Abstract
Aberrant activation of NLRP3 inflammasome causes the progression of various inflammation-related diseases, but the small-molecule inhibitors of NLRP3 are not currently available for clinical use. Tabersonine (Tab) is a natural product derived from a traditional Chinese herb Catharanthus roseus that is usually used as an anti-tumor agent. In this study we investigated the anti-inflammatory effects and molecular targets of Tab. We first screened 151 in-house natural compounds for their inhibitory activity against IL-1β production in BMDMs. We found that Tab potently inhibited NLRP3-mediated IL-1β production with an IC50 value of 0.71 μM. Furthermore, we demonstrated that Tab suppressed the assembly of NLRP3 inflammasome, especially the interaction between NLRP3 and ASC. Interestingly, we found that Tab directly bound to NLRP3 NACHT domain, thereby reducing the self-oligomerization of NLRP3. In addition, we showed that administration of Tab significantly ameliorated NLRP3-driven diseases, such as peritonitis, acute lung injury, and sepsis in mouse models. The preventive effects of Tab were not observed in the models of NLRP3 knockout mouse. In conclusion, we have identified Tab as a natural NLRP3 inhibitor and a lead compound for the design and discovery of novel NLRP3 inhibitors.

Similar content being viewed by others
Introduction
The nucleotide-oligomerization domain-like receptor family pyrin domain-containing 3 (NLRP3) is a unique and important component of NLRP3 inflammasome, which consists of the innate immune sensor NLRP3, adaptor protein apoptosis-associated speck-like protein containing a CARD (ASC), and effector protein caspase-1 [1]. NLRP3 inflammasome activation mediates the cleavage of caspase-1, which cleaves pro-IL-1β, pro-IL-18, and GSDMD, leading to pyroptosis and the maturation of pro-inflammatory cytokines IL-1β and IL-18 [2, 3]. NLRP3 inflammasome can be sensitized by stimulation of pathogen-associated molecular patterns, such as nigericin, and death-associated molecular patterns such as extracellular ATP and monosodium urate crystals [4, 5]. In addition, never in mitosis A-related kinase-7 (NEK7) has been characterized as a license for the activation of NLRP3 inflammasome, which directly binds to NLRP3 [6]. NLRP3 inflammasome activation is aberrantly associated with the progression of inflammation-related diseases, such as acute lung injury (ALI) [7], peritonitis [8], and sepsis [9]. Recently, many small molecular inhibitors, such as MCC950 [10] and CY-09 [11], have been shown to inhibit inflammasome by targeting NLRP3. Various natural products also have a potential inhibitory effect on NLRP3 inflammasome activation to suppress inflammation in vivo [12]. Taken together, the pharmacological inhibition of NLRP3 is a potential target for the treatment of inflammatory diseases. The discovery of novel NLRP3-inhibiting molecules may contribute to the design and development of NLRP3 inhibitors for the treatment of NLRP3-driven diseases.
Natural products represent a crucial resource in drug discovery because they provide a large number of structures and lead compounds for drug design [13]. Tabersonine (Tab) is a natural compound isolated from the medicinal plant Catharanthus roseus [14], a traditional Chinese herb usually used in anti-tumor treatment [15]. Recently, Tab has been shown to possess anti-inflammatory activity by suppressing TRAF6 ubiquitination [16]. Our laboratory also found that Tab attenuates cardiac remodeling and dysfunction through inhibiting TAK1 [17]. However, the effects of Tab on NLRP3 inflammasome activation are still unclear, and the precise target proteins of Tab remain unknown.
In this study, Tab was identified as an efficient inhibitor of NLRP3 inflammasome from a bank of 151 in-house natural products. It was found that Tab directly bound to NLRP3 NACHT domain, inhibited NLRP3 oligomerization, and then suppressed the activation of NLRP3 inflammasome. It was also observed that Tab efficiently alleviated NLRP3-driven diseases in mouse models like peritonitis, ALI, and sepsis.
Materials and methods
Reagents
Lipopolysaccharides (LPS, #L2880), adenosine triphosphate (ATP, #A3377) and aluminum potassium sulfate dodecahydrate (Alum, #237086) were bought from Sigma (St. Louis, MO, USA). Tab (#AB0928) was purchased from Abphyto Biotech Co., Ltd (Chengdu, China). Protein A + G agarose (#P2012) was obtained from Beyotime (Shanghai, China). Anti-NLRP3 (#AG-20B-0014) and anti-mouse caspase-1 (#AG-20B-0042; for WB) antibodies were purchased from Adipogen (San Diego, CA, USA). Anti-NEK7 (#ab133514), anti-GSDMD (#ab209845), and anti-caspase-1 (#ab179515; for IHC) antibodies were obtained from Abcam (Cambridge, UK). Anti-mouse IL-1β (#AF-401-NA) antibody was supplied by R&D Systems (Minneapolis, MN, USA). Anti-FLAG (#20543-1-AP), anti-HA (#66006-2-Ig), and anti-β-actin (#66009-1-Ig) antibodies were purchased from Proteintech (Rosemont, IL, USA).
Cell culture
Bone marrow-derived macrophages (BMDMs) were isolated and cultured from 8-week-old mice as described previously [18]. BMDMs were cultured for 7 days in Dulbecco’s modified Eagle’s medium (DMEM, #C11995500BT; Gibco, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS, #10270-106; Gibco), 20% supernatants of L929 mouse fibroblasts, 100 U/ml penicillin, and 100 μg/ml streptomycin (#C100C5; NCM Biotech, Suzhou, China). L929 mouse fibroblasts (#GNM28), THP-1 cells (#SCPS-567), and HEK-293T cells (#GNHu17) were supplied by the Shanghai Institute of Biochemistry and Cell Biology (Shanghai, China). L929 cells were cultured in minimum essential medium α (#SH30265.01; Hyclone, Logan, UT, USA) supplemented with 10% FBS and penicillin-streptomycin. THP-1 cells were cultured in the Roswell Park Memorial Institute 1640 medium (RPMI-1640, #C11875500BT; Gibco) supplemented with 10% FBS and antibiotics. HEK-293T cells were cultured in DMEM supplemented with 10% FBS and penicillin-streptomycin.
Animal experiments
Gempharmatech Co., Ltd provided C57BL/6J mice for this study. C57BL/6-Nlrp3KO mice (Strain No.: VSM40005) were purchased from Beijing Viewsolid Biotech Co., Ltd. All animal care and experimental protocols were approved by the Wenzhou Medical University Animal Policy and Welfare Committee (approved No.: wydw-2021-0353). All animal experiments conformed the NIH guidelines (Guide for the care and use of laboratory animals). Animals were housed under a 12:12 h light/dark cycle at a constant room temperature and provided with food and water.
For modeling peritonitis, 10-week-old male C57BL/6 mice were treated via gavage with Tab (10, 20, or 40 mg/kg) dissolved in 0.5% carboxymethylcellulose sodium (CMC-Na) or vehicle before intraperitoneal injection of Alum (1 mg per mouse). After 6 h, the mice were sacrificed and 6 ml of PBS was used for peritoneal lavage three times. Peritoneal lavage fluids were centrifuged, and the supernatants were used to test the level of IL-1β, and the monocyte and neutrophils were analyzed by flow cytometry with staining different markers. APC-CD45.2 (#109813), Per-CP-CD11b (#101229), FITC-Ly6C (#128005), and PE-Ly6G (#127607) antibodies were purchased from BioLegend (San Diego, CA, USA).
For modeling ALI, 10-week-old male C57BL/6 and Nlrp3KO mice were intragastrically administered Tab (10 mg/kg) dissolved in 0.5% CMC-Na three times daily before intratracheal instillation of LPS (5 mg/kg). After 6 h, mice were sacrificed. Serum, bronchoalveolar lavage fluid (BALF), and lung tissue samples were collected. The same lung sections were weighed, dried at 60 °C for 48 h, and weighed again. The protein concentrations in the BALF were measured using Bradford assay. The levels of IL-1β in BALF and serum samples were tested using enzyme-linked immunosorbent assay (ELISA). The same lung sections were used for hematoxylin and eosin staining and IHC staining. Other lung sections were used to measure the levels of IL-1β and p20 by Western blotting.
For modeling sepsis, 10-week-old male C57BL/6 and Nlrp3KO mice were injected intraperitoneally with Tab (10 mg/kg) before intraperitoneal injection of Escherichia coli (E. coli, 1 × 109 CFU/mouse) in 0.4 ml of PBS. The E. coli strain DH5α was cultured in Luria broth media and the density was determined at 600 nm. The survival of mice was recorded every 6 h for 48 h.
For modeling LPS-challenged mice, 8–10-week-old male C57BL/6 mice were intraperitoneally injected with Tab (10 mg/kg) or vehicles. After 1 h, mice were intraperitoneally injected with LPS (10 mg/kg) or PBS as vehicles. After 4 h, serum samples were collected, and cytokines were measured using ELISA.
Inflammasome stimulation
To activate NLRP3 inflammasome, 1 × 106/ml of BMDMs were plated in 6-well plates. After overnight incubation, the BMDMs were challenged with 500 ng/ml LPS for 3 h. Subsequently, the cells were treated with Tab for 30 min, and then stimulated with ATP (2.5 mM) for 0.5 h, nigericin (10 μM) for 30 min, or Alum (300 μg/ml) for 4 h.
THP-1 cells were plated in 6-well plates and treated with 100 ng/ml PMA overnight. To stimulate NLRP3 inflammasome, the cells were treated with 500 ng/ml LPS and different doses of Tab for 3 h, and then challenged with nigericin (10 μM) for 30 min.
ELISA
Supernatants from cell culture, peritoneal lavage fluids, BALF, lung homogenate, and serum were measured for mouse IL-1β (#88-7013-77), TNF-α (#88-7324-76), and IL-6 (#88-7064-76) using commercial ELISA kits (Invitrogen, Carlsbad, USA) according to the manufacturer’s instructions.
Immunofluorescence (IHC) staining
BMDMs were plated overnight in glass-bottomed cell culture dishes at 1 × 105/ml density. After overnight incubation, BMDMs were stimulated as described in “inflammasome stimulation”. After stimulation, the cells were washed thrice with PBS, and fixed with 4% paraformaldehyde for 20 min at room temperature. After washing thrice with PBS containing 0.05% Tween 20, the cells were permeabilized with 0.25% Triton X-100 for 10 min and blocked with 5% bovine serum albumin for 30 min. Then the antibodies were incubated at 4 °C overnight. The following day, the cells were incubated with secondary antibodies and then counterstained with 4’, 6-diamidino-2-phenylindole (DAPI, #36308ES11; Yeasen, Shanghai, China).
Western blotting and co-immunoprecipitation
Cells or tissue were lysed by lysis buffer, and the total protein concentration was determined using the Bradford assay. The samples were separated by 12% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and then transferred to polyvinylidene fluoride membranes. After blocking with 5% non-fat milk in Tris-buffered saline containing 0.05% Tween 20 for 1.5 h at room temperature, the membranes were incubated with primary antibodies overnight at 4 °C. Protein bands were detected by incubation with horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence reagent (Bio-Rad, Hercules, CA, USA) the following day.
In co-immunoprecipitation assays, cell lysates treated as described in “inflammasome stimulation” were incubated with antibodies overnight at 4 °C, and immunoprecipitated with protein A + G agarose beads at 4 °C for 6 h. Immunoprecipitation samples were further analyzed by immunoblotting for the detection of co-precipitated proteins. Total cell lysates were analyzed by Western blotting as an input control.
NLRP3 oligomerization assay
BMDMs prepared following treatments were lysed using lysis buffer. After centrifugation, the precipitate was resuspended in 1 × sample buffer and electrophoresed on a vertical 1.5% agarose gel in 1 × Tris-borate EDTA (TBE) and 0.1% SDS for 1 h. The proteins were then transferred to polyvinylidene fluoride membranes for immunoblotting as previously described. The lysis buffer contained 0.5% Triton X-100, 50 mM Tris-HCl, 150 mM NaCl, 10% glycerol, and protease inhibitor cocktail. The sample buffer consisted of 0.5 × TBE, 10% glycerol, and 2% SDS containing 0.0025% bromophenol blue.
ASC oligomerization assay
BMDMs prepared in the above treatments were lysed using 0.5% Triton X-100 with protease inhibitor cocktail. After centrifugation, the precipitate was resuspended in PBS containing 2 mM suberic acid bis (3-sulfo-N-hydroxysuccinimide ester) sodium salt (BS3, #S855494; Macklin, Shanghai, China). After incubation at room temperature for 30 min and centrifugation, the pellets were resuspended in SDS sample loading buffer and analyzed by immunoblotting, as previously described [18].
Lactate dehydrogenase (LDH) assay
The release of LDH from LPS + ATP-challenged BMDMs was measured using the LDH Cytotoxicity Assay Kit (#C0016, Beyotime) following the manufacturer’s instructions.
NLRP3 ATPase activity assay
Human NLRP3 immunoprecipitated from plasmid-transfected HEK-293T cells were incubated with different concentrations of Tab for 40 min, then ultra-pure ATP was added to the reaction buffer and incubated at 37 °C for 40 min. The amount of ATP converted into ADP was determined by luminescent ATP detection with ADP-Glo Kinase Assay Kit (#V6930; Promega, WI, USA) according to the manufacturer’s protocol.
Drug affinity responsive target stability (DARTS) assay
BMDMs were primed with LPS for 3 h, and HEK-293T cells were harvested 24 h after transfection. After lysed, the total cell lysates were centrifuged. The protein concentration was then measured using Bradford assay. Protein lysates were incubated with Tab at different doses overnight at 4 °C. The protease pronase (25 ng of enzyme per μg of protein, Sigma) was added to the lysates (20 μg of protein lysate per reaction) and incubated for 5 min at room temperature. Pronase was stopped by the addition of SDS loading buffer and heated at 100 °C for 10 min. The samples were analyzed by immunoblotting.
Molecular docking
Molecular docking of NLRP3 NACHT domain–Tab complex was performed using AutoDock 4.2.6. The crystal structure of human NLRP3 (PDB code: 6NPY) was obtained from Protein Data Bank (PDB). The AutoDock Tools version 1.5.6 package was used to create the input files and to analyze the docking results. A 60 × 60 × 60-point grid box with 0.375 Å spacing between the grid points was implemented. AutoGrid was used to calculate affinity maps of the protein. One hundred Lamarckian genetic algorithm runs with default parameter settings. Then the interactions between the protein and ligand in the complex were analyzed.
Statistical analysis
Data are presented as mean ± standard error of the mean. The statistical significance of differences between groups was determined using Student’s t test or one-way ANOVA multiple comparisons in GraphPad Prism 8 (GraphPad, San Diego, CA, USA). One-way ANOVA followed by Dunnett’s post hoc test was used to compare more than two groups of data. Differences were considered statistically significant at P < 0.05.
Results
Tab inhibits NLRP3 inflammasome activation and NLRP3-mediated pyroptosis
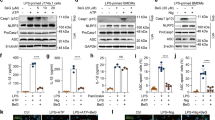
The level of LPS + ATP-induced IL-1β production in BMDMs has been used as an indicator of NLRP3 inflammasome activation [19]. To identify the anti-inflammatory natural compounds that suppress NLRP3 inflammasome activation, 151 natural products were screened in our in-house natural compound bank (Supplementary Table S1) for their inhibitory activity against IL-1β production in LPS + ATP-challenged BMDMs at 10 μM. The results found that some natural compounds could inhibit NLRP3 inflammasome activation in different degree (Fig. 1a). Interestingly, among these 151 natural compounds, Tab showed an exceptional inhibitory rate (>95%) of IL-1β production (Fig. 1b and Supplementary Fig. S1a, IC50 = 0.71 μM). It was also found that Tab had no obvious cytotoxicity in BMDMs (Fig. 1c). Whether Tab could inhibit IL-1β and caspase-1 (p20) levels in BMDMs was then tested, which confirms the activation of NLRP3 inflammasome. As shown in Fig. 1d, e, Tab suppressed p20 cleavage and IL-1β secretion in a dose-dependent manner (1, 5, and 10 μM). Similar inhibition of IL-1β production by Tab was observed in THP-1 cells (Supplementary Fig. S1b). Previous studies have reported that Tab has anti-inflammatory effects on NF-κB signaling [16], the priming step of NLRP3 and pro-IL-1β expression. The inhibitory ability of Tab on LPS priming for NLRP3 inflammasome activation was examined. When treated with Tab before LPS challenge, the cells also showed inhibited IL-1β in the culture supernatants of BMDMs (Fig. 1f). However, the TNF-α (Fig. 1g) and IL-6 (Supplementary Fig. S1c) levels could not be altered by Tab treatment at 1, 5, and 10 μM, as well as NLRP3 and pro-IL-1β expression (Fig. 1h). The level of p20 was also suppressed by Tab (Fig. 1h), suggesting that Tab inhibited NLRP3 activation via an NF-κB-independent manner. These results were also validated in mouse serum. The results showed that Tab treatment significantly reduced serum IL-1β production, but not TNF-α production, in LPS-challenged mice (Supplementary Fig. S1d, e). Furthermore, Tab inhibited the release of LDH (Fig. 1i) and cell death (Supplementary Fig. S2a, b). To verify the patterns of the cell death, we examined the level of the cleavage of GSDMD (GSDMD-NT). The data showed that Tab significantly reduced the production of GSDMD-NT, indicating that Tab suppresses LPS + ATP-induced pyroptosis in BMDMs (Supplementary Fig. S2c). These results indicate that Tab inhibited NLRP3 inflammasome activation and NLRP3-mediated pyroptosis in an NF-κB-independent manner.
a BMDMs were primed with LPS for 3 h and stimulated with ATP for 0.5 h after treatment with 10 μM natural products library (151 compounds) for 0.5 h, ELISA of IL-1β in culture supernatant (SN). b Structure of Tab. c Cell viability of 151 compounds. d, e LPS-primed BMDMs were treated with or without Tab in different doses for 0.5 h, and then stimulated with ATP for 0.5 h. IL-1β production was assessed using ELISA in SN (d). n = 3. Western blotting analysis of IL-1β and caspase-1 (p20) levels in culture SN and pro-IL-1β, caspase-1, NLRP3, ASC, and GAPDH in lysates (Input) of BMDMs (e). n = 3. f–h BMDMs were treated with Tab in different doses for 0.5 h, challenged with LPS for 3 h and then stimulated with ATP for 0.5 h. IL-1β production was assessed using ELISA in SN (f). n = 3. TNF-α (g) secretion was assessed using ELISA in SN. n = 3. Western blotting analysis of IL-1β and p20 levels in culture SN and pro-IL-1β, caspase-1, NLRP3, ASC, and GAPDH in Input of BMDMs (h). n = 3. i Assay of LDH release in the culture supernatants of LPS-primed BMDMs treated with different doses of Tab for 0.5 h and then stimulated with ATP for 1 h. Data are presented as the mean ± SEM. *P < 0.05, compared to the LPS + ATP group; ns not significant.
Tab inhibits the formation of NLRP3 inflammasome
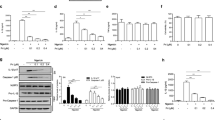
Next, we explored whether Tab could suppress NLRP3 inflammasome activation induced by other activators, such as Alum and nigericin. It was found that Tab treatment also inhibited p20 and IL-1β release from the culture supernatants (Fig. 2a, b). Caspase-1 is an effector protein in NLRP3 inflammasome that cleaves pro-IL-1β and pro-IL-18 to generate functional IL-1β and IL-18 [20]. The cleavage of caspase-1 is a unique indicator of NLRP3 inflammasome activation. The levels of caspase-1 activation were then tested in BMDMs by immunofluorescence. As shown in Fig. 2c, d, the fluorescence spots representing caspase-1 activation were suppressed by Tab treatment, indicating that Tab inhibits NLRP3 inflammasome activation above the cleavage of caspase-1. Tab treatment also decreased the level of caspase-1 activity in the cells culture supernatants (Supplementary Fig. S3). Thus, it was hypothesized that Tab inhibits NLRP3 inflammasome assembly. The formation of ASC specks participates in the activation of NLRP3 inflammasome, which is a critical step in subsequent caspase-1 cleavage [21]. Therefore, the effect of Tab on ASC specks in BMDMs was examined. It was discovered that the fluorescence spots representing ASC specks were suppressed by Tab treatment (Fig. 2e, f). It was also observed that Tab inhibited ASC oligomerization (Fig. 2g). These results indicate that Tab inhibits NLRP3 inflammasome activation, possibly by suppressing inflammasome assembly.
a, b LPS-primed BMDMs were treated with or without Tab (10 μM) for 0.5 h and then stimulated with ATP for 0.5 h, nigericin for 0.5 h, or Alum for 4 h. Western blotting analysis of mature IL-1β and p20 levels in SN (a) or ELISA of IL-1β production in SN (b). n = 3. c–f Immunofluorescence assay of caspase-1 activation (c) and ASC speck (e) in LPS-primed BMDMs treated with different doses of Tab for 0.5 h and then stimulated with ATP for 0.5 h. Quantitative data of 2c (d) and 2e (f). n = 3. g Western blotting analysis of ASC speck (Pellets) of LPS-primed BMDMs treated with or without Tab for 0.5 h and stimulated with ATP for 0.5 h. n = 3. Data are presented as the mean ± SEM. *P < 0.05, compared to the LPS + ATP group; ns not significant.
Tab targets NLRP3 NACHT domain
In order to determine the mechanism by which Tab inhibits the assembly of NLRP3 inflammasome, how Tab inhibits the interactions between proteins composed of inflammasomes was tested. First, the interaction between NLRP3 and ASC expression was validated. As shown in Fig. 3a, LPS + ATP induced the formation of the NLRP3–ASC complex, which was reduced by Tab treatment. A previous study showed that NEK7 plays a critical role in the activation of NLRP3 inflammasome by binding to NLRP3 [6, 22]. The effect of Tab on the interaction between NLRP3 and NEK7 was then examined. The results showed that Tab failed to alter this interaction (Fig. 3b). Based on these results, it was concluded that Tab might target NLRP3 or ASC to inhibit NLRP3 inflammasome activation. Furthermore, the DARTS experiment was employed to confirm the target protein of Tab [23]. It was observed that NLRP3, rather than ASC, was protected from pronase-driven hydrolysis in BMDMs by Tab (Fig. 3c). The results were validated in HEK-293T cells transfected with plasmids encoding human NLRP3 or ASC (Fig. 3d, e).
a Co-immunoprecipitation (Co-IP) with ASC antibody and Western blotting analysis to evaluate the NLRP3-ASC interaction in LPS-primed BMDMs treated with or without Tab for 0.5 h and stimulated with ATP for 0.5 h. n = 3. b Co-IP with NLRP3 antibody and Western blotting analysis to evaluate the NLRP3-NEK7 interaction in LPS-primed BMDMs treated with or without Tab for 0.5 h and stimulated with ATP for 0.5 h. n = 3. c DARTS assay of NLRP3 and ASC in LPS-primed BMDMs by Western blotting. n = 3. DARTS assay of FLAG-NLRP3 (d) and FLAG-ASC (e) in HEK-293T cells transfected plasmids. n = 3. DARTS assay of FLAG-NLRP3-LRR (f), FLAG-NLRP3-PYD (g), and FLAG-NLRP3-NACHT (h) in the cell lysates of HEK-293T cells transfected with high expression plasmids. n = 3. i Molecular docking analysis of Tab bound to NLRP3. Tab (sticks model) in the binding site of NLRP3 (cartoon, PDB ID: 6NPY). j 2D pose view of the interaction between Tab and NLRP3 in the molecular docking model. k Western blotting analysis of NLRP3 by SDD–AGE or SDS–PAGE assay in LPS-primed BMDMs treated with Tab for 0.5 h and stimulated with ATP for 0.5 h. n = 3. l Co-IP with FLAG antibody and Western blotting analysis to evaluate the NLRP3-NLRP3 interaction in HEK-293T cells transfected with high expression plasmids. n = 3. m ATPase activity assay for NLRP3 proteins in the presence of different concentrations of Tab. n = 3. Data are presented as the mean ± SEM. *P < 0.05, compared to the 0 μM Tab group.
NLRP3 consists of three domains with different functions: LRR for recognition and self-inhibition, NACHT for ATPase activity and oligomerization to form complexes, and PYD for interactions with adaptor protein ASC. Furthermore, plasmids encoding different domains were constructed to identify the Tab-targeting domains of NLRP3. The results showed that Tab suppressed the enzymatic hydrolysis stability of the NACHT domain instead of the LRR or PYD domain (Fig. 3f–h). Based on these results, a molecular model was simulated using docking software (Fig. 3i). The results showed that Phe297, Arg335, Lys322, and Ile295 formed a hydrophobic pocket that fit with Tab (Fig. 3j). Phe297 showed strong π–π stacking interactions (Fig. 3j). Therefore, these residues may be crucial amino acid residues in the Tab-NLRP3 interaction.
Based on the function of NACHT in NLRP3 oligomerization [24] self-oligomerization during the activation of NLRP3 inflammasome was tested through semi-denaturing detergent agarose gel electrophoresis. As expected, Tab inhibited NLRP3 oligomerization in BMDMs in a dose-dependent manner (Fig. 3k). The inhibition in HEK-293T cells was also verified, and it was found that Tab reduced the complexity of the different-tags NLRP3 (Fig. 3l). The effect of Tab on ATPase activity of endogenous NLRP3 was then examined. The data showed that Tab suppressed NLRP3 ATPase activity (Fig. 3m), indicating that Tab blocked NLRP3 oligomerization through an ATPase-dependent way. These results suggest that Tab inhibits the activation of NLRP3 inflammasome by targeting the NACHT domain, then inhibiting ATPase activity.
Tab prevents peritonitis via inhibiting NLRP3 inflammasome activation
Previous studies have shown that NLRP3 inflammasome can be activated by alum crystals to drive the development of peritonitis in vivo [25]. To assess the protective efficacy of Tab in vivo, a mouse model of alum-induced acute peritonitis was created (Fig. 4a). As expected, the alum-induced total cell number increased in the peritoneal fluid of wild-type (WT) mice, whereas Tab treatment significantly decreased the total cell number (Fig. 4b). Tab treatment also decreased NLRP3-dependent IL-1β release into the peritoneal fluid (Fig. 4c). The migration of immunocytes into the abdominal cavity was then tested using flow cytometry. The results showed that monocyte (CD45.2+CD11b+Ly6C+) and neutrophil (CD45.2+CD11b+Ly6G+) counts were significantly suppressed by Tab treatment (Fig. 4d, e). These results indicate that Tab prevented peritonitis in a mouse model by inhibiting NLRP3 inflammasome activation.
a Schematic showing the process of peritonitis in mouse model. b Total cells in peritoneal fluids. n = 6 per group. c IL-1β level in peritoneal fluids. n = 6 per group. FACS analysis of monocyte (d) and neutrophil (e) ratios in the peritoneal cavity. n = 6 per group. Data are presented as the mean ± SEM. *P < 0.05, compared to the Alum + Vehicle group; ns not significant.
Tab alleviated LPS-induced acute lung injury in mouse model
We examined the anti-inflammasome effects of Tab in a mouse model of LPS-induced ALI. As shown in the pathological section of the lung, LPS challenge induced hypercellularity, inflammatory cell proliferation, alveolar wall thickening, and normal lung tissue structure destruction (Fig. 5a and Supplementary Fig. S4a). As expected, Tab treatment at 10 mg/kg reversed all events in the lungs (Fig. 5a and Supplementary Fig. S4a). The wet/dry ratio of the lung tissue (Fig. 5b), protein concentration (Fig. 5c), and total cells in BALF (Fig. 5d) showed that Tab administration significantly mitigated LPS-induced pulmonary edema and damage to the air-blood barrier. In addition, the migration of neutrophils (Ly6G+) and macrophages (F4/80+) in the lung tissue was tested using immunohistochemistry. As shown in Supplementary Fig. S4b, Tab treatment significantly decreased LPS-induced inflammation.
a Representative histological images of lung tissues showing H&E-stained sections. Original magnification ×200. Panels showing lung wet/dry ratio (b), amount of protein in BALF (c), numbers of total cells in BALF (d). e Western blot analysis of IL-1β, pro-IL-1β, p20, pro-caspase-1, and GAPDH of tissue homogenates from the lung of WT mice. f IL-1β level in BALF. g Western blot analysis of IL-1β, pro-IL-1β, p20, caspase-1, and GAPDH of tissue homogenates from the lung of Nlrp3KO mice. Data are presented as the mean ± SEM. n = 5 per group. *P < 0.05; ns not significant.
The markers of inflammasome activation in lung tissue were also tested to confirm whether Tab inhibited the activation of NLRP3 inflammasome. The data expressed in Fig. 5e show that the levels of p20 and IL-1β decreased in the group treated with Tab in WT mice. Furthermore, Tab treatment alleviated the IL-1β level in BALF (Fig. 5f) and serum (Supplementary Fig. S4c). As expected, NLRP3 deletion reversed the LPS-induced ALI phenotypes and inflammasome activation in mice (Fig. 5a–g and Supplementary Fig. S4a–g). However, pharmacological effects were not observed when LPS-challenged Nlrp3KO mice were treated with Tab (Fig. 5a–g and Supplementary Fig. S4a–g). In summary, the in vivo data showed that Tab prevented LPS-induced ALI in an NLRP3-dependent manner.
Tab promotes survival in septic mice
Finally, we explored the anti-inflammatory effect of Tab on septic mice by evaluating their survival after infection with live E. coli bacteria. As shown in Fig. 6a, all mice in the E. coli group died within 48 h of septic shock. In mice receiving Tab at 10 mg/kg prior to E. coli treatment, the survival rate was significantly increased to 60%. As expected, NLRP3 knockout significantly increased the survival rate of mice following E. coli infection (Fig. 6b). No additive pharmacological effects were observed when bacterial-challenged Nlrp3KO mice were treated with Tab (Fig. 6b). Thus, Tab treatment prolongs survival in the NLRP3-driven septic model.
WT (a) or Nlrp3KO (b) mice were treated with Tab (10 mg/kg) or vehicle prior to infection with viable E. coli (1 × 109 CFU/mouse). Mouse survival was monitored every 6 h for 48 h. Kaplan–Meier survival curves were used to analyze the data (n = 10 mice per group). The significance was evaluated by the log rank (Mantel–Cox) test. *P < 0.05, compared to the DH5α-challenged group.
Discussion
In this study, Tab was found to be a novel NLRP3 inhibitor. We discovered that Tab directly binds to NLRP3 NACHT domain thereby decreasing the ATPase activity, reducing the self-oligomerization of NLRP3, and inhibiting the assembly of NLRP3 inflammasome. We also found that Tab has a great protective capacity against NLRP3-driven peritonitis, ALI, and sepsis in the mouse model. This study provides Tab as a new lead compound for the design and discovery of NLRP3 inhibitors.
It has been reported that NLRP3 inflammasome activation plays a critical role in several inflammatory diseases [26,27,28]. Aberrant activation of NLRP3 inflammasome leads to atherosclerosis [29], type 2 diabetes [26], ulcerative colitis [28], and gouty arthritis [27]. In this study, we created ALI, peritonitis, and sepsis models to confirm the protective effect of Tab treatment in NLRP3-driven disease models. During ALI, extracellular histones secreted by neutrophils in the lung have been characterized as activators of NLRP3 inflammasome [30]. As shown in Fig. 5f, IL-1β levels in BALF decreased by ~50% in Nlrp3KO mice, indicating that NLRP3 participates in the entire development of ALI. As shown in Fig. 6a, b, the same results were observed in the sepsis model of Nlrp3KO mice. In the above disease model in mice, Tab showed great protective effect in WT group rather than in the Nlrp3KO group. Based on the excellent inhibition efficiency on LPS + ATP-challenged BMDMs and their great protective capacity against NLRP3-driven disease, Tab shows good development and clinical application prospects. As factors that cannot be ignored, the therapeutic window and pharmacokinetics in the human body of Tab should be determined before its development.
Pharmacological inhibition of NLRP3 inflammasome appears to be an effective therapeutic approach. MCC950, a great small-molecule synthesized, has been proven to block NLRP3 activation at nanomolar concentrations by inhibiting the ATPase activity of NACHT [10, 31]. Several natural products separated from Chinese herbs have been proven to be substantial and specific inhibitor of NLRP3 inflammasome activation. Wang et al. reported that cardamonin suppresses NLRP3 inflammasome activation by inhibiting ASC speck [32]. Our laboratory has found that costunolide covalently targets NACHT domain of NLRP3 to inhibit inflammasome activation [18]. Our screening data showed that parthenolide and oridonin exhibited great inhibition on NLRP3 activation, which were consistent with previous publications [33, 34]. We selected Tab for further investigations, since Tab exhibited an exceptional inhibitory rate (>95%, the highest among the 151 natural products) in the screening. In addition, the chemical structure of Tab is completely different from that of currently known NLRP3 inhibitors. Therefore, Tab may promote a new leading skeleton for the drug design of new NLRP3 inhibitors. A previous study showed that the NACHT domain of NLRP3 has ATPase activity that is vital for NLRP3 self-association and its function [35]. According to the previous studies and our results, NLRP3 NACHT domain may be the most significant and potential pharmacological target for NLRP3. Therefore, pharmacological inhibition of its NACHT domain and ATPase activity is common and important in NLRP3 inhibitors. The development of NLRP3 inhibitors should focus on the drug design based on NACHT and ATPase pockets. We believe that, further drug design and chemical modification based on Tab will obtain specific small-molecule inhibitors with a much higher affinity with NLRP3.
In the docking model, we found that the Tab site might be close to the ATP hydrolysis (Walker B) motifs in NACHT domain, which confirmed the inhibition of NLRP3 oligomerization and its ATPase. As shown in Fig. 3j, the interaction between NLRP3 and Tab stabilizes the drug in the pocket. As we discovered first, Phe297 plays an important role not only near the formation of the Walker B motifs but also in the π–π stack interactions with Tab, which means that Phe297 might be a critical residue in inhibiting NLRP3 inflammasome activation. The exact effect of Phe297 on the pharmacological inhibition of NLRP3 inflammasome should be determined. However, we failed to create a crystal structure of Tab–NLRP3 complex, and the complete NLRP3 protein structure was unavailable. The interaction mode of Tab–NACHT complex could only be understood by molecular simulation using a docking software. The precise mechanism of NLRP3 activation is a future research objective and attempts to create a co-crystal structure of the Tab–NLRP3 or Tab–NACHT complex will be continued.
Tab is a well-known indole alkaloid, that is one of the major types of natural products inherent in Chinese herbs [14]. Tab has been reported to exhibit anti-inflammatory activities in different diseases. Our laboratory has reported that Tab can attenuate cardiac remodeling and dysfunction by targeting TAK1 [17]. Zhang et al. found that Tab suppresses LPS-induced activation and nuclear translocation of NF-κB by suppressing TRAF6 ubiquitination [16]. Sun et al. showed that Tab ameliorated osteoblast apoptosis by regulating the Nrf2/ROS/Bax signaling pathway[36]. However, no significant decline in TNF-α and IL-6 levels was observed at doses below 10 μM in the cell experiments, suggesting that the concentrations and treatment times of Tab required to inhibit NLRP3 inflammasome activation were substantially lower than those required for NF-κB signaling suppression. Tab is also considered to have multiple targets for its anti-inflammatory actions in different disease models. The relationship between targeting NLRP3 and inhibiting other inflammatory signals of Tab is worthy of further investigation.
Taken together, a natural product, Tab, was characterized as a potential and effective inhibitor of the NLRP3 inflammasome by targeting the NLRP3 NACHT domain. Tab effectively inhibited NLRP3 inflammasome activation in macrophages and significantly prevented NLRP3-driven diseases in mouse models. Tab is also a potential lead compound for future drug design and the development of novel NLRP3 inhibitors.
References
Davis BK, Wen H, Ting JP. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol. 2011;29:707–35.
Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153–8.
Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–65.
Kelley N, Jeltema D, Duan Y, He Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019;20:3328.
Jiang H, Gong T, Zhou R. The strategies of targeting the NLRP3 inflammasome to treat inflammatory diseases. Adv Immunol. 2020;145:55–93.
Shi H, Wang Y, Li X, Zhan X, Tang M, Fina M, et al. NLRP3 activation and mitosis are mutually exclusive events coordinated by NEK7, a new inflammasome component. Nat Immunol. 2016;17:250–8.
Feng Y, Li M, Yangzhong X, Zhang X, Zu A, Hou Y, et al. Pyroptosis in inflammation-related respiratory disease. J Physiol Biochem. 2022;78:721–37.
Pourcet B, Duez H. Circadian control of inflammasome pathways: implications for circadian medicine. Front Immunol. 2020;11:1630.
Pfalzgraff A, Weindl G. Intracellular lipopolysaccharide sensing as a potential therapeutic target for sepsis. Trends Pharmacol Sci. 2019;40:187–97.
Coll RC, Robertson AA, Chae JJ, Higgins SC, Muñoz-Planillo R, Inserra MC, et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015;21:248–55.
Jiang H, He H, Chen Y, Huang W, Cheng J, Ye J, et al. Identification of a selective and direct NLRP3 inhibitor to treat inflammatory disorders. J Exp Med. 2017;214:3219–38.
Zou J, Wang SP, Wang YT, Wan JB. Regulation of the NLRP3 inflammasome with natural products against chemical-induced liver injury. Pharmacol Res. 2021;164:105388.
Atanasov AG, Zotchev SB, Dirsch VM, Supuran CT. Natural products in drug discovery: advances and opportunities. Nat Rev Drug Discov. 2021;20:200–16.
Qu Y, Easson ML, Froese J, Simionescu R, Hudlicky T, De Luca V. Completion of the seven-step pathway from tabersonine to the anticancer drug precursor vindoline and its assembly in yeast. Proc Natl Acad Sci USA. 2015;112:6224–9.
Fernández-Pérez F, Almagro L, Pedreño MA, Gómez Ros LV. Synergistic and cytotoxic action of indole alkaloids produced from elicited cell cultures of Catharanthus roseus. Pharm Biol. 2013;51:304–10.
Zhang D, Li X, Hu Y, Jiang H, Wu Y, Ding Y, et al. Tabersonine attenuates lipopolysaccharide-induced acute lung injury via suppressing TRAF6 ubiquitination. Biochem Pharmacol. 2018;154:183–92.
Dai C, Luo W, Chen Y, Shen S, Wang Z, Chen R, et al. Tabersonine attenuates Angiotensin II-induced cardiac remodeling and dysfunction through targeting TAK1 and inhibiting TAK1-mediated cardiac inflammation. Phytomedicine. 2022;103:154238.
Xu H, Chen J, Chen P, Li W, Shao J, Hong S, et al. Costunolide covalently targets NACHT domain of NLRP3 to inhibit inflammasome activation and alleviate NLRP3-driven inflammatory diseases. Acta Pharm Sin B. 2022. https://doi.org/10.1016/j.apsb.2022.09.014.
Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–25.
Franchi L, Eigenbrod T, Muñoz-Planillo R, Nuñez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009;10:241–7.
Duncan JA, Bergstralh DT, Wang Y, Willingham SB, Ye Z, Zimmermann AG, et al. Cryopyrin/NALP3 binds ATP/dATP, is an ATPase, and requires ATP binding to mediate inflammatory signaling. Proc Natl Acad Sci USA. 2007;104:8041–6.
Sharif H, Wang L, Wang WL, Magupalli VG, Andreeva L, Qiao Q, et al. Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome. Nature. 2019;570:338–43.
Chang J, Kim Y, Kwon HJ. Advances in identification and validation of protein targets of natural products without chemical modification. Nat Prod Rep. 2016;33:719–30.
Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–32.
Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–56.
Masters SL, Dunne A, Subramanian SL, Hull RL, Tannahill GM, Sharp FA, et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat Immunol. 2010;11:897–904.
Szekanecz Z, Szamosi S, Kovács GE, Kocsis E, Benkő S. The NLRP3 inflammasome-interleukin 1 pathway as a therapeutic target in gout. Arch Biochem Biophys. 2019;670:82–93.
Zhen Y, Zhang H. NLRP3 inflammasome and inflammatory bowel disease. Front Immunol. 2019;10:276.
Grebe A, Hoss F, Latz E. NLRP3 inflammasome and the IL-1 pathway in atherosclerosis. Circ Res. 2018;122:1722–40.
Grailer JJ, Canning BA, Kalbitz M, Haggadone MD, Dhond RM, Andjelkovic AV, et al. Critical role for the NLRP3 inflammasome during acute lung injury. J Immunol. 2014;192:5974–83.
Coll RC, Hill JR, Day CJ, Zamoshnikova A, Boucher D, Massey NL, et al. MCC950 directly targets the NLRP3 ATP-hydrolysis motif for inflammasome inhibition. Nat Chem Biol. 2019;15:556–9.
Wang Z, Xu G, Gao Y, Zhan X, Qin N, Fu S, et al. Cardamonin from a medicinal herb protects against LPS-induced septic shock by suppressing NLRP3 inflammasome. Acta Pharm Sin B. 2019;9:734–44.
Juliana C, Fernandes-Alnemri T, Wu J, Datta P, Solorzano L, Yu JW, et al. Anti-inflammatory compounds parthenolide and Bay 11-7082 are direct inhibitors of the inflammasome. J Biol Chem. 2010;285:9792–802.
He H, Jiang H, Chen Y, Ye J, Wang A, Wang C, et al. Oridonin is a covalent NLRP3 inhibitor with strong anti-inflammasome activity. Nat Commun. 2018;9:2550.
Swanson KV, Deng M, Ting JP. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19:477–89.
Sun X, Gan L, Li N, Sun S, Li N. Tabersonine ameliorates osteoblast apoptosis in rats with dexamethasone-induced osteoporosis by regulating the Nrf2/ROS/Bax signalling pathway. AMB Express. 2020;10:165.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (21961142009 to GL and 82000793 to WL), Thailand Research Fund Grant (DBG6280006 to NC), Natural Science Foundation of Zhejiang Province (LY22H070004 to WL), Wenzhou Scientific Project in China(Y20210213 to WL), and Zhejiang Provincial Key Scientific Project (2021C03041 to GL).
Author information
Authors and Affiliations
Contributions
GL, WL, and DW contributed to the literature search and study design. GL, WL, and HWX participated in the drafting of the article. HWX, WFL, SSH, JJS, and JHC carried out the experiments. GL and NC revised the manuscript. HWX and WFL contributed to data collection and analysis.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, Hw., Li, Wf., Hong, Ss. et al. Tabersonine, a natural NLRP3 inhibitor, suppresses inflammasome activation in macrophages and attenuate NLRP3-driven diseases in mice. Acta Pharmacol Sin 44, 1252–1261 (2023). https://doi.org/10.1038/s41401-022-01040-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-022-01040-z
- Springer Nature Singapore Pte Ltd.
Keywords
This article is cited by
-
Britannin as a novel NLRP3 inhibitor, suppresses inflammasome activation in macrophages and alleviates NLRP3-related diseases in mice
Acta Pharmacologica Sinica (2024)