Abstract
Bipolar disorder (BD) presents a significant challenge due to its chronic and relapsing nature, with its underlying pathogenesis remaining elusive. This study employs Mendelian randomization (MR), a widely recognized genetic approach, to unveil intricate causal associations between proteins and BD, leveraging protein quantitative trait loci (pQTL) as key exposures. We integrate pQTL data from brain, cerebrospinal fluid (CSF), and plasma with genome-wide association study (GWAS) findings of BD within a comprehensive systems analysis framework. Our analyses, including two-sample MR, Steiger filtering, and Bayesian colocalization, reveal noteworthy associations. Elevated levels of AGRP, FRZB, and IL36A in CSF exhibit significant associations with increased BD_ALL risk, while heightened levels of CTSF and LRP8 in CSF, and FLRT3 in plasma, correlate with decreased BD_ALL risk. Specifically for Bipolar I disorder (BD_I), increased CSF AGRP levels are significantly linked to heightened BD_I risk, whereas elevated CSF levels of CTSF and LRP8, and plasma FLRT3, are associated with reduced BD_I risk. Notably, genes linked to BD-related proteins demonstrate substantial enrichment in functional pathways such as “antigen processing and presentation,” “metabolic regulation,” and “regulation of myeloid cell differentiation.” In conclusion, our findings provide beneficial evidence to support the potential causal relationship between IL36A, AGRP, FRZB, LRP8 in cerebrospinal fluid, and FLRT3 in plasma, and BD and BD_I, providing insights for future mechanistic studies and therapeutic development.
Similar content being viewed by others
Introduction
Bipolar disorder (BD) is a chronic and recurrent mental illness characterized by alternating episodes of mania and depression [1]. Globally, BD affects approximately 2.4% of the population [2], and within 20 years of diagnosis, over 6% of patients succumb to suicide, making it one of the most challenging mental health disorders currently [3]. According to the World Mental Health Survey Initiative report, the estimated lifetime and 12-month prevalence rates for BD are 2.4% and 1.5%, respectively [4]. Widely recognized as one of the most heritable psychiatric disorders, BD is often divided into Bipolar I disorder (BD_I) with at least one manic episode and Bipolar II disorder (BD_II) with no manic episode, one or more hypomanic episodes, and one or more major depressive episodes. Different types have obvious differences in treatment and prognosis [5, 6].
The rapid development of Genome-Wide Association Studies (GWAS) significantly advances the discovery of genetic susceptibility loci for BD. Utilizing modern molecular biology tools such as genetic and epigenetic research, scientists have made significant progress at the genetic level [7]. Studies by Eli A Stahl and others identified 30 loci associated with BD occurrence [8], and research by Budde and colleagues detected significant genetic associations in 18 regions across the entire genome, replicated in multiple independent studies [9]. Unraveling the functional basis of these risk loci is a crucial step in formulating testable hypotheses about the biological processes involved in BD development. However, GWAS designs alone cannot reliably pinpoint potential pathogenic genes to explain the heritability of BD.
Mendelian randomization (MR) is a method that utilizes genetic variation as instrumental variable (IV) to infer whether an exposure has a causal impact on outcomes. Its essence lies in leveraging the inherent characteristics of genetic variation, specifically identifying genome-wide significant single nucleotide polymorphisms (SNPs) closely associated with the exposure, to investigate potential causal relationships between exposure and disease [10, 11]. Baum AE et al., through GWAS studies on BD, suggested that BD may be a polygenic disease [12]. The latest GWAS database constructed by PGC for BD highlighted CACNA1C as a risk gene for both SCZ and BD, although the specific pathophysiological mechanisms remain unclear [13]. Zandi et al. found that over 50% of significant genomic loci for BD contain expression quantitative trait loci (eQTL), potentially indicating specific genes and pathways contributing to the pathophysiology of BD [14]. Chen et al., by combining human brain eQTL with BD GWAS, identified 16 functional SNPs located at 9 risk loci [15]. Liu et al., through whole-genome association of brain proteomics (pQTL), suggested that genetic risk variants may confer risk for psychiatric disorders by regulating mRNA expression and protein abundance of specific genes [16]. Based on the above research background, taking into account that many genetic variants only change the transcription level but not the protein level [17]. In this study, we choose the SNPs associated with the abundance of a protein (protein quantitative trait loci, pQTLs) as exposure and utilize the BD as the outcome. By employing a two-sample MR method, Steiger filter analysis, Bayesian colocalization analysis, and Protein Interaction analysis, we aimed to deeply investigate the potential causal relationship between proteins and BD. This study is expected to elucidate the pathogenic mechanisms of BD and provide important insights to guide the development of treatments [18].
Subjects and methods
Ethics
This study utilized publicly available de-identified data from participant studies and therefore no separate ethics approval was required in this study. All participants included in this study belong to European ancestry, and detailed information regarding the datasets is presented in Supplementary Table S1.
pQTL dataset
The pQTLs data source includes 380 brain samples (1079 proteins), 529 plasma samples (931 proteins), and 835 cerebrospinal fluid samples (713 proteins), collected by Dr. Yang C and colleagues at Washington University School of Medicine [19]. The study included 1537 participants from Washington University School of Medicine in St. Louis, including participants with Alzheimer’s disease (AD) and healthy controls of European ancestry. Demographic characteristics of participants are detailed in Supplementary Table S2. Dr. Yang C’s team utilized an aptamer-based platform to measure protein abundance in cerebrospinal fluid, plasma and brain (parietal cortex) samples. Then, to identify the association between genotype and protein levels within each tissue, genome-wide association analyses of 14.06 million imputed autosomal common variants (minor allele frequency ≥0.02) were performed against protein levels in each tissue. It is worth mentioning that although the study included cognitively normal elderly people and AD patients, after rigorous statistical analysis and research, the results showed that associations of the genetic variants with protein levels were not disease-specific, and most of the pQTLs were not age-specific. The detailed methods can be found in the original study.
BD dataset
Summary data for BD genome-wide association studies (GWAS) were obtained from the Psychiatric Genomics Consortium (PGC) (https://www.med.unc.edu/pgc/results-and-downloads). The dataset corresponds to F31 in the International Classification of Diseases, 10th edition (ICD-10), with different subtypes corresponding to different secondary codes. The GWAS dataset involved meta-analysis of 57 BD cohorts collected from Europe, North America, and Australia, comprising a total of 41,917 BD cases and 371,549 European ancestry controls. Cases met the international consensus standards for lifetime BD (DSM-IV, ICD-9, or ICD-10), established through structured diagnostic interviews, clinically managed checklists, or medical record reviews [20].
Instrumental variable (IV) selection
To select pQTLs as instrumental variables (IVs), the following criteria were employed: i) To ensure genetic variations are directly associated with the exposure, exposure-related SNP screening was limited to a p < 5E−08. ii) To minimize weak instrument bias, a large sample size meta-analysis of GWAS was utilized, and only SNPs with an F statistic greater than 10 were considered as IVs. (see Supplementary Table S3 in the Supplementary) [21] iii) To reduce linkage disequilibrium, criteria were set with [LD] R2 < 0.001, and variants with an LD proxy <1 MB were selected. iv) To ensure that there was no direct correlation between IV and BD, each SNP was searched in the PhenoScanner V2 database (see Supplementary Table S4 in the Supplementary) [22, 23]. The study framework is depicted in Fig. 1.
Mendelian randomization (MR) analysis
Following the aforementioned selection criteria, the majority of pQTLs had a maximum of 2 eligible IVs (specifically, 346 pQTLs have 1 IV, 28 pQTLs have 2 IVs, and 1 pQTL has 3 IVs). In such cases, the Wald ratio method (for pQTLs with 1 IV) and the Inverse Variance Weighting (IVW) method (for pQTLs with 2 IVs) were chosen for MR analysis. No other MR analyses, including weighted median regression and MR-Egger, were conducted. Additionally, due to the limited number of IVs, heterogeneity testing analyses (Cochran’s Q test, leave-one-out analysis) and horizontal pleiotropy testing (MR-Egger intercept test) were not performed. Results of MR analysis were considered significant when they were below the Bonferroni-corrected p value (p < 0.05/number of proteins analyzed), indicating strong evidence, and p < 0.05 was considered suggestive evidence.
Steiger filtering analysis
In Mendelian Randomization causal relationship analysis, when assessing the causal relationship between exposure A and outcome B, the correlation of instrumental variable (IV) with exposure A should be greater than with outcome B, to avoid reverse causation. To verify the directionality of the association between proteins and BD, Steiger filtering analysis was introduced to determine whether there is a reverse causal relationship for IVs outside those significantly associated with the outcome (p < 5E−08) [24]. If the Steiger filtering analysis result’s p value < 0.05, it indicates that the direction of the effect is from exposure to outcome. The aforementioned steps were performed utilizing the “TwoSampleMR” R package (version 0.5.8, github.com/MRCIEU/TwoSampleMR; R software, version 4.3.2) [25].
Bayesian co-localization analysis
Bayesian co-localization analysis was employed to assess the probability that two phenotypes share the same causal variation, involving posterior probabilities for five hypotheses [26]. Bayesian co-localization analysis was performed to evaluate whether two associated signals (pQTLs and BD GWAS) shared the same causal variation. In this study, we tested the posterior probability of hypothesis 4 (PPH4), and evidence of exposure and outcome variation co-localization was considered when PPH4 > 80% [27, 28]. The aforementioned steps were performed utilizing the “coloc” R package (https://github.com/chr1swallace/coloc).
Functional and network prediction of BD-associated proteins
We employed GeneMANIA (http://www.genemania.org) to predict the functions and networks of genes associated with BD. The specific settings of the GeneMANIA website are as follows: For protein network construction, we selected six types: “Physical Interactions”, “Predicted”, “Genetic Interactions”, “Pathway”, “Co-localization” and “Shared protein domains”.In the custom advanced options, we set the “Maximum Result Gene Parameter” to 20, the “Maximum Result Attribute” to 10, and the query relevance weight selected “Automatically select weighting method”. Detailed information about the datasets included in GeneMANIA is described elsewhere [29, 30]. Enriched functional pathways were considered significant with a false discovery rate <0.05.
External validation of proteins
Only potential proteins derived from the preliminary analysis were repeated for external MR validation, with exactly the same parameters and methods as the preliminary analysis. UK Biobank and FinnGen meta-datasets were selected to validate potential proteins in BD_ALL (Ncases = 8171; Nsum = 756,729). For further subtype analysis, the BD_I dataset published by Jiang et al. was selected (Ncases = 702; Nsum = 108,781). We considered the odds ratio (OR) between the validation cohort and the preliminary results to be consistent as a success, and a p value of less than 0.5 as strong evidence of validation. The above BD_ALL dataset can be downloaded from https://finngen.gitbook.io/documentation/data-download, and the BD_I dataset can be downloaded from the study of Liang et al. [31].
Results
Associations between genetically pQTLs with BD_ALL
After multiple testing corrections, we identified genes determining protein levels in brain (p < 7.14E−04 [0.05/70]), CSF (p < 2.63E−04 [0.05/190]), and plasma (p < 3.82E−04 [0.05/131]) that were significantly associated with the risk of BD_ALL (Table 1 and Supplementary Table S5 in the Supplementary). Notably, increased levels of AGRP (OR = 3.407469, 95% CI: 2.006013–5.788024; p = 5.75E−06), FRZB (OR = 2.803016, 95% CI: 1.773841–4.429314; p = 1.01E−05), IL36A (OR = 1.698281, 95% CI: 1.332097–2.165128; p = 1.92E−05), and MMEL1 (OR = 1.507243, 95% CI: 1.208567–1.879732; p = 2.71E−04) in CSF were significantly associated with increased BD_ALL risk. Conversely, increased levels of CTSF (OR = 0.115262, 95% CI: 0.055057–0.241299; p = 9.95E−09) and LRP8 (OR = 0.451058, 95% CI: 0.314002–0.647935; p = 1.64E−05) in CSF and increased levels of FLRT3 (OR = 0.698616, 95% CI: 0.584332–0.835253; p = 8.31E−05) and LYZ (OR = 0.454988, 95% CI: 0.297324–0.696258; p = 2.86E−04) in plasma were associated with decreased BD_ALL risk.
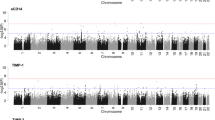
Steiger filtering analysis supported the causal directionality from protein levels to BD_ALL for the identified genes in CSF and plasma. However, for CSF MMEL1 (PPH4 = 0.79) and plasma LYZ (PPH4 = 0.76), the associations were less robust, suggesting potential confounding due to linkage disequilibrium or pleiotropy. Several other gene loci were confirmed to be mediated by shared variants (PPH4 > 0.8), providing strong evidence for their potential causal relationship with BD_ALL (Table 1). Additionally, suggestive associations were found for 20 gene-determined protein levels with BD_ALL risk changes (p < 0.05) (Table 1, Fig. 2A–C), and Steiger filtering analysis supported their suggestive causal direction from protein levels to BD_ALL.
A brain pQTL and BD_ALL. B CSF pQTL and BD_ALL. C plasma pQTL and BD_ALL. D brain pQTL and BD_I. E CSF pQTL and BD_I. F plasma pQTL and BD_I. G brain pQTL and BD_II. H CSF pQTL and BD_II. I plasma pQTL and BD_II. The dots colored in green or red represent the genes that associated with changes in risk for BD_ALL, BD_I, and BD_II. (p < 0.05).
Associations between genetically pQTLs with BD_I
After multiple testing corrections, genes determining protein levels in brain (p < 7.14E−04), CSF (p < 2.63E−04), and plasma (p < 3.82E−04) were found to be significantly associated with the risk of BD_I (Table 1 and Supplementary Table S6 in the Supplementary). Notably, increased levels of AGRP in CSF (OR = 4.901996, 95% CI: 2.536748–9.472589; p = 2.25E−06) were significantly associated with increased BD_I risk. Conversely, increased levels of CTSF (OR = 0.098038, 95% CI: 0.039321–0.24444; p = 6.28E−07) and LRP8 (OR = 0.412842, 95% CI: 0.263025–0.647995; p = 1.20E−04) in CSF, and increased levels of FLRT3 (OR = 0.663554, 95% CI: 0.532524–0.826825; p = 2.58E−04) and LYZ (OR = 0.382629, 95% CI: 0.226341–0.646833; p = 3.35E−04) in plasma were associated with decreased BD_I risk.
Steiger filtering analysis supported the causal directionality from protein levels to BD_I for the identified genes in CSF and plasma. However, for plasma LYZ (PPH4 = 0.78), no co-localization evidence was found, while other gene loci were confirmed to be mediated by shared variants (PPH4 > 0.8), providing strong evidence for their potential causal relationship with BD_I (Table 1). Additionally, suggestive associations were found for 7 gene-determined protein levels in brain, 19 in CSF, and 17 in plasma with BD_I risk changes (p < 0.05) (Fig. 2D–F), and Steiger filtering analysis supported their suggestive causal direction from protein levels to BD_I.
Associations between genetically pQTLs with BD_II
After multiple testing corrections, no genes determining protein levels in brain (p < 7.14E−04), CSF (p < 2.63E−04), and plasma (p < 3.82E−04) were found to be significantly associated with the risk of BD_II (Supplementary Table S7 in the Supplementary). However, suggestive associations were found for 3 gene-determined protein levels in brain, 2 in CSF, and 2 in plasma with BD_II risk changes (p < 0.05) (Fig. 2G–I)., and Steiger filtering analysis supported their suggestive causal direction from protein levels to BD_II.
Functional and network prediction of BD-associated proteins
The identified proteins associated with both BD_ALL and BD_I exhibit a network, particularly through physical interactions (Figs. 3 and 4). Notably, genes associated with proteins related to BD_I show significant enrichment in various functional pathways (Fig. 4, Supplementary Table S8 in the Supplementary), including “antigen processing and presentation”, “metabolic regulation”, and “regulation of myeloid cell differentiation”.
External validation of causal proteins for BD and BD_I
We repeated the two-sample MR analysis for the proteins with potential causal relationships in the preliminary analysis, specifically 6 BD_ALL candidate proteins and 4 BD_I candidate proteins that met the colocalization analysis (PPH4 > 0.80). After verification, our results showed that the directional effects of all potential proteins were consistent between the main cohort and the validation cohort, and 4 proteins were confirmed in both BD_ALL and BD_I, specifically CTSF, AGRP, LRP8 in CSF and FLRT3 in plasma. In addition, the results of 2 proteins in CSF were replicated in BD_ALL at p < 0.05. From a tissue perspective, we found that CTSF and LRP8 in the CSF of BD_ALL patients showed strong replication evidence (Fig. 5).
Discussion
Despite significant advances in genetic sequencing methods and bioinformatics tools, the mechanistic understanding of BD remains largely unknown. This study combined MR, Steiger filtering analysis and Bayesian colocalization analysis with pQTL datasets derived from the brain, CSF, and plasma to identify novel proteins potentially associated with BD onset. We provide supporting evidence demonstrating a causal relationship between genetically determined protein levels of IL36A, CTSF, AGRP, FRZB, LRP8 in CSF, and FLRT3 in plasma with BD. Due to distinct symptoms, signs, and prognosis of the two common subtypes of BD, further subgroup analysis was conducted. We observed a significant causal relationship between genetically determined protein levels of CTSF, AGRP, LRP8 in CSF, and FLRT3 in plasma with the risk of BD_I, while no significant association was observed with BD_II risk. Subsequent analyses revealed enrichment of BD_I associated proteins in biological processes related to “antigen processing and presentation”, “metabolic regulation”, and “regulation of myeloid cell differentiation”.
First, our study utilized several independent but complementary methods to identify new BD-related proteins, including MR analysis for discovering potential causal relationships, Steiger filtering analysis for ensuring the correct direction of association, and Bayesian colocalization analysis for verifying that linkage disequilibrium (LD) and pleiotropy do not distort potential causal relationships. Second, we integrated pQTL datasets from multiple tissues (brain, cerebrospinal fluid, and plasma) and the most recently published and extensive BD GWAS dataset from the PGC Consortium to comprehensively explore key proteins involved in the pathogenesis of BD. Finally, we supplemented the BD_ALL meta-dataset from the UK Biobank and the FinnGen database and the BD_I dataset from the study by Jiang et al. as external validation to ensure the credibility of the research results [31].
The proteins identified in our study have been underexplored in the context of BD, yet some evidence supports our findings. Clinical studies have indicated an association between elevated levels of pro-inflammatory cytokines such as IL-1, IL-6, TNFα in the serum of individuals with psychiatric disorders [32,33,34,35]. These inflammatory factors may also impact regions associated with neurodevelopmental psychiatric disorders, including SCZ and autism [36]. Manic and depressive episodes in BD are often associated with activation of inflammatory, cell-mediated, and negative immune-regulatory cytokines [37]. IL36A, belonging to the IL-1 family, plays a crucial role in the immune system by stimulating innate and adaptive immune responses, and its expression changes may lead to sustained pro-inflammatory reactions [38]. Our study found that an increase in IL36A in CSF is associated with an increased risk of BD_ALL, but no causal relationship was observed between IL36A and BD_I or BD_II in subsequent analyses. Therefore, further research is needed to elucidate the mechanistic role of IL36A in BD and its feasibility as a potential therapeutic target.
AGRP allelic variants have been identified as risk factors for anorexia nervosa in humans [39]. AGRP is a powerful appetite enhancer that inhibits the binding and activity of the anorectic hormone α-melanocyte stimulating hormone by binding to melanocortin 3, 4, and 5 receptors [40]. In addition, AGRP can inhibit the secretion of thyroid hormones and reduce energy expenditure by affecting the hypothalamic-pituitary-thyroid axis, thereby promoting anabolism [41]. AGRP neurons in the hypothalamus participate in the regulation of circadian rhythms, fertility, and pain perception by sending extensive projections to targets inside and outside the hypothalamus (including the paraventricular hypothalamic nucleus and the lateral hypothalamic area) [42, 43]. In addition, AGRP neurons regulate stereotyped responses under abnormal nutritional conditions [44]. Therefore, the AGRP neural circuit may link peripheral hormone signals to metabolically sensitive psychological functions [45]. Zhang et al.’s study showed that reducing iron overload in hypothalamic AgRP neurons can improve obesity and related metabolic dysfunction [46, 47]. Metabolic-related diseases such as obesity have local genetic correlations with BD [48]. Studies have shown that BD patients often have metabolic imbalances and are related to mitochondrial dysfunction [49, 50]. Parlak et al.’s study found that the serum AGRP level of patients with manic BD was lower than that of the control group, while the serum AGRP level of patients with emotionally stable BD was not significantly different from that of the control group [40]. However, a study by Özkorumak K et al. showed that AGRP levels in emotionally stable BD patients were higher than those in healthy controls [51]. Our results suggest that increased abundance of AGRP protein in cerebrospinal fluid increases the risk of BD_ALL, and further analysis found that increased levels of AGRP protein in cerebrospinal fluid increase the risk of BD_I. The current research results seem to be contradictory, but the relationship between AGRP regulation of body metabolism and BD deserves further study.
This study also found that the reduction of LRP8 protein abundance in cerebrospinal fluid led to an increased risk of BD_ALL and BD_I. Li M et al. conducted genetic analysis in a large-scale sample, and the results showed that LRP8 is an important susceptibility gene for psychosis (schizophrenia and BD), which is involved in a common molecular network that regulates the risk of psychosis and can physically interact with RELN and APOE proteins [52]. Researchers have found that Reelin regulates neuron positioning and migration in the brain cortex structure by binding to very low-density lipoprotein receptor (Vldlr) and LRP8 [53]. As a cell surface receptor for Reelin, variations in LRP8 may potentially affect brain development by disrupting the Reelin signaling pathway, leading to the onset of SCZ or BD [54]. LRP8 is a receptor for Reelin (a protein encoded by RELN). Studies by Xiao et al. have shown that compared with controls, the expression of RELN messenger RNA (mRNA) and protein in brain tissue of SCZ patients is reduced, and in an meta-analysis in the European population, rs5177 in the 3′ untranslated region (3′UTR) of LRP8 is significantly associated with schizophrenia [54]. In addition, studies have found that the expression of LRP8 can increase the transcriptional response of Wnt signal transduction and promote the formation of the Wnt signal transduction pathway [55]. The Wnt pathway is currently widely believed to inhibit GSK-3 signal transduction. GSK-3 is essentially a serine/threonine kinase, which may induce psychiatric diseases such as Parkinson’s disease, AD, and BD through enzymatic reactions or phosphorylation pathways [56,57,58]. It is worth mentioning that some studies have pointed out that the protein encoded by FRZB can inhibit the operation of the Wnt signaling pathway as an antagonist of the Wnt signaling pathway [59, 60]. The Wnt signaling mechanism plays a role in neuroprotection and synaptic plasticity [61, 62]. Because of the importance of the Wnt cascade in regulating brain function, its dysregulation or inhibition may be related to the pathophysiology of BD [63]. Therefore, whether LRP8 and FRZB can induce psychiatric diseases such as BD through the Wnt pathway will be an interesting research direction. In summary, it is necessary to further explore how changes in the abundance of LRP8 and FRZB proteins in cerebrospinal fluid affect the pathophysiology of BD.
FLRT3 plays a crucial role in cell adhesion and/or receptor signaling and is expressed in various tissues, including the kidney, brain, pancreas, skeletal muscle, lungs, liver, placenta, and heart [64]. Neuronal migration is a hallmark of nervous system development, attracting neurons from diverse sources to assemble functional neuronal circuits [65]. Netrin-1 is one of the most influential axon guidance cues in the development of axons in the central nervous system of vertebrates [66]. Studies suggest that FLRT3 may enhance axon growth by modulating the signaling of Robo1, possibly through the upregulation of protein kinase A (PKA) activity in the cell membrane level of colorectal carcinoma receptor, thus attracting Netrin-1 and promoting axon growth [67]. Additionally, research indicates that FLRT3 may contribute to axon regeneration and memory formation following axon injury [68]. Furthermore, our research results suggest that a reduction in FLRT3 levels in plasma is associated with an increased risk of both BD_ALL and BD_I. Given the important role of FLRT3 in neuronal development, it is of great significance to analyze the causal relationship between changes in FLRT3 content in the brain or cerebrospinal fluid and BD. Unfortunately, the pQTL data source utilized in this study did not have IVs that met the screening criteria for FLRT3 analysis in brain or cerebrospinal fluid pQTL. Nevertheless, exploring the mechanism of BD by monitoring the abundance of FLRT3 protein in plasma is of great significance for the development of new treatments.
Several limitations of this study should be acknowledged. First, the sample size of protein pQTL sequencing in brain, cerebrospinal fluid, and plasma tissues is currently limited, which may lead to the omission of potential target proteins. Second, the GWAS database samples analyzed in this study are from European populations, which to some extent limits the general applicability of the results to other ethnic groups or races. Mendelian randomization (MR) requires strict filtering conditions to select instrumental variables (IVs) to ensure that they are closely related to exposure, but most pQTLs have only 1 or 2 available IVs after selection, which limits further sensitivity analysis and heterogeneity tests. Therefore, our study avoids weak instrument bias by ensuring that the F statistic of the selected SNPs is greater than 10, and increases the robustness of the results through Steiger filtering, Bayesian colocalization, and external validation. Then, the pQTL dataset utilized in our study comes from AD cases and cognitively normal elderly people, although Dr. Yang C’s team found through rigorous statistical analysis that genetic variants associated with protein levels are not disease-specific and most pQTLs are not age-specific [19]. However, since multiple factors, including genetic factors, shorten the life expectancy of BD patients, the results of a single MR analysis may be affected by factors related to life span, thereby overestimating or underestimating the impact of a specific protein on BD. Therefore, the release of pQTL datasets from healthy people of different ages in the future is expected to further promote MR studies of BD. Last but not least, the BD GWAS database utilized in this study did not mention whether the included BD patients received drug treatment and the specific therapeutical program. In order to avoid the potential drug treatment affecting our research results, we searched the latest drug guide for BD [69] and searched for potential targets of the therapeutic drugs mentioned therein (including aripiprazole, quetiapine, risperidone, asenapine, and cariprazine, commonly utilized drugs for the treatment of mania and mixed episodes; quetiapine, lurasidone, cariprazine, lamotrigine and lithium, commonly utilized drugs for the treatment of bipolar depression; lithium, quetiapine, aripiprazole, lamotrigine, etc., commonly utilized drugs for the bipolar disorder prevention (maintenance)) (https://go.drugbank.com/). No interactions were found between these drugs and the positive proteins in this study (Supplementary Table S9 in the Supplementary). In the future, with the publication of more and more extensive clinical drug target-related articles, this may provide a basis for further research on BD.
In conclusion, this study provides valuable insights into potential protein markers associated with BD, emphasizing the need for further research to explore their roles in the pathogenesis and potential therapeutic interventions for BD.
Conclusions
In conclusion, we identified supporting evidence for a causal relationship between IL36A, AGRP, FRZB, LRP8 in CSF, and FLRT3 in plasma and BD and BD_I, which can provide insights for future mechanistic studies to develop novel therapies for this disease. In addition, our results did not identify proteins that have a potential causal relationship with BD_II, so studies with larger sample sizes, more tissue sources, and more types of proteins are needed.
Data availability
The pQTL dataset is from a study by Yang C and colleagues at Washington University School of Medicine. Summary data from genome-wide association studies of BD are from the Psychiatric Genomics Consortium (PGC) (https://www.med.unc.edu/pgc/results-and-downloads). The BD_ALL dataset used for external validation can be downloaded from https://finngen.gitbook.io/documentation/data-download, and the BD_I dataset can be downloaded from a study by Liang J et al.
References
Vieta E, Berk M, Schulze TG, Carvalho AF, Suppes T, Calabrese JR, et al. Bipolar disorders. Nat Rev Dis Primers. 2018;4:18008.
Bonnín CDM, Reinares M, Martínez-Arán A, Jiménez E, Sánchez-Moreno J, Solé B, et al. Improving Functioning, Quality of Life, and Well-being in Patients With Bipolar Disorder. Int J Neuropsychopharmacol. 2019;22:467–77.
Anderson IM, Haddad PM, Scott J. Bipolar disorder. BMJ. 2012;345:e8508.
Merikangas KR, Jin R, He J-P, Kessler RC, Lee S, Sampson NA, et al. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry. 2011;68:241–51.
Barnett JH, Smoller JW. The genetics of bipolar disorder. Neuroscience. 2009;164:331–43.
Renk K, White R, Lauer B-A, McSwiggan M, Puff J, Lowell A. Bipolar disorder in children. Psychiatry J. 2014;2014:928685.
Carvalho AF, Firth J, Vieta E. Bipolar Disorder. N Engl J Med. 2020;383:58–66.
Stahl EA, Breen G, Forstner AJ, McQuillin A, Ripke S, Trubetskoy V, et al. Genome-wide association study identifies 30 loci associated with bipolar disorder. Nat Genet. 2019;51:793–803.
Budde M, Friedrichs S, Alliey-Rodriguez N, Ament S, Badner JA, Berrettini WH, et al. Efficient region-based test strategy uncovers genetic risk factors for functional outcome in bipolar disorder. Eur Neuropsychopharmacol. 2019;29:156–70.
Bowden J, Holmes MV. Meta-analysis and Mendelian randomization: A review. Res Synth Methods. 2019;10:486–96.
Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7:e34408.
Baum AE, Akula N, Cabanero M, Cardona I, Corona W, Klemens B, et al. A genome-wide association study implicates diacylglycerol kinase eta (DGKH) and several other genes in the etiology of bipolar disorder. Mol Psychiatry. 2008;13:197–207.
Psychiatric GWAS Consortium Bipolar Disorder Working Group. Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nat Genet. 2011;43:977–83.
Zandi PP, Jaffe AE, Goes FS, Burke EE, Collado-Torres L, Huuki-Myers L, et al. Amygdala and anterior cingulate transcriptomes from individuals with bipolar disorder reveal downregulated neuroimmune and synaptic pathways. Nat Neurosci. 2022;25:381–9.
Chen R, Yang Z, Liu J, Cai X, Huo Y, Zhang Z, et al. Functional genomic analysis delineates regulatory mechanisms of GWAS-identified bipolar disorder risk variants. Genome Med. 2022;14:53.
Gu X, Dou M, Su W, Jiang Z, Duan Q, Cao B, et al. Identifying novel proteins underlying schizophrenia via integrating pQTLs of the plasma, CSF, and brain with GWAS summary data. BMC Med. 2022;20:474.
Sun BB, Maranville JC, Peters JE, Stacey D, Staley JR, Blackshaw J, et al. Genomic atlas of the human plasma proteome. Nature. 2018;558:73–79.
Pirmohamed M. Pharmacogenomics: current status and future perspectives. Nat Rev Genet. 2023;24:350–62.
Yang C, Farias FHG, Ibanez L, Suhy A, Sadler B, Fernandez MV, et al. Genomic atlas of the proteome from brain, CSF and plasma prioritizes proteins implicated in neurological disorders. Nat Neurosci. 2021;24:1302–12.
Mullins N, Forstner AJ, O’Connell KS, Coombes B, Coleman JRI, Qiao Z, et al. Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nat Genet. 2021;53:817–29.
Pierce BL, Ahsan H, Vanderweele TJ. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int J Epidemiol. 2011;40:740–52.
Kamat MA, Blackshaw JA, Young R, Surendran P, Burgess S, Danesh J, et al. PhenoScanner V2: an expanded tool for searching human genotype-phenotype associations. Bioinformatics. 2019;35:4851–3.
Staley JR, Blackshaw J, Kamat MA, Ellis S, Surendran P, Sun BB, et al. PhenoScanner: a database of human genotype-phenotype associations. Bioinformatics. 2016;32:3207–9.
Hemani G, Tilling K, Davey Smith G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet. 2017;13:e1007081.
Deng Y-T, Ou Y-N, Wu B-S, Yang Y-X, Jiang Y, Huang Y-Y, et al. Identifying causal genes for depression via integration of the proteome and transcriptome from brain and blood. Mol Psychiatry. 2022;27:2849–57.
Zou X, Wang L, Wang S, Zhang Y, Ma J, Chen L et al. Promising therapeutic targets for ischemic stroke identified from plasma and cerebrospinal fluid proteomes: A multicenter mendelian randomization study. Int J Surg. 2024;110:766–76.
Lin J, Zhou J, Xu Y. Potential drug targets for multiple sclerosis identified through Mendelian randomization analysis. Brain. 2023;146:3364–72.
Zakharov AF, Bedel’baeva KA, Baranovskaia LI. Variability in the expression of the fragile site of the (fra)X chromosome in 2 consecutive cell cycles. Biull Eksp Biol Med. 1986;102:738–41.
Warde-Farley D, Donaldson SL, Comes O, Zuberi K, Badrawi R, Chao P, et al. The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 2010;38:W214–20.
Franz M, Rodriguez H, Lopes C, Zuberi K, Montojo J, Bader GD, et al. GeneMANIA update 2018. Nucleic Acids Res. 2018;46:W60–4.
Jiang L, Zheng Z, Fang H, Yang J. A generalized linear mixed model association tool for biobank-scale data. Nat Genet. 2021;53:1616–21.
Gill J, Luckenbaugh D, Charney D, Vythilingam M. Sustained elevation of serum interleukin-6 and relative insensitivity to hydrocortisone differentiates posttraumatic stress disorder with and without depression. Biol Psychiatry. 2010;68:999–1006.
Spivak B, Shohat B, Mester R, Avraham S, Gil-Ad I, Bleich A, et al. Elevated levels of serum interleukin-1 beta in combat-related posttraumatic stress disorder. Biol Psychiatry. 1997;42:345–8.
Maes M, Lin AH, Delmeire L, Van Gastel A, Kenis G, De, et al. Elevated serum interleukin-6 (IL-6) and IL-6 receptor concentrations in posttraumatic stress disorder following accidental man-made traumatic events. Biol Psychiatry. 1999;45:833–9.
Gola H, Engler H, Sommershof A, Adenauer H, Kolassa S, Schedlowski M, et al. Posttraumatic stress disorder is associated with an enhanced spontaneous production of pro-inflammatory cytokines by peripheral blood mononuclear cells. BMC Psychiatry. 2013;13:40.
Williams JA, Burgess S, Suckling J, Lalousis PA, Batool F, Griffiths SL, et al. Inflammation and Brain Structure in Schizophrenia and Other Neuropsychiatric Disorders: A Mendelian Randomization Study. JAMA Psychiatry. 2022;79:498–507.
Modabbernia A, Taslimi S, Brietzke E, Ashrafi M. Cytokine alterations in bipolar disorder: a meta-analysis of 30 studies. Biol Psychiatry. 2013;74:15–25.
Gresnigt MS, van de Veerdonk FL. Biology of IL-36 cytokines and their role in disease. Semin Immunol. 2013;25:458–65.
Dardennes RM, Zizzari P, Tolle V, Foulon C, Kipman A, Romo L, et al. Family trios analysis of common polymorphisms in the obestatin/ghrelin, BDNF and AGRP genes in patients with Anorexia nervosa: association with subtype, body-mass index, severity and age of onset. Psychoneuroendocrinology. 2007;32:106–13.
Parlak N, Görgülü Y, Köse Çinar R, Sönmez MB, Parlak E. Serum agouti-related protein (AgRP) levels in bipolar disorder: Could AgRP be a state marker for mania? Psychiatry Res. 2018;260:36–40.
Fekete C, Sarkar S, Rand WM, Harney JW, Emerson CH, Bianco AC, et al. Agouti-related protein (AGRP) has a central inhibitory action on the hypothalamic-pituitary-thyroid (HPT) axis; comparisons between the effect of AGRP and neuropeptide Y on energy homeostasis and the HPT axis. Endocrinology. 2002;143:3846–53.
Alhadeff AL, Su Z, Hernandez E, Klima ML, Phillips SZ, Holland RA, et al. A Neural Circuit for the Suppression of Pain by a Competing Need State. Cell. 2018;173:140–52.e15
Padilla SL, Qiu J, Nestor CC, Zhang C, Smith AW, Whiddon BB, et al. AgRP to Kiss1 neuron signaling links nutritional state and fertility. Proc Natl Acad Sci USA. 2017;114:2413–8.
Dietrich MO, Zimmer MR, Bober J, Horvath TL. Hypothalamic Agrp neurons drive stereotypic behaviors beyond feeding. Cell. 2015;160:1222–32.
Xia G, Han Y, Meng F, He Y, Srisai D, Farias M, et al. Reciprocal control of obesity and anxiety-depressive disorder via a GABA and serotonin neural circuit. Mol Psychiatry. 2021;26:2837–53.
Zhang Y, Chen L, Xuan Y, Zhang L, Tian W, Zhu Y, et al. Iron overload in hypothalamic AgRP neurons contributes to obesity and related metabolic disorders. Cell Rep. 2024;43:113900.
de Souza GO, Dos Santos WO, Donato J. Ironing out obesity. Trends Endocrinol Metab. 2024;35:456–8.
Fanelli G, Franke B, Fabbri C, Werme J, Erdogan I, De Witte W et al. Local patterns of genetic sharing challenge the boundaries between neuropsychiatric and insulin resistance-related conditions. medRxiv. 2024.
Rosso G, Cattaneo A, Zanardini R, Gennarelli M, Maina G, Bocchio-Chiavetto L. Glucose metabolism alterations in patients with bipolar disorder. J Affect Disord. 2015;184:293–8.
Kato T. Mitochondrial dysfunction as the molecular basis of bipolar disorder: therapeutic implications. CNS Drugs. 2007;21:1–11.
Özkorumak Karagüzel E, Kural BV, Tiryaki A, Keleş Altun İ, Özer SY, Civil Arslan F. Blood levels of agouti-related peptide (AgRP), obestatin, corticosteroid-binding globulin (CBG), and cortisol in patients with bipolar disorder (BD): a case–control study. Psychiatry Clin Psychopharmacol. 2019;29:14–20.
Li M, Huang L, Grigoroiu-Serbanescu M, Bergen SE, Landén M, Hultman CM, et al. Convergent Lines of Evidence Support LRP8 as a Susceptibility Gene for Psychosis. Mol Neurobiol. 2016;53:6608–19.
Iwata K, Izumo N, Matsuzaki H, Manabe T, Ishibashi Y, Ichitani Y, et al. Vldlr overexpression causes hyperactivity in rats. Mol Autism. 2012;3:11.
Xiao X, Yu H, Li J, Wang L, Li L, Chang H, et al. Further evidence for the association between LRP8 and schizophrenia. Schizophr Res. 2020;215:499–505.
Zhang J, Zhang X, Zhang L, Zhou F, van Dinther M, Ten Dijke P. LRP8 mediates Wnt/β-catenin signaling and controls osteoblast differentiation. J Bone Miner Res. 2012;27:2065–74.
Marmol F. Lithium: bipolar disorder and neurodegenerative diseases Possible cellular mechanisms of the therapeutic effects of lithium. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1761–71.
Hoseth EZ, Krull F, Dieset I, Mørch RH, Hope S, Gardsjord ES, et al. Exploring the Wnt signaling pathway in schizophrenia and bipolar disorder. Transl Psychiatry. 2018;8:55.
Snitow ME, Bhansali RS, Klein PS. Lithium and Therapeutic Targeting of GSK-3. Cells. 2021;10:255.
Wang S, Krinks M, Lin K, Luyten FP, Moos M. Frzb, a secreted protein expressed in the Spemann organizer, binds and inhibits Wnt-8. Cell. 1997;88:757–66.
Lories RJ, Corr M, Lane NE. To Wnt or not to Wnt: the bone and joint health dilemma. Nat Rev Rheumatol. 2013;9:328–39.
Shimogori T, VanSant J, Paik E, Grove EA. Members of the Wnt, Fz, and Frp gene families expressed in postnatal mouse cerebral cortex. J Comp Neurol. 2004;473:496–510.
Tissir F, Goffinet AM. Expression of planar cell polarity genes during development of the mouse CNS. Eur J Neurosci. 2006;23:597–607.
Hu LW, Kawamoto EM, Brietzke E, Scavone C, Lafer B. The role of Wnt signaling and its interaction with diverse mechanisms of cellular apoptosis in the pathophysiology of bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:11–17.
Lacy SE, Bönnemann CG, Buzney EA, Kunkel LM. Identification of FLRT1, FLRT2, and FLRT3: a novel family of transmembrane leucine-rich repeat proteins. Genomics. 1999;62:417–26.
Fleitas C, Marfull-Oromí P, Chauhan D, Del Toro D, Peguera B, Zammou B, et al. FLRT2 and FLRT3 Cooperate in Maintaining the Tangential Migratory Streams of Cortical Interneurons during Development. J Neurosci. 2021;41:7350–62.
Bradford D, Cole SJ, Cooper HM. Netrin-1: diversity in development. Int J Biochem Cell Biol. 2009;41:487–93.
Leyva-Díaz E, del Toro D, Menal MJ, Cambray S, Susín R, Tessier-Lavigne M, et al. FLRT3 is a Robo1-interacting protein that determines Netrin-1 attraction in developing axons. Curr Biol. 2014;24:494–508.
Tsuji L, Yamashita T, Kubo T, Madura T, Tanaka H, Hosokawa K, et al. FLRT3, a cell surface molecule containing LRR repeats and a FNIII domain, promotes neurite outgrowth. Biochem Biophys Res Commun. 2004;313:1086–91.
Goes FS. Diagnosis and management of bipolar disorders. BMJ. 2023;381:e073591.
Acknowledgements
We would like to thank all the authors who contributed to the original GWAS dataset and the three tissues pQTLs dataset studies in this article.
Funding
This work was sponsored by the Fujian Minimally Invasive Medical Center Foundation (Grant No. 080270211 to RW).
Author information
Authors and Affiliations
Contributions
ZX: Lead the writing of the original draft of the project and responsible for overall project management, including resource allocation and progress tracking. Offer innovative suggestions and strategic guidance during the conceptualization phase and contribute to the initial drafting of project content. NZ: Responsible for collecting and organizing relevant data to provide reliable research support. Assist in developing formal analysis methods and standards to ensure accuracy and completeness. HC and ZY: Lead the investigation phase, ensuring the project is based on thorough facts and information and responsible for reviewing and editing written content. ZL and RW: Lead the overall conceptualization of the project, defining its core ideas and goals, provide professional guidance and support in methodology to ensure the team works according to specified methods and participate in the review and editing process, ensuring the quality and coherence of the final written material. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xiao, Z., Zheng, N., Chen, H. et al. Identifying novel proteins underlying bipolar disorder via integrating pQTLs of the plasma, CSF, and brain with GWAS summary data. Transl Psychiatry 14, 344 (2024). https://doi.org/10.1038/s41398-024-03056-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-024-03056-x
- Springer Nature Limited