Abstract
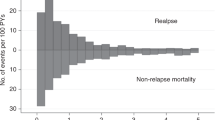
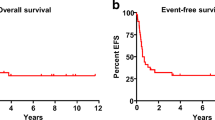
We studied 232 consecutive children transplanted between 1990 and 2011 with relapse after first hematopoietic cell transplant (HCT). Kaplan–Meier survival and hazard ratios for mortality were calculated for factors known at time of relapse using Cox proportional hazards models. The median (range) age at time of first HCT was 10.9 (0.5–20.9) years, time to relapse was 6.1 (0.2–89.5) months after HCT, and age at relapse was 11.7 (0.7–23.6) years. The 3-year overall survival (OS) after relapse was 13% (95% confidence interval (CI): 9%, 18%).The median (range) follow-up for the 18 surviving patients was 7.2 (3.0–24.4) years after relapse. The remaining 214 died after a median of 3 months (0.02–190.4). OS was not significantly different for patients with ALL as compared to AML. Fifty-one patients proceeded to second transplant of whom nine survive. Factors associated with improved survival included late relapse (>12 months), ALL in first CR at the time of first transplant and chemotherapy-based first conditioning regimens. These results can be used to counsel patients at the time of relapse after first transplant and as a baseline for comparison as to the effectiveness of newer therapies which are greatly needed for treatment of post-transplant relapse.



Similar content being viewed by others
References
Shiba N, Ohki K, Kobayashi T, Hara Y, Yamato G, Tanoshima R, et al. High PRDM16 expression identifies a prognostic subgroup of pediatric acute myeloid leukaemia correlated to FLT3-ITD, KMT2A-PTD, and NUP98-NSD1: the results of the Japanese Paediatric Leukaemia/Lymphoma Study Group AML-05 trial. Br J Haematol. 2016;172:581–91.
Chen IM, Harvey RC, Mullighan CG, Gastier-Foster J, Wharton W, Kang H, et al. Outcome modeling with CRLF2, IKZF1, JAK, and minimal residual disease in pediatric acute lymphoblastic leukemia: a Children’s Oncology Group study. Blood. 2012;119:3512–22.
Borowitz MJ, Shuster J, Carroll AJ, Nash M, Look AT, Camitta B, et al. Prognostic significance of fluorescence intensity of surface marker expression in childhood B-precursor acute lymphoblastic leukemia. Blood. 1997;89:3960–6.
Roberts KG, Mullighan CG. Genomics in acute lymphoblastic leukaemia: insights and treatment implications. Nat Rev Clin Oncol. 2015;12:344–57.
Schultz KR, Pullen DJ, Sather HN, Shuster JJ, Devidas M, Borowitz MJ, et al. Risk- and response-based classification of childhood B-precursor acute lymphoblastic leukemia: a combined analysis of prognostic markers from the Pediatric Oncology Group (POG) and Children’s Cancer Group (CCG). Blood. 2007;109:926–35.
van der Linden MH, Valsecchi MG, De Lorenzo P, Moricke A, Janka G, Leblanc TM, et al. Outcome of congenital acute lymphoblastic leukemia treated on the Interfant-99 protocol. Blood. 2009;114:3764–8.
Rubnitz JE, Inaba H, Dahl G, Ribeiro RC, Bowman WP, Taub J, et al. Minimal residual disease-directed therapy for childhood acute myeloid leukaemia: results of the AML02 multicentre trial. Lancet Oncol. 2010;11:543–52.
Lange BJ, Smith FO, Feusner J, Barnard DR, Dinndorf P, Feig S, et al. Outcomes in CCG-2961, a children’s oncology group phase 3 trial for untreated pediatric acute myeloid leukemia: a report from the children’s oncology group. Blood. 2008;111:1044–53.
Meshinchi S, Alonzo TA, Stirewalt DL, Zwaan M, Zimmerman M, Reinhardt D, et al. Clinical implications of FLT3 mutations in pediatric AML. Blood. 2006;108:3654–61.
Woolfrey AE, Anasetti C, Storer B, Doney K, Milner LA, Sievers EL, et al. Factors associated with outcome after unrelated marrow transplantation for treatment of acute lymphoblastic leukemia in children. Blood. 2002;99:2002–8.
Yusuf U, Frangoul HA, Gooley TA, Woolfrey AE, Carpenter PA, Andrews RG, et al. Allogeneic bone marrow transplantation in children with myelodysplastic syndrome or juvenile myelomonocytic leukemia: the Seattle experience. Bone Marrow Transplant. 2004;33:805–14.
Nemecek ER, Gooley TA, Woolfrey AE, Carpenter PA, Matthews DC, Sanders JE. Outcome of allogeneic bone marrow transplantation for children with advanced acute myeloid leukemia. Bone Marrow Transplant. 2004;34:799–806.
Nemecek ER, Ellis K, He W, Bunin NJ, Bajwa RS, Cheerva A, et al. Outcome of myeloablative conditioning and unrelated donor hematopoietic cell transplantation for childhood acute lymphoblastic leukemia in third remission. Biol Blood Marrow Transplant. 2011;17:1833–40.
Woolfrey AE, Gooley TA, Sievers EL, Milner LA, Andrews RG, Walters M, et al. Bone marrow transplantation for children less than 2 years of age with acute myelogenous leukemia or myelodysplastic syndrome. Blood. 1998;92:3546–56.
Woodard P, Carpenter PA, Davies SM, Gross TG, He W, Zhang MJ, et al. Unrelated donor bone marrow transplantation for myelodysplastic syndrome in children. Biol Blood Marrow Transplant. 2011;17:723–8.
Schechter T, Gassas A, Chen H, Pollard J, Meshinchi S, Zaidman I, et al. The outcome of allogeneic hematopoietic cell transplantation for children with FMS-like tyrosine kinase 3 internal tandem duplication-positive acute myelogenous leukemia. Biol Blood Marrow Transplant. 2015;21:172–5.
Sanders JE, Im HJ, Hoffmeister PA, Gooley TA, Woolfrey AE, Carpenter PA, et al. Allogeneic hematopoietic cell transplantation for infants with acute lymphoblastic leukemia. Blood. 2005;105:3749–56.
Strahm B, Nollke P, Zecca M, Korthof ET, Bierings M, Furlan I, et al. Hematopoietic stem cell transplantation for advanced myelodysplastic syndrome in children: results of the EWOG-MDS 98 study. Leukemia. 2011;25:455–62.
Loh ML, Zhang J, Harvey RC, Roberts K, Payne-Turner D, Kang H, et al. Tyrosine kinome sequencing of pediatric acute lymphoblastic leukemia: a report from the Children’s Oncology Group TARGET Project. Blood. 2013;121:485–8.
Mann G, Attarbaschi A, Schrappe M, De Lorenzo P, Peters C, Hann I, et al. Improved outcome with hematopoietic stem cell transplantation in a poor prognostic subgroup of infants with mixed-lineage-leukemia (MLL) -rearranged acute lymphoblastic leukemia: results from the Interfant-99 Study. Blood. 2010;116:2644–50.
Bar M, Wood BL, Radich JP, Doney KC, Woolfrey AE, Delaney C, et al. Impact of minimal residual disease, detected by flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute lymphoblastic leukemia. Leuk Res Treat. 2014;2014:421723.
Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. New Engl J Med. 2010;363:2091–101.
Bader P, Kreyenberg H, Henze GH, Eckert C, Reising M, Willasch A, et al. Prognostic value of minimal residual disease quantification before allogeneic stem-cell transplantation in relapsed childhood acute lymphoblastic leukemia: the ALL-REZ BFM Study Group. J Clin Oncol. 2009;27:377–84.
Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81.
Cox DR. Regression models and life tables (with discussion). J R Stat Soc,Ser B. 1972;34:187–220.
Eapen M, Giralt SA, Horowitz MM, Klein JP, Wagner JE, Zhang MJ, et al. Second transplant for acute and chronic leukemia relapsing after first HLA-identical sibling transplant. Bone Marrow Transplant. 2004;34:721–7.
Radich JP, Sanders JE, Buckner CD, Martin PJ, Petersen FB, Bensinger W, et al. Second allogeneic marrow transplantation for patients with recurrent leukemia after initial transplant with total-body irradiation-containing regimens. J Clin Oncol. 1993;11:304–13.
Shaw BE, Mufti GJ, Mackinnon S, Cavenagh JD, Pearce RM, Towlson KE, et al. Outcome of second allogeneic transplants using reduced-intensity conditioning following relapse of haematological malignancy after an initial allogeneic transplant. Bone Marrow Transplant. 2008;42:783–9.
Bajwa R, Schechter T, Soni S, Gassas A, Doyle J, Sisler I, et al. Outcome of children who experience disease relapse following allogeneic hematopoietic SCT for hematologic malignancies. Bone Marrow Transplant. 2013;48:661–5.
Meshinchi S, Leisenring WM, Carpenter PA, Woolfrey AE, Sievers EL, Radich JP, Sanders JE. Survival after second hematopoietic stem cell transplantation for recurrent pediatric acute myeloid leukemia. Biol Blood Marrow Transplant. 2003;9:706–13.
Mielcarek M, Storer BE, Flowers MED, Storb R, Sandmaier BM, Martin PJ. Outcomes among patients with recurrent high-risk hematologic malignancies after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2007;13:1160–8.
Milano F, Gooley T, Wood B, Woolfrey A, Flowers ME, Doney K, et al. Cord blood transplant in patients with minimal residual disease. New Engl J Med. 2016;375:944–53.
Salit RB, Milano F, Delaney C. Outcomes of cord blood transplantation as salvage therapy after graft failure or relapse after prior allogeneic transplantation. Biol Blood Marrow Transplant. 2016;22:339–43.
Nemecek ER, Guthrie KA, Sorror ML, Wood BL, Doney KC, Hilger RA, et al. Conditioning with treosulfan and fludarabine followed by allogeneic hematopoietic cell transplantation for high-risk hematologic malignancies. Biol Blood Marrow Transplant. 2011;17:341–50.
Hill BT, Bolwell BJ, Rybicki L, Dean R, Kalaycio M, Pohlman B, et al. Nonmyeloablative second transplants are associated with lower nonrelapse mortality and superior survival than myeloablative second transplants. Biol Blood Marrow Transplant. 2010;16:1738–46.
Arfons LM, Tomblyn M, Rocha V, Lazarus HM. Second hematopoietic stem cell transplantation in myeloid malignancies. Curr Opin Hematol. 2009;16:112–23.
Menon NN, Jenkins LM, Cui H, Jenkins C, Anwer F, Yeager AM, et al. Factors associated with improved outcomes after second allogeneic hematopoietic cell transplantation for relapsed pediatric leukemia. Ann Hematol. 2016;95:637–44.
Tarlock K, Chang B, Cooper T, Gross T, Gupta S, Neudorf S, et al. Sorafenib treatment following hematopoietic stem cell transplant in pediatric FLT3/ITD acute myeloid leukemia. Pediatr Blood Cancer. 2015;62:1048–54.
Antar A, Kharfan-Dabaja MA, Mahfouz R, Bazarbachi A. Sorafenib maintenance appears safe and improves clinical outcomes in FLT3-ITD acute myeloid leukemia after allogeneic hematopoietic cell transplantation. Clin Lymphoma Myeloma Leuk. 2015;15:298–302.
Carpenter PA, Snyder DS, Flowers MED, Sanders JE, Gooley TA, Martin PJ, et al. Prophylactic administration of imatinib after hematopoietic cell transplantation for high-risk Philadelphia chromosome-positive leukemia. Blood. 2007;109:2791–3.
Foster JB, Maude SL. New developments in immunotherapy for pediatric leukemia. Curr Opin Pediatr. 2018;30:25–9.
Rytting M, Triche L, Thomas D, O’Brien S, Kantarjian H. Initial experience with CMC-544 (inotuzumab ozogamicin) in pediatric patients with relapsed B-cell acute lymphoblastic leukemia. Pediatr Blood Cancer. 2014;61:369–72.
Gamis AS, Alonzo TA, Meshinchi S, Sung L, Gerbing RB, Raimondi SC, et al. Gemtuzumab ozogamicin in children and adolescents with de novo acute myeloid leukemia improves event-free survival by reducing relapse risk: results from the randomized phase III Children’s Oncology Group trial AAML0531. J Clin Oncol. 2014;32:3021–32.
Maude SL, Teachey DT, Porter DL, Grupp SA. CD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Blood. 2015;125:4017–23.
Gardner RA, Finney O, Annesley C, Brakke H, Summers C, Leger K, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. 2017;129:3322–31.
Maude SL, Teachey DT, Rheingold SR, Shaw PA, Aplenc R, Barrett DM, et al. Sustained remissions with CD19-specific chimeric antigen receptor (CAR)-modified T cells in children with relapsed/refractory ALL. J Clin Oncol. 2016;34:3011–3011. (abstract)
Acknowledgements
MB is supported through NHLBI RO1 HL121568–04, Alex’s Lemonade Stand Foundation Biotherapeutic Impact Grant, Leukemia and Lymphoma Society Translational Research Program 6519–17, Cookies for Kids Cancer and ACCR SU2C Innovative Research Grant 14–17. C.D. is supported by K23HL077446 and RC2HL101844. AW is supported by NHLBI P01 HL 122173–01 and NHLBI P01 HL 036444–30.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Dahlberg, A., Leisenring, W., Bleakley, M. et al. Prognosis of relapse after hematopoietic cell transplant (HCT) for treatment of leukemia or myelodysplastic syndrome (MDS) in children. Bone Marrow Transplant 54, 1337–1345 (2019). https://doi.org/10.1038/s41409-019-0438-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-019-0438-z
- Springer Nature Limited
This article is cited by
-
Outcomes of pediatric patients who relapse after first HCT for acute leukemia or MDS
Bone Marrow Transplantation (2021)