects of aged macrophages on age-related tissue damage through stem cell therapies or growth factors.
Neutrophil ageing
Neutrophil ageing brings about significant changes, including functional decline, morphological alterations, and gene expression shifts. Aged neutrophils have reduced abilities in chemotaxis, phagocytosis, and oxidative burst, making them less effective at fighting infections. Dysregulated signaling pathways and gene expression patterns underlie the molecular mechanism of neutrophil ageing, affecting chemokine and cytokine signaling, microbiome-influenced Toll-like receptor signaling, and responsiveness to inflammation.105
Beyond the cellular level, neutrophil ageing impacts overall health and disease susceptibility in ageing individuals. Compromised neutrophils elevate infection risk, potentially leading to more severe and prolonged infections. Reverse transendothelial migration in aged neutrophils can cause tissue damage in remote organs, increasing the risk of organ dysfunction following inflammatory events.106 Addressing neutrophil ageing clinically involves targeting specific molecular mechanisms. Modulating microRNAs, addressing dysregulated chemokine and cytokine signaling pathways, and microbiota interventions can potentially restore neutrophil responsiveness to inflammation.107 These strategies hold promise for rejuvenating neutrophil function in ageing individuals and enhancing their ability to combat infections and maintain immune balance.
Natural killer (NK) cell ageing
NK cell ageing involves changes in both phenotype and functionality, characterized by increased inhibitory receptors and decreased activating receptors, leading to reduced cytotoxicity, impaired cytokine secretion, and compromised immune responses. This vulnerability to infections and neoplastic transformations is accentuated by the accumulation of memory-like NK cell subsets, such as NK2.1 cells, observed in conditions like COVID-19.108 Molecular mechanisms behind NK cell ageing include altered gene expression profiles affecting receptors and effector functions. Activation of the SASP amplifies inflammation, impairing NK cell function. Aged cells can influence NK cell behavior and response, contributing to NK cell dysfunction.
Clinically, aged NK cells, with their reduced function, increase susceptibility to infections and neoplastic transformations, worsening disease severity. They play a role in various age-related diseases, exacerbating their progression and severity. Addressing NK cell ageing requires innovative therapeutic strategies. Immunotherapies enhancing NK cell function or replacing aged NK cells can improve immune surveillance in the elderly. Senolytic agents may rejuvenate the immune system by eliminating aged cells.109 Integrative therapies combining NK cell-based immunotherapies with senolytics or alternative methods offer a holistic solution to target both aged cells and dysfunctional NK cells, countering the clinical consequences of NK cell ageing and promoting healthier ageing.
Dendritic cells (DCs) ageing
DC ageing involves intricate molecular mechanisms influenced by various factors, including epigenetic regulation and tissue microenvironment stiffness. These changes impact DC gene expression, function, maturation, cytokine production, and immune responses.110 DC ageing leads to reduced immune efficacy, making individuals more vulnerable to infections, neoplastic transformations, and autoimmune diseases. It also hampers the clinical effects of immunotherapies and vaccinations in the ageing population by compromising antigen presentation, cytokine production, and T cell activation.111 Therapeutic strategies for DC ageing hold significant clinical potential. These approaches aim to restore or enhance DC function in ageing individuals through targeted immunotherapies like senolytics, epigenetic and metabolic modulation, and interventions maintaining tissue microenvironments conducive to optimal DC function. The goal is to mitigate the clinical consequences of DC ageing, boost immune responses, and improve health outcomes in ageing individuals.
In summary, age-related alterations in the immune system encompass shifts in immune organs and the initiation of ageing of a variety of immune cells (Fig. 3). These changes have significant clinical consequences, including reduced immune responsiveness to novel pathogens and vaccinations as well as increased susceptibility to infections, heightened risk of autoimmune diseases, and chronic inflammation—all of which collectively contribute to the overall decline in health associated with increasing age. A better understanding of the underlying mechanisms involved in immunoageing and the bidirectional relationship between the immune and the skeletal system is essential for the development of new therapeutic strategies to promote healthy ageing and prevent or mitigate age-related diseases.
Age-related alterations affect both the adaptive and innate immune systems. In the adaptive immune system, there is a decline in the population of naive CD4+ T cells, CD8+ T cells, Treg cells, and B cells, whereas memory cells become more prevalent. In the innate immune system, the functionality of monocytes/macrophages is compromised, and neutrophils exhibit reduced capabilities in terms of NET formation, phagocytosis, and chemotaxis, along with an increased susceptibility to apoptosis. Furthermore, there is an increase in CD56dim NK cells and a decrease in CD56bright NK cells. The population of dendritic cells also decreases, and their anti-phagocytic abilities weaken with age
Impact of ageing on the bidirectional communication between the immune system and the skeleton
The crosstalk between the immune and skeletal systems is a complex and finely tuned process, essential for the maintenance of both bone health and immune function throughout an individual’s life. However, this bidirectional communication undergoes significant changes with age. In this section, we delve into the intricate interplay between the immune and the skeletal system with increasing age, shedding light on the molecular and cellular mechanisms that underlie these interactions.
Impact of age-related bone changes on immune systems
The bone marrow serves as the primary lymphoid organ housing hematopoietic stem cells, immune cell progenitors, and mature immune cells. However, with increasing age, the functional capacity of the bone marrow declines due to changes in the hematopoietic stem cell environment, reduced osteoblast function, and increased adipocyte deposition.112 These changes culminate in an impaired lymphopoiesis with reduced output of naive B and T cells.113,114 Age-related bone loss also reduces the available space for hematopoiesis. The aged bone marrow microenvironment shows increased levels of oxidative stress and inflammation, altering the expression of cytokines and survival factors that support immune cell maintenance. Specifically, aged HSCs exert profound effects on the immune system, contributing to immunoageing characterized by the differentiation of aged HSCs into dysfunctional immune cells, which process leads to an imbalanced myeloid/megakaryocytic differentiation bias, altering the composition of immune cells.115 Aged HSCs also induce inflammaging, a chronic inflammatory state marked by the release of pro-inflammatory cytokines, increasing the risk of various age-related diseases.116 The diminished capacity for self-renewal and regeneration in aged HSCs results in an overall decline in immune function, making individuals more susceptible to infections. Metabolic shifts in HSCs, accompanied by increased ROS, further impact their functionality. Ultimately, the consequences of aged HSCs extend to an impaired ability to mount diverse and effective immune responses, contributing to the higher incidence of infections and hematologic malignancies among the elderly. Reversing HSC aging involves multiple strategies. Transplantation of aged HSCs into a young bone marrow niche partially restores transcriptional profiles but not DNA methylation patterns, indicating intrinsic cellular aging factors that are not mitigated by a youthful environment alone.117 Engineering the matrix stiffness of bone marrow niche using biophysical cues like soft hydrogels can promote HSC rejuvenation by affecting MSCs behavior and improving multilineage reconstitution capacity.118 Additionally, manipulating soluble factors such as cytokines and implementing biomaterial-based scaffolds can enhance HSC maintenance and stemness by mimicking natural bone marrow microenvironments.119,120 These approaches highlight the complex interplay between extrinsic niche factors and intrinsic stem cell properties in HSC aging and rejuvenation.
In addition, aged MSCs exhibit altered behavior, particularly in the secretion of various pro-inflammatory molecules collectively referred to as the SASP, which include cytokines such as IL-6, IL-8, and IL-15, among others. The heightened levels of IL-6 play a pivotal role in influencing macrophage polarization and T cell differentiation, thereby creating a favorable environment for inflammation.121,122 Notably, previous research has shown that increased IL-15 levels promote the development of aged CD28− CD8+ T cells, while decreased IL-7 levels support the maintenance of naive T cells. This imbalance contributes to the accumulation of highly differentiated effector T cells.123 Furthermore, the elevated levels of proinflammatory IL-6 synergize with IL-15 to drive the differentiation of CD8+ T cells. Additionally, IL-8, induced by aged MSCs, hinders the maturation and functioning of DCs, leading to reduced adhesion, migration, and antigen presentation by DCs.124 The age-related alteration in the bone marrow microenvironment in mice also leads to a reduction in B cell production and diversity of immunoglobulins. These changes are primarily attributed to extrinsic factors that affect the V(D)J recombination process in pro-B cells and hinder their progression to the pre-B cell stage, which may be related to age-associated osteoporosis and compromised marrow stem cell generation.125 Additionally, the loss of MMP14 on the cell surface of aged MSCs further impedes the maturation of B cells.126 Decreased levels of TGFβ in aged MSCs negatively affect NK cells by suppressing their natural cytotoxicity, expression of activating receptors, and IFNγ production, ultimately compromising the overall functionality of NK cells.127 Lastly, the deficiency of RUNX1 in aged MSCs results in heightened inflammation in neutrophils, with dysregulated Toll-like receptor 4 (TLR4) and Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathways originating in granulocyte-monocyte progenitors (GMPs).128 (Fig. 4) Downregulation of CXCL12 and proliferation-inducing ligands in the aged bone marrow impairs plasma cell survival.129 Overall, the aged bone marrow supports increased survival of aged and exhausted T cells while impairing central memory T cells and antibody-producing plasma cells. This skews the adaptive immune system towards a profile dominated by highly differentiated, yet dysfunctional T cells and reduces humoral immunity mediated by long-lived plasma cells. Pharmacological targeting of bone-immune interactions may help rejuvenate immunoageing by improving the maintenance of immunological memory in the bone marrow niche. For example, leverageing antioxidants could mitigate the effects of inflammageing, potentially restoring expression patterns of survival factors. Therapies that target aged T cells, modulate CXCL12, or promote plasma cell survival could also help rebalance the aged bone marrow microenvironment to better support functional immune cell populations.
In summary, understanding the age-related changes in the bone marrow microenvironment and their impact on immune cells is crucial for developing interventions that can mitigate the decline in immune function associated with aging. Furthermore, targeted strategies aimed at preserving or restoring the functional capacity of stem cells and promoting a healthy bone marrow environment may offer potential avenues for enhancing immune competence in the elderly population. As we delve deeper into the intricacies of bone marrow aging, we can explore innovative approaches to rejuvenate the immune system, potentially contributing to improved overall health and resilience in the aging population.
Impact of age-related immune system changes on bone
The aged immune system exerts a profound and far-reaching impact on bone health. This influence manifests in the form of heightened bone resorption and diminished bone formation processes. As a consequence, this imbalance results in the gradual weakening and fragility of bones over time. The intricate interplay between immune system ageing and bone underscores the importance of understanding and addressing these age-related changes to maintain skeletal integrity and overall health (Fig. 5).
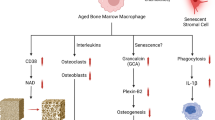
Immune cell ageing is marked by distinctive features, including mitochondrial dysfunction, DNA damage, cell cycle arrest, telomere dysfunction, and elevated levels of reactive oxygen species (ROS) and SASP factors. Moreover, aged immune cells release detrimental cytokines, contributing to an increased presence of osteoclasts and adipocytes while diminishing osteocyte numbers, consequently resulting in the development of bone fragility
With increasing age, T cell output from the thymus declines, resulting in reduced naive T cell numbers and contracted T cell receptor diversity. Individuals with osteoporosis exhibit a higher presence of aged CD4+ CD28− T cells compared to healthy individuals.130 The accumulation of aged T cells leads to increased inflammatory cytokine production, which promotes bone loss through several mechanisms. Pro-inflammatory cytokines such as TNFα and IL-6 stimulate osteoclast activity, thereby shifting the balance towards bone resorption. Further, TNFα directly inhibits osteoblast differentiation and bone formation. T cells are an important source of TNFα that drives bone loss in postmenopausal osteoporosis models.6 And aged cells promotes naive T cells differentiation to Th17 cell, which induce synovial fibroblasts ageing and impair OA.131 Inflamm-ageing impairs the anabolic response in bones to parathyroid hormone by reducing T cell production of Wnt10b.132 Additionally, it disrupts the bone marrow niches that support osteoprogenitors and lymphopoiesis through its effects on mesenchymal stromal cells and increased oxidative stress. Aged T cells may displace long-lived plasma cells within the bone marrow, contributing to reduced humoral immunity. Moreover, they occupy niche space required by hematopoietic stem cells, thus impairing lymphopoiesis.59 Furthermore, Cytomegalovirus (CMV)-specific T cells accumulate in the ageing bone marrow and, with their biased T cell receptor usage, displace polyclonal naive T cells. Ultimately, immunoageing creates an osteoimmunological environment dominated by dysfunctional effector T cells that promote bone loss. In summary, the accumulation of aged T cells and the associated inflamm-ageing process create a complex osteoimmunological environment. The impact on bone health is multifaceted, affecting both bone resorption and formation through various pathways.
The ageing process has a profound impact on neutrophils, critical players in the innate immune system, and their interactions within the bone marrow environment. Aged neutrophils exhibit impaired migration, reduced ROS production, and an increased tendency for neutrophil extracellular trap (NET) formation. NETs contain inflammatory molecules and tissue-damageing proteins. By stimulating macrophages and dendritic cells, NETs promote chronic inflammation and osteoclastogenesis, as well as directly induce osteoblast apoptosis and inhibit differentiation.133 The loss of CXCR2 on aged neutrophils impairs regeneration of blood vessels in the bone marrow and compromises their bone-protective functions.134 Additionally, impaired signals mediated by granulocyte colony-stimulating factor (G-CSF) contribute to the reduced mobilization of neutrophils within the bone marrow, further compromising their ability to respond effectively to immune challenges. Furthermore, Li et al. reported that TGFβ1+ CCR5+ neutrophils are highly increased in the bone marrow of aged mice, where they induce significant bone loss by promoting the degradation of TRAF3 in mesenchymal progenitor cells (MPCs).135 Aged pro-inflammatory macrophages accumulate in the bone marrow and produce TNFα, IL-1β, IL-6, and RANKL, creating an inflammatory milieu conducive to osteoclast differentiation. The impaired phagocytic capacity of aged macrophages leads to the accumulation of apoptotic cells and cellular debris within the bone marrow. This accumulation perpetuates inflammation, contributing to the overall disruption of bone homeostasis. For instance, elderly individuals face more complications during fracture healing due to inflammatory dysregulation associated with aging. Research showed that preventing macrophage infiltration at fracture sites in old mice improved healing, suggesting that age-related changes in macrophages contribute to delayed bone repair.136,137 Recent findings provide support for the accumulation of aged macrophages and neutrophils in the bone marrow during ageing, contributing to skeletal ageing through the activation of granulocyte-specific protein grancalcin and plexin B2 (plexinB2).138 In summary, the ageing-related alterations in neutrophils and macrophages collectively contribute to the dysregulation of immune responses within the bone marrow. The imbalance created by impaired neutrophil functions, altered macrophage activity, and the inflammatory microenvironment accelerates the ageing of the skeletal system, emphasizing the intricate connections between the immune and skeletal systems during the ageing process.
The ageing process exerts notable effects on B cells, influencing various aspects of their functionality and, consequently, impacting the delicate balance of bone homeostasis. Aged B cells present shortened telomeres, elevated ROS, and altered chemokine receptor profiles, all of which compromise their bone marrow homing capabilities. Further, aged B cells display decreased production of interleukin-10 (IL-10), a key anti-inflammatory cytokine, and impaired affinity maturation. This compromises the overall effectiveness of the humoral immune response. The decline in the number of long-lived plasma cells within the bone marrow further hampers humoral immunity, reducing the capacity to mount robust responses against pathogens. In addition to these immunological changes, aged B cells produce proinflammatory agents like TNFα or RANKL, as well as low-affinity autoantibodies, directly stimulating osteoclastogenesis.139 Impaired generation of immune complexes reduces osteoclast FcR signaling, which is crucial for maintenance of skeletal mass. Beyond effects on osteoclasts, aged B cells have intrinsic effects on osteoblasts. While B cell-derived IL-10 and the chemokine CXCL13 typically stimulate bone formation, their diminished levels during B cell ageing may suppress osteoblast function. Additionally, a decrease in the production of osteoprotegerin (OPG) by aged B cells creates an environment conducive to heightened osteoclastogenesis, further disrupting the balance between bone resorption and formation.140 Furthermore, the increased production of RANKL by B cells, along with their reduced numbers and impaired function, contributes to an imbalance with OPG, exacerbating bone resorption and leading to age-associated bone loss.141 In summary, the ageing-related changes in B cells encompass not only immunological aspects but also direct implications for bone metabolism.
DCs ageing diminishes the ability to prime naive T cells, and age-related changes in innate cells contribute to the breakdown of self-tolerance, potentially leading to autoimmunity and sterile inflammation. The presence of autoantibodies the elderly, combined with decreased levels of IL6 and TNF-α, can furthermore directly stimulate osteoclastogenesis.142 Age-related changes in invariant NK cells, innate lymphoid cells, and myeloid-derived suppressor cells may further disrupt the balanced regulation of immune responses and contribute to bone loss by favoring bone resorption over formation.143 The compromised priming ability of DCs, coupled with the breakdown of self-tolerance and alterations in key immune cell populations, creates an environment where the elderly are more susceptible to autoimmune conditions, sterile inflammation, and bone-related issues.
Therefore, the aging-associated alterations in immune cell populations, encompassing T cells, neutrophils, macrophages, B cells, and dendritic cells, collectively contribute to the intricate pathogenesis of skeletal aging. These collective changes create a complex osteoimmunological milieu, emphasizing the need for targeted therapeutic approaches to address immune dysregulation and mitigate the adverse effects of aging on skeletal health.
In conclusion, the bidirectional communication between the immune and the skeletal system is a dynamic and tightly regulated process that undergoes significant age-related changes. These changes have profound clinical implications, impacting immune responses, bone health, and the susceptibility to age-related diseases. Understanding the molecular and cellular mechanisms underlying this complex interplay is essential for developing strategies to promote healthier ageing and reduce the burden of age-related bone disorders.
Clinical implications
Potential interventions to promote healthy ageing of the immune system and bone
The age-related decline in immune function and bone health can have profound clinical implications, including but not limited to an increased risk of infections, neoplastic transformations, osteoporosis and OA. Therefore, identifying potential therapeutic approaches to prevent or attenuate age-related changes in the immune and skeletal system is of high scientific interest. One potential intervention is the use of immunomodulatory drugs to enhance immune function in the ageing population. For example, the use of interleukin-7 (IL-7) has been shown to enhance immune function in ageing mice and humans.144 Senolytic drugs, such as rapamycin, metformin, dasatinib, and quercetin, have also proven beneficial in eliminating aged cells and preventing aging (Table 1).145,146,147 Additionally, antioxidants and natural products may offer support. For instance, Geng et al. reported that pyrroloquinoline quinone inhibits oxidative stress and the release of SASP, thereby promoting osteoblastic bone formation.40
Prior to initiating therapeutic agents specifically tailored to modulate the immune and skeletal system, patients should first be advised to implement lifestyle modifications, such as regular physical exercise and a healthy diet in their daily routine. Exercise has been shown to improve immune function and bone health, potentially by reducing inflammation and promoting the production of growth factors such as reticulocalbin that promote bone formation.148 Similarly, a healthy diet rich in calcium, vitamin D, and other nutrients can promote bone health and reduce the risk of aged-bone related disease (Table 2). Finally, the use of regenerative medicine approaches, such as stem cell therapy and tissue engineering, may hold promise for promoting healthy ageing of the immune and skeletal system.149
Emerging areas of research and potential avenues for future investigation
The intricate relationship between bone ageing and immunology is a field experiencing rapid advancements, unveiling several emerging areas of research and potential avenues for deeper investigation. One promising area of research is the identification of specific immune cell populations and cytokines that regulate bone remodeling with ageing. Recent studies have identified the involvement of various immune cell populations, such as T cells, B cells, and myeloid cells in age-related bone loss.150 Additionally, pro-inflammatory cytokines such as IL-6, TNF-α, and RANKL have been implicated in the regulation of bone remodeling in the ageing population. Future research in this area may focus on the development of targeted interventions aiming to modulate these immune cell populations and cytokines to promote healthy ageing of the skeletal system. Another area of emerging research is the role of the cellular ageing. Aged cells accumulate with ageing and have been implicated in various age-related diseases, including osteoporosis and overall impaired function of the immune system. Recent studies have shown that aged cells can promote bone loss and impede immune functionality through various mechanisms, such as the production of pro-inflammatory cytokines. Future research in this area may focus on the development of interventions targeting these aged cells to ultimately promote healthy ageing of the immune and skeletal system.151 Finally, the development of novel image techniques and biomarkers may provide valuable insights into the bone-immune axis and the effects of ageing on these systems. For example, high-resolution image techniques such as micro-CT and PET can provide detailed information on bone structure and metabolism, while biomarkers such as bone turnover markers and cytokine profiles can provide information on bone remodeling and immune function.152,153 Future research may focus on the development of novel image techniques and biomarkers that can provide more detailed and accurate information on the bone-immune axis during ageing.
Conclusions
In conclusion, the complex network of bidirectional communication between the skeletal and immune system, termed the bone-immune axis, plays a crucial role in the regulation of bone remodeling and immune function. The ageing process is associated with alterations in the production and activity of inflammatory cytokines, chemokines, and growth factors, leading to dysregulation of the bone-immune axis, thereby laying the foundation for age-related diseases such as osteoporosis and immune dysfunction. Further research is needed to better understand the molecular mechanisms underlying the bone-immune axis to identify novel therapeutic targets for the prevention and treatment of age-related diseases. A better understanding of the bone-immune axis will improve our ability to promote healthy ageing and improve the quality of life for the ageing population.
References
Hou, Y. et al. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 15, 565–581 (2019).
Tsukasaki, M. & Takayanagi, H. Osteoimmunology: evolving concepts in bone-immune interactions in health and disease. Nat. Rev. Immunol. 19, 626–642 (2019).
Barbe-Tuana, F. et al. The interplay between immunosenescence and age-related diseases. Semin. Immunopathol. 42, 545–557 (2020).
Lian, J., Yue, Y., Yu, W. & Zhang, Y. Immunosenescence: a key player in cancer development. J. Hematol. Oncol. 13, 151 (2020).
Chandra, A. & Rajawat, J. Skeletal aging and osteoporosis: mechanisms and therapeutics. Int. J. Mol. Sci. 22, 3553 (2021).
Fischer, V. & Haffner-Luntzer, M. Interaction between bone and immune cells: implications for postmenopausal osteoporosis. Semin. Cell Dev. Biol. 123, 14–21 (2022).
Fitzpatrick, E. A., Han, X., Xiao, Z. & Quarles, L. D. Role of fibroblast growth factor-23 in innate immune responses. Front. Endocrinol. (Lausanne) 9, 320 (2018).
Martin-Marquez, B. T. et al. Osteopontin: a bone-derived protein involved in rheumatoid arthritis and osteoarthritis immunopathology. Biomolecules 13, 502 (2023).
Clezardin, P. et al. Bone metastasis: mechanisms, therapies, and biomarkers. Physiol. Rev. 101, 797–855 (2021).
Zioupos, P., Kirchner, H. O. K. & Peterlik, H. Ageing bone fractures: the case of a ductile to brittle transition that shifts with age. Bone 131, 115176 (2020).
Yokota, K. et al. Characterization and function of tumor necrosis factor and interleukin-6-induced osteoclasts in rheumatoid arthritis. Arthritis Rheumatol. 73, 1145–1154 (2021).
Takeuchi, T., Yoshida, H. & Tanaka, S. Role of interleukin-6 in bone destruction and bone repair in rheumatoid arthritis. Autoimmun. Rev. 20, 102884 (2021).
Johannesdottir, F. et al. Age-related changes in bone density, microarchitecture, and strength in postmenopausal black and white women: the SWAN longitudinal HR-pQCT study. J. Bone Min. Res. 37, 41–51 (2022).
Burr, D. B. Changes in bone matrix properties with aging. Bone 120, 85–93 (2019).
Alvarenga, J. C., Caparbo, V. F., Domiciano, D. S. & Pereira, R. M. R. Age-related reference data of bone microarchitecture, volumetric bone density, and bone strength parameters in a population of healthy Brazilian men: an HR-pQCT study. Osteoporos. Int. 33, 1309–1321 (2022).
Pignolo, R. J. et al. Defects in telomere maintenance molecules impair osteoblast differentiation and promote osteoporosis. Aging Cell 7, 23–31 (2008).
Guo, Y. et al. Sirt3-mediated mitophagy regulates AGEs-induced BMSCs senescence and senile osteoporosis. Redox Biol. 41, 101915 (2021).
Liu, F. et al. LRRc17 controls BMSC senescence via mitophagy and inhibits the therapeutic effect of BMSCs on ovariectomy-induced bone loss. Redox Biol. 43, 101963 (2021).
Li, H. et al. FOXP1 controls mesenchymal stem cell commitment and senescence during skeletal aging. J. Clin. Invest. 127, 1241–1253 (2017).
Weng, Z. et al. Mesenchymal stem/stromal cell senescence: hallmarks, mechanisms, and combating Strategies. Stem Cells Transl. Med. 11, 356–371 (2022).
Luo, H. et al. Cadmium exposure induces osteoporosis through cellular senescence, associated with activation of NF-kappaB pathway and mitochondrial dysfunction. Environ. Pollut. 290, 118043 (2021).
Xiao, Y. et al. Splicing factor YBX1 regulates bone marrow stromal cell fate during aging. EMBO J. 42, e111762 (2023).
Hu, M. et al. NAP1L2 drives mesenchymal stem cell senescence and suppresses osteogenic differentiation. Aging Cell 21, e13551 (2022).
Yang, R. et al. 1,25-Dihydroxyvitamin D protects against age-related osteoporosis by a novel VDR-Ezh2-p16 signal axis. Aging Cell 19, e13095 (2020).
Lei, Q. et al. Extracellular vesicles deposit PCNA to rejuvenate aged bone marrow-derived mesenchymal stem cells and slow age-related degeneration. Sci. Transl. Med. 13, eaaz8697 (2021).
Chen, S. et al. lncRNA Xist regulates osteoblast differentiation by sponging miR-19a-3p in aging-induced osteoporosis. Aging Dis. 11, 1058–1068 (2020).
Sherk, V. D. & Rosen, C. J. Senescent and apoptotic osteocytes and aging: exercise to the rescue? Bone 121, 255–258 (2019).
Ding, P. et al. Osteocytes regulate senescence of bone and bone marrow. Elife 11, e81480 (2022).
Cui, J. et al. Osteocytes in bone aging: Advances, challenges, and future perspectives. Ageing Res. Rev. 77, 101608 (2022).
Eckhardt, B. A. et al. Accelerated osteocyte senescence and skeletal fragility in mice with type 2 diabetes. JCI Insight 5, e135236 (2020).
Zhang, Y. et al. Neuronal Induction of Bone-Fat Imbalance through Osteocyte Neuropeptide Y. Adv. Sci. (Weinh.) 8, e2100808 (2021).
Kassem, M. & Marie, P. J. Senescence-associated intrinsic mechanisms of osteoblast dysfunctions. Aging Cell 10, 191–197 (2011).
Kim, H. N. et al. DNA damage and senescence in osteoprogenitors expressing Osx1 may cause their decrease with age. Aging Cell 16, 693–703 (2017).
Xu, P. et al. VDR activation attenuates osteoblastic ferroptosis and senescence by stimulating the Nrf2/GPX4 pathway in age-related osteoporosis. Free Radic. Biol. Med. 193, 720–735 (2022).
Kim, H. J. et al. ROS-induced PADI2 downregulation accelerates cellular senescence via the stimulation of SASP production and NFkappaB activation. Cell Mol. Life Sci. 79, 155 (2022).
Chen, A. et al. mTORC1 induces plasma membrane depolarization and promotes preosteoblast senescence by regulating the sodium channel Scn1a. Bone Res. 10, 25 (2022).
Lian, W. S. et al. MicroRNA-29a mitigates osteoblast senescence and counteracts bone loss through oxidation resistance-1 control of FoxO3 Methylation. Antioxid. (Basel) 10, 1248 (2021).
Kaur, J. et al. MicroRNA-19a-3p decreases with age in mice and humans and inhibits osteoblast senescence. JBMR 7, e10745 (2023).
Kim, H. N. et al. Osteocyte RANKL is required for cortical bone loss with age and is induced by senescence. JCI Insight 5, e138815 (2020).
Geng, Q. et al. Pyrroloquinoline quinone prevents estrogen deficiency-induced osteoporosis by inhibiting oxidative stress and osteocyte senescence. Int. J. Biol. Sci. 15, 58–68 (2019).
Wang, X. et al. Enhancement of bone-forming ability on beta-tricalcium phosphate by modulating cellular senescence mechanisms using senolytics. Int J. Mol. Sci. 22, 12415 (2021).
Gorissen, B. et al. Hypoxia negatively affects senescence in osteoclasts and delays osteoclastogenesis. J. Cell Physiol. 234, 414–426 (2018).
Chen, W. et al. Novel pycnodysostosis mouse model uncovers cathepsin K function as a potential regulator of osteoclast apoptosis and senescence. Hum. Mol. Genet. 16, 410–423 (2007).
Pimenta-Lopes, C. et al. Inhibition of C5AR1 impairs osteoclast mobilization and prevents bone loss. Mol. Ther. 31, 2507–2523 (2023).
Kim, H. N. et al. Elimination of senescent osteoclast progenitors has no effect on the age-associated loss of bone mass in mice. Aging Cell 18, e12923 (2019).
Samakkarnthai, P. et al. In vitro and in vivo effects of zoledronic acid on senescence and senescence-associated secretory phenotype markers. Aging (Albany NY) 15, 3331–3355 (2023).
Li, Z. et al. Lipolysis of bone marrow adipocytes is required to fuel bone and the marrow niche during energy deficits. Elife 11, e78496 (2022).
Liu, X. et al. Oxylipin-PPARgamma-initiated adipocyte senescence propagates secondary senescence in the bone marrow. Cell Metab. 35, 667–684.e666 (2023).
Ungvari, Z. et al. Mechanisms of vascular aging, a geroscience perspective: JACC focus seminar. J. Am. Coll. Cardiol. 75, 931–941 (2020).
Kusumbe, A. P., Ramasamy, S. K. & Adams, R. H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 507, 323–328 (2014).
Tuckermann, J. & Adams, R. H. The endothelium-bone axis in development, homeostasis and bone and joint disease. Nat. Rev. Rheumatol. 17, 608–620 (2021).
Kusumbe, A. P. et al. Age-dependent modulation of vascular niches for haematopoietic stem cells. Nature 532, 380–384 (2016).
Xiao, X. et al. Mesenchymal stem cell-derived small extracellular vesicles mitigate oxidative stress-induced senescence in endothelial cells via regulation of miR-146a/Src. Signal Transduct. Target Ther. 6, 354 (2021).
Saul, D. et al. Modulation of fracture healing by the transient accumulation of senescent cells. Elife 10, e69958 (2021).
Zeng, X. et al. Fecal microbiota transplantation from young mice rejuvenates aged hematopoietic stem cells by suppressing inflammation. Blood 141, 1691–1707 (2023).
Kasbekar, M., Mitchell, C. A., Proven, M. A. & Passegue, E. Hematopoietic stem cells through the ages: A lifetime of adaptation to organismal demands. Cell Stem Cell 30, 1403–1420 (2023).
Wang, M. et al. Genotoxic aldehyde stress prematurely ages hematopoietic stem cells in a p53-driven manner. Mol. Cell 83, 2417–2433.e2417 (2023).
Bogeska, R. et al. Inflammatory exposure drives long-lived impairment of hematopoietic stem cell self-renewal activity and accelerated aging. Cell Stem Cell 29, 1273–1284.e1278 (2022).
Shevyrev, D., Tereshchenko, V., Berezina, T. N. & Rybtsov, S. Hematopoietic stem cells and the immune system in development and aging. Int. J. Mol. Sci. 24, 5862 (2023).
Ambrosi, T. H. et al. Aged skeletal stem cells generate an inflammatory degenerative niche. Nature 597, 256–262 (2021).
Ramalingam, P. et al. Restoring bone marrow niche function rejuvenates aged hematopoietic stem cells by reactivating the DNA Damage Response. Nat. Commun. 14, 2018 (2023).
Nakamura-Ishizu, A., Ito, K. & Suda, T. Hematopoietic stem cell metabolism during development and aging. Dev. Cell 54, 239–255 (2020).
Ji, M. L. et al. Sirt6 attenuates chondrocyte senescence and osteoarthritis progression. Nat. Commun. 13, 7658 (2022).
Zhang, H. et al. Mechanical overloading promotes chondrocyte senescence and osteoarthritis development through downregulating FBXW7. Ann. Rheum. Dis. 81, 676–686 (2022).
Cao, H. et al. MYL3 protects chondrocytes from senescence by inhibiting clathrin-mediated endocytosis and activating of Notch signaling. Nat. Commun. 14, 6190 (2023).
Shen, P. et al. LncRNA AC006064.4-201 serves as a novel molecular marker in alleviating cartilage senescence and protecting against osteoarthritis by destabilizing CDKN1B mRNA via interacting with PTBP1. Biomark. Res. 11, 39 (2023).
Gong, Z. et al. CircZSWIM6 mediates dysregulation of ECM and energy homeostasis in ageing chondrocytes through RPS14 post-translational modification. Clin. Transl. Med. 13, e1158 (2023).
Chen, X. et al. METTL3-mediated m(6)A modification of ATG7 regulates autophagy-GATA4 axis to promote cellular senescence and osteoarthritis progression. Ann. Rheum. Dis. 81, 87–99 (2022).
Liu, F. et al. Identification of FOXO1 as a geroprotector in human synovium through single-nucleus transcriptomic profiling. Protein Cell 15, 441–459 (2023).
Zhou, J. et al. Identification of aging-related biomarkers and immune infiltration characteristics in osteoarthritis based on bioinformatics analysis and machine learning. Front. Immunol. 14, 1168780 (2023).
Pessler, F. et al. A histomorphometric analysis of synovial biopsies from individuals with Gulf War Veterans’ Illness and joint pain compared to normal and osteoarthritis synovium. Clin. Rheumatol. 27, 1127–1134 (2008).
Li, Y. S., Luo, W., Zhu, S. A. & Lei, G. H. T. Cells in osteoarthritis: alterations and beyond. Front. Immunol. 8, 356 (2017).
Ko, C. Y. et al. Omentin-1 ameliorates the progress of osteoarthritis by promoting IL-4-dependent anti-inflammatory responses and M2 macrophage polarization. Int J. Biol. Sci. 19, 5275–5289 (2023).
Grieshaber-Bouyer, R. et al. Ageing and interferon gamma response drive the phenotype of neutrophils in the inflamed joint. Ann. Rheum. Dis. 81, 805–814 (2022).
Barbouti, A. et al. Implications of oxidative stress and cellular senescence in age-related thymus involution. Oxid. Med. Cell Longev. 2020, 7986071 (2020).
Spits, H. Development of alphabeta T cells in the human thymus. Nat. Rev. Immunol. 2, 760–772 (2002).
Pinho, S. & Frenette, P. S. Haematopoietic stem cell activity and interactions with the niche. Nat. Rev. Mol. Cell Biol. 20, 303–320 (2019).
Mei, Y. et al. Bone marrow-confined IL-6 signaling mediates the progression of myelodysplastic syndromes to acute myeloid leukemia. J. Clin. Invest. 132, e152673 (2022).
Soto-Heredero, G., Gomez de Las Heras, M. M., Escrig-Larena, JI., & Mittelbrunn, M. Extremely differentiated T cell subsets contribute to tissue deterioration during aging. Annu. Rev. Immunol. 41, 181–205 (2023).
Zhang, H. et al. Aging-associated HELIOS deficiency in naive CD4+ T cells alters chromatin remodeling and promotes effector cell responses. Nat. Immunol. 24, 96–109 (2023).
Bignon, A. et al. DUSP4-mediated accelerated T-cell senescence in idiopathic CD4 lymphopenia. Blood 125, 2507–2518 (2015).
Hasegawa, T. et al. Cytotoxic CD4+ T cells eliminate senescent cells by targeting cytomegalovirus antigen. Cell 186, 1417–1431.e1420 (2023).
Lorenzo, E. C. et al. Senescence-induced changes in CD4 T cell differentiation can be alleviated by treatment with senolytics. Aging Cell 21, e13525 (2022).
Nian, Y. et al. Targeting age-specific changes in CD4+ T cell metabolism ameliorates alloimmune responses and prolongs graft survival. Aging Cell 20, e13299 (2021).
Xiong, Y. et al. hPMSCs-derived exosomal miRNA-21 protects against aging-related oxidative damage of CD4+ T cells by targeting the PTEN/PI3K-Nrf2 axis. Front. Immunol. 12, 780897 (2021).
Song, Y. et al. T-cell immunoglobulin and ITIM domain contributes to CD8+ T-cell immunosenescence. Aging Cell 17, e12716 (2018).
Callender, L. A. et al. Human CD8+ EMRA T cells display a senescence-associated secretory phenotype regulated by p38 MAPK. Aging Cell 17, e12675 (2018).
Marin, I. et al. Cellular senescence is immunogenic and promotes antitumor immunity. Cancer Discov. 13, 410–431 (2023).
Delpoux, A. et al. FOXO1 constrains activation and regulates senescence in CD8 T cells. Cell Rep. 34, 108674 (2021).
Gustafson, C. E. et al. Functional pathways regulated by microRNA networks in CD8 T-cell aging. Aging Cell 18, e12879 (2019).
Ye, J. et al. Human regulatory T cells induce T-lymphocyte senescence. Blood 120, 2021–2031 (2012).
Guo, Z. et al. DCAF1 regulates Treg senescence via the ROS axis during immunological aging. J. Clin. Invest. 130, 5893–5908 (2020).
Li, L. et al. TLR8-mediated metabolic control of human Treg function: a mechanistic target for cancer immunotherapy. Cell Metab. 29, 103–123.e105 (2019).
Danileviciute, E. et al. PARK7/DJ-1 promotes pyruvate dehydrogenase activity and maintains T(reg) homeostasis during ageing. Nat. Metab. 4, 589–607 (2022).
Cancro, M. P. Age-associated B cells. Annu. Rev. Immunol. 38, 315–340 (2020).
Riley, R. L. et al. Deficient B lymphopoiesis in murine senescence: potential roles for dysregulation of E2A, Pax-5, and STAT5. Semin. Immunol. 17, 330–336 (2005).
Cepeda, S. et al. Age-associated decline in thymic B cell expression of aire and aire-dependent self-antigens. Cell Rep. 22, 1276–1287 (2018).
Zhang, H. et al. Polyamines control eIF5A hypusination, TFEB translation, and autophagy to reverse B cell senescence. Mol. Cell 76, 110–125.e119 (2019).
de Mol, J., Kuiper, J., Tsiantoulas, D. & Foks, A. C. The dynamics of B cell aging in health and disease. Front. Immunol. 12, 733566 (2021).
Landin, A. M. et al. E47 retroviral rescue of intrinsic B-cell defects in senescent mice. Aging Cell 10, 327–337 (2011).
Rawji, K. S. et al. Immunosenescence of microglia and macrophages: impact on the ageing central nervous system. Brain 139, 653–661 (2016).
Wang, Q. et al. Diabetes fuels periodontal lesions via GLUT1-driven macrophage inflammaging. Int. J. Oral. Sci. 13, 11 (2021).
Schloesser, D. et al. Senescent cells suppress macrophage-mediated corpse removal via upregulation of the CD47-QPCT/L axis. J. Cell Biol. 222, e202207097 (2023).
Wang, H. et al. BRD4 contributes to LPS-induced macrophage senescence and promotes progression of atherosclerosis-associated lipid uptake. Aging (Albany NY) 12, 9240–9259 (2020).
Barkaway, A. et al. Age-related changes in the local milieu of inflamed tissues cause aberrant neutrophil trafficking and subsequent remote organ damage. Immunity 54, 1494–1510.e1497 (2021).
Minton, K. Neutrophils hit reverse in ageing. Nat. Rev. Immunol. 21, 408–409 (2021).
Ward, J. R. et al. Regulation of neutrophil senescence by microRNAs. PLoS One 6, e15810 (2011).
Guo, C. et al. Single-cell transcriptomics reveal a unique memory-like NK cell subset that accumulates with ageing and correlates with disease severity in COVID-19. Genome Med. 14, 46 (2022).
Bai, Z. et al. Combining adoptive NK cell infusion with a dopamine-releasing peptide reduces senescent cells in aged mice. Cell Death Dis. 13, 305 (2022).
Chakraborty, M. et al. Mechanical stiffness controls dendritic cell metabolism and function. Cell Rep. 34, 108609 (2021).
Wong, C. & Goldstein, D. R. Impact of aging on antigen presentation cell function of dendritic cells. Curr. Opin. Immunol. 25, 535–541 (2013).
Young, K. et al. Decline in IGF1 in the bone marrow microenvironment initiates hematopoietic stem cell aging. Cell Stem Cell 28, 1473–1482.e1477 (2021).
Valletta, S. et al. Micro-environmental sensing by bone marrow stroma identifies IL-6 and TGFbeta1 as regulators of hematopoietic ageing. Nat. Commun. 11, 4075 (2020).
Ratliff, M. et al. In aged mice, low surrogate light chain promotes pro-B-cell apoptotic resistance, compromises the PreBCR checkpoint, and favors generation of autoreactive, phosphorylcholine-specific B cells. Aging Cell 14, 382–390 (2015).
Colom Diaz, P. A., Mistry, J. J. & Trowbridge, J. J. Hematopoietic stem cell aging and leukemia transformation. Blood 142, 533–542 (2023).
Li, X. et al. Inflammation and aging: signaling pathways and intervention therapies. Signal Transduct. Target Ther. 8, 239 (2023).
Kuribayashi, W. et al. Limited rejuvenation of aged hematopoietic stem cells in young bone marrow niche. J. Exp. Med. 218, e20192283 (2021).
Zhang, X. et al. Harnessing matrix stiffness to engineer a bone marrow niche for hematopoietic stem cell rejuvenation. Cell Stem Cell 30, 378–395.e378 (2023).
Maryanovich, M. et al. Adrenergic nerve degeneration in bone marrow drives aging of the hematopoietic stem cell niche. Nat. Med. 24, 782–791 (2018).
Yu, Z. et al. Endothelial cell-derived angiopoietin-like protein 2 supports hematopoietic stem cell activities in bone marrow niches. Blood 139, 1529–1540 (2022).
Chen, J. et al. Interleukin 6-regulated macrophage polarization controls atherosclerosis-associated vascular intimal hyperplasia. Front. Immunol. 13, 952164 (2022).
Yan, Y. et al. Interleukin-6 produced by enteric neurons regulates the number and phenotype of microbe-responsive regulatory T cells in the gut. Immunity 54, 499–513.e495 (2021).
Pritz, T., Weinberger, B. & Grubeck-Loebenstein, B. The aging bone marrow and its impact on immune responses in old age. Immunol. Lett. 162, 310–315 (2014).
Liu, S. et al. Endothelial IL-8 induced by porcine circovirus type 2 affects dendritic cell maturation and antigen-presenting function. Virol. J. 16, 154 (2019).
Labrie, J. E. 3rd, Borghesi, L. & Gerstein, R. M. Bone marrow microenvironmental changes in aged mice compromise V(D)J recombinase activity and B cell generation. Semin. Immunol. 17, 347–355 (2005).
Jin, G. et al. MT1-MMP cleaves Dll1 to negatively regulate Notch signalling to maintain normal B-cell development. EMBO J. 30, 2281–2293 (2011).
Petillo, S. et al. NEDD8-activating enzyme inhibition potentiates the anti-myeloma activity of natural killer cells. Cell Death Dis. 14, 438 (2023).
Xiong, Y. et al. Metal-organic frameworks and their composites for chronic wound healing: From bench to bedside. Adv. Mater. e2302587 (2023)
Benet, Z., Jing, Z. & Fooksman, D. R. Plasma cell dynamics in the bone marrow niche. Cell Rep. 34, 108733 (2021).
Fessler, J. et al. Senescent T-cells promote bone loss in rheumatoid arthritis. Front. Immunol. 9, 95 (2018).
Faust, H. J. et al. IL-17 and immunologically induced senescence regulate response to injury in osteoarthritis. J. Clin. Invest. 130, 5493–5507 (2020).
Li, J. Y. et al. The sclerostin-independent bone anabolic activity of intermittent PTH treatment is mediated by T-cell-produced Wnt10b. J. Bone Min. Res. 29, 43–54 (2014).
O’Neil, L. J. et al. Neutrophil extracellular trap-associated carbamylation and histones trigger osteoclast formation in rheumatoid arthritis. Ann. Rheum. Dis. 82, 630–638 (2023).
Hale, S. J. et al. CXCR2 modulates bone marrow vascular repair and haematopoietic recovery post-transplant. Br. J. Haematol. 169, 552–564 (2015).
Li, J. et al. TGFbeta1+ CCR5+ neutrophil subset increases in bone marrow and causes age-related osteoporosis in male mice. Nat. Commun. 14, 159 (2023).
Clark, D. et al. Age-related changes to macrophages are detrimental to fracture healing in mice. Aging Cell 19, e13112 (2020).
Gibon, E. et al. Aging affects bone marrow macrophage polarization: relevance to bone healing. Regen. Eng. Transl. Med. 2, 98–104 (2016).
Li, C. J. et al. Senescent immune cells release grancalcin to promote skeletal aging. Cell Metab. 33, 1957–1973.e1956 (2021).
Titanji, K. et al. Dysregulated B cell expression of RANKL and OPG correlates with loss of bone mineral density in HIV infection. PLoS Pathog. 10, e1004497 (2014).
Titanji, K. Beyond antibodies: B cells and the OPG/RANK-RANKL pathway in health, non-HIV disease and HIV-induced bone loss. Front. Immunol. 8, 1851 (2017).
Li, Y. et al. B cell production of both OPG and RANKL is significantly increased in aged mice. Open Bone J. 6, 8–17 (2014).
Grolleau-Julius, A. et al. Effect of aging on bone marrow-derived murine CD11c+CD4−CD8alpha− dendritic cell function. J. Gerontol. A Biol. Sci. Med. Sci. 61, 1039–1047 (2006).
Farr, J. N. et al. Identification of senescent cells in the bone microenvironment. J. Bone Min. Res. 31, 1920–1929 (2016).
Shin, M. S., Park, H. J., Young, J. & Kang, I. Implication of IL-7 receptor alpha chain expression by CD8+ T cells and its signature in defining biomarkers in aging. Immun. Ageing 19, 66 (2022).
Dhanabalan, K. M. et al. Intra-articular injection of rapamycin microparticles prevent senescence and effectively treat osteoarthritis. Bioeng. Transl. Med. 8, e10298 (2023).
Liu, W. et al. Alpl prevents bone ageing sensitivity by specifically regulating senescence and differentiation in mesenchymal stem cells. Bone Res. 6, 27 (2018).
Wang, Y. et al. Repurpose dasatinib and quercetin: targeting senescent cells ameliorates postmenopausal osteoporosis and rejuvenates bone regeneration. Bioact. Mater. 25, 13–28 (2023).
Peng, H. et al. A mechanosensitive lipolytic factor in the bone marrow promotes osteogenesis and lymphopoiesis. Cell Metab. 34, 1168–1182.e1166 (2022).
Yang, X. et al. T cell-depleting nanoparticles ameliorate bone loss by reducing activated T cells and regulating the Treg/Th17 balance. Bioact. Mater. 6, 3150–3163 (2021).
Chen, Y. et al. Single-cell RNA landscape of the osteoimmunology microenvironment in periodontitis. Theranostics 12, 1074–1096 (2022).
Farr, J. N. et al. Targeting cellular senescence prevents age-related bone loss in mice. Nat. Med. 23, 1072–1079 (2017).
Cheng, A. et al. Early systemic immune biomarkers predict bone regeneration after trauma. Proc. Natl. Acad. Sci. USA 118, e2017889118 (2021).
Shields, A. F. Imaging bone-marrow activity with (18)F-fluorothymidine PET. Lancet Haematol. 5, e8–e9 (2018).
Yousefzadeh, M. J. et al. An aged immune system drives senescence and ageing of solid organs. Nature 594, 100–105 (2021).
Yan, S. et al. Metformin regulates chondrocyte senescence and proliferation through microRNA-34a/SIRT1 pathway in osteoarthritis. J. Orthop. Surg. Res. 18, 198 (2023).
Landry, D. A. et al. Metformin prevents age-associated ovarian fibrosis by modulating the immune landscape in female mice. Sci. Adv. 8, eabq1475 (2022).
Yang, J. et al. The effect of metformin on senescence of T lymphocytes. Immun. Ageing 20, 73 (2023).
Liu, J. et al. Age-associated callus senescent cells produce TGF-beta1 that inhibits fracture healing in aged mice. J. Clin. Invest. 132, e148073 (2022).
Dai, H. et al. Eliminating senescent chondrogenic progenitor cells enhances chondrogenesis under intermittent hydrostatic pressure for the treatment of OA. Stem Cell Res. Ther. 11, 199 (2020).
Iske, J. et al. Senolytics prevent mt-DNA-induced inflammation and promote the survival of aged organs following transplantation. Nat. Commun. 11, 4289 (2020).
Griveau, A. et al. The JAK1/2 inhibitor ruxolitinib delays premature aging phenotypes. Aging Cell 19, e13122 (2020).
Ledesma-Colunga, M. G. et al. Transferrin receptor 2 deficiency promotes macrophage polarization and inflammatory arthritis. Redox Biol. 60, 102616 (2023).
Xu, M. et al. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc. Natl. Acad. Sci. USA 112, E6301–E6310 (2015).
Hambright, W. S. et al. The senolytic drug fisetin attenuates bone degeneration in the Zmpste24−/− progeria mouse model. J. Osteoporos. 2023, 5572754 (2023).
Jia, Q. et al. Fisetin, via CKIP-1/REGgamma, limits oxidized LDL-induced lipid accumulation and senescence in RAW264.7 macrophage-derived foam cells. Eur. J. Pharm. 865, 172748 (2019).
Chang, J. et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med. 22, 78–83 (2016).
Miura, Y., Endo, K., Komori, K. & Sekiya, I. Clearance of senescent cells with ABT-263 improves biological functions of synovial mesenchymal stem cells from osteoarthritis patients. Stem Cell Res. Ther. 13, 222 (2022).
Yang, H. et al. Navitoclax (ABT263) reduces inflammation and promotes chondrogenic phenotype by clearing senescent osteoarthritic chondrocytes in osteoarthritis. Aging (Albany NY) 12, 12750–12770 (2020).
Chung, L. et al. Interleukin 17 and senescent cells regulate the foreign body response to synthetic material implants in mice and humans. Sci. Transl. Med. 12, eaax3799 (2020).
Hernandez-Silva, D. et al. Senescence-independent anti-inflammatory activity of the senolytic drugs dasatinib, navitoclax, and venetoclax in zebrafish models of chronic inflammation. Int. J. Mol. Sci. 23, 10468 (2022).
Peterson, C. T., Rodionov, D. A., Osterman, A. L. & Peterson, S. N. B vitamins and their role in immune regulation and cancer. Nutrients 12, 3380 (2020).
Clements, M. et al. A 2-year randomized controlled trial with low-dose B-vitamin supplementation shows benefits on bone mineral density in adults with lower B12 status. J. Bone Min. Res. 37, 2443–2455 (2022).
Hong, H. et al. Associations of homocysteine, folate, and vitamin B12 with osteoarthritis: a mendelian randomization study. Nutrients 15, 1636 (2023).
Carr, A. C. & Maggini, S. Vitamin C and immune function. Nutrients 9, 1211 (2017).
Thaler, R. et al. Vitamin C epigenetically controls osteogenesis and bone mineralization. Nat. Commun. 13, 5883 (2022).
Blouin, S. et al. Vitamin C deficiency deteriorates bone microarchitecture and mineralization in a sex-specific manner in adult mice. J. Bone Min. Res. 38, 1509–1520 (2023).
Fantini, C. et al. Vitamin D as a shield against aging. Int. J. Mol. Sci. 24, 4546 (2023).
van Driel, M. & van Leeuwen, J. Vitamin D and bone: a story of endocrine and auto/paracrine action in osteoblasts. Nutrients 15, 480 (2023).
Lewis, E. D., Meydani, S. N. & Wu, D. Regulatory role of vitamin E in the immune system and inflammation. IUBMB Life 71, 487–494 (2019).
Nazrun Shuid, A., Das, S. & Mohamed, I. N. Therapeutic effect of Vitamin E in preventing bone loss: an evidence-based review. Int. J. Vitam. Nutr. Res. 89, 357–370 (2019).
Cui, A. et al. Associations between vitamin E status and bone mineral density in children and adolescents aged 8-19 years: evidence based on NHANES 2005-2006, 2017-2018. PLoS One 18, e0283127 (2023).
Hatanaka, H. et al. Effects of vitamin K3 and K5 on proliferation, cytokine production, and regulatory T cell-frequency in human peripheral-blood mononuclear cells. Life Sci. 99, 61–68 (2014).
Mandatori, D. et al. The dual role of vitamin K2 in “Bone-Vascular Crosstalk”: opposite effects on bone loss and vascular calcification. Nutrients 13, 1222 (2021).
Tsugawa, N. & Shiraki, M. Vitamin K nutrition and bone health. Nutrients 12, 1909 (2020).
Yang, L. et al. Amino acid metabolism in immune cells: essential regulators of the effector functions, and promising opportunities to enhance cancer immunotherapy. J. Hematol. Oncol. 16, 59 (2023).
Lv, Z., Shi, W. & Zhang, Q. Role of essential amino acids in age-induced bone loss. Int. J. Mol. Sci. 23, 11281 (2022).
Bachem, A. et al. Microbiota-derived short-chain fatty acids promote the memory potential of antigen-activated CD8+ T Cells. Immunity 51, 285–297.e285 (2019).
Tan, J. K., Macia, L. & Mackay, C. R. Dietary fiber and SCFAs in the regulation of mucosal immunity. J. Allergy Clin. Immunol. 151, 361–370 (2023).
Yang, K. L. et al. Short chain fatty acids mitigate osteoclast-mediated arthritic bone remodelling. Arthritis Rheumatol. 76, 647–659 (2023).
Lucas, S. et al. Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat. Commun. 9, 55 (2018).
Hidalgo, M. A., Carretta, M. D. & Burgos, R. A. Long chain fatty acids as modulators of immune cells function: Contribution of FFA1 and FFA4 receptors. Front. Physiol. 12, 668330 (2021).
Abshirini, M., Ilesanmi-Oyelere, B. L. & Kruger, M. C. Potential modulatory mechanisms of action by long-chain polyunsaturated fatty acids on bone cell and chondrocyte metabolism. Prog. Lipid Res. 83, 101113 (2021).
Acknowledgements
This work was supported by the National Key R&D Program of China (2021YFA1101500), Wuhan Science and Technology Bureau (2022020801020464). This work was also partially supported by University Grants Committee, Research Grants Council of the Hong Kong Special Administrative Region, China (14113723, N_CUHK472/22, T13-402/17-N and AoE/M-402/20) and Direct Grant of CUHK (2022.042). The authors thank Medpeer (www.medpeer.cn) and Servier Medical Art (smart.servier.com) for helping create the Figures.
Author information
Authors and Affiliations
Contributions
B.M. write original draft, review and editing, drawing and editing illustrations. Y.X. literature search and classification and editing. S.K. literature search and classification and editing. M.K. literature search and classification and editing. A.P. review and editing. H.W. review and editing. G.L. review and editing. S.L., G.L. conceptualization, project administration, funding acquisition, review and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mi, B., Xiong, Y., Knoedler, S. et al. Ageing-related bone and immunity changes: insights into the complex interplay between the skeleton and the immune system. Bone Res 12, 42 (2024). https://doi.org/10.1038/s41413-024-00346-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41413-024-00346-4
- Springer Nature Limited