Abstract
Several mental disorders emerge during childhood or adolescence and are often characterized by socioemotional difficulties, including alterations in emotion perception. Emotional facial expressions are processed in discrete functional brain modules whose connectivity patterns encode emotion categories, but the involvement of these neural circuits in psychopathology in youth is poorly understood. This study examined the associations between activation and functional connectivity patterns in emotion circuits and psychopathology during development. We used task-based fMRI data from the Philadelphia Neurodevelopmental Cohort (PNC, N = 1221, 8–23 years) and conducted generalized psycho-physiological interaction (gPPI) analyses. Measures of psychopathology were derived from an independent component analysis of questionnaire data. The results showed positive associations between identifying fearful, sad, and angry faces and depressive symptoms, and a negative relationship between sadness recognition and positive psychosis symptoms. We found a positive main effect of depressive symptoms on BOLD activation in regions overlapping with the default mode network, while individuals reporting higher levels of norm-violating behavior exhibited emotion-specific lower functional connectivity within regions of the salience network and between modules that overlapped with the salience and default mode network. Our findings illustrate the relevance of functional connectivity patterns underlying emotion processing for behavioral problems in children and adolescents.
Similar content being viewed by others
Introduction
Socioemotional difficulties, including deficits in emotion perception, are a common characteristic of several mental disorders that emerge during childhood and adolescence [1, 2]. Emotional facial expressions are processed in functional brain modules, including frontoparietal (FP), medial prefrontal-posterior cingulate (mPFC/PCC), and subcortical-posterior insular (SPI) modules, whose connectivity patterns are sensitive to distinct emotion categories [3]. These modules represent communities or clusters of strongly connected regions and overlap with the central executive, default mode, and salience networks, respectively. Interestingly, reduced functional segregation between the default mode and both salience and central executive network has been associated with psychopathology [4]. Analyzing task-based functional magnetic resonance imaging (fMRI) data from a large sample of children and adolescents, the present study aimed to examine the relationships between brain activation and connectivity during processing of emotional facial stimuli and distinct dimensions of psychopathology in youth.
The ability to differentiate between perceived emotions is present in infancy and continues to develop and improve throughout childhood and adolescence [5, 6]. Recognizing facial expressions of distinct basic emotions is an important social skill to help infer the emotional state of others and react accordingly [6, 7]. Early childhood sees development of this skill from being valence-based (feels good vs feels bad) to revolving around identifying distinct categories (angry, sadness, fear) of emotional states [8], while more fine-grained recognition skills develop throughout childhood [6, 9]. Social interactions become increasingly complex during adolescence, paralleling further refinement of socioemotional abilities during the transition from childhood to adulthood [10, 11].
Several mental disorders, including mood, anxiety, and conduct disorders, typically emerge during childhood and adolescence [2], and have been linked to socioemotional processing difficulties. Emotion recognition difficulties have been reported in adult patients with depression [12], anxiety [13], psychosis [14, 15], and among aggression-prone individuals [16]. Less is known about the role of emotion recognition in psychopathology in children and adolescents. General psychopathology was linked to poorer anger recognition and faster fear recognition in a small sample of 12-year-olds [17], and a review reported associations between emotion recognition abilities and mood disorders, schizophrenia, eating disorders, anxiety, ADHD, and conduct disorder among youth [1].
Several studies also indicate, although not consistently, that the processing of certain emotions is more affected than others in different dimensions of psychopathology. A so-called negativity bias, i.e., an attentional or interpretation bias toward negative emotions has been associated with various mental disorders. Adolescents with depressive symptoms tend to exhibit atypical perception of sadness, happiness, and anger (for a review see Nyquist & Luebbe [18]). Impaired or biased interpretation of social cues has also been reported in youth with anxiety disorders; compared to healthy controls, anxious youth made more errors in anger recognition [19]. Additionally, youth with disruptive mood dysregulation disorder showed a tendency of misidentifying happy stimuli as more angry or fearful [20]. This bias toward identifying stimuli as angry has also been found in youth with bipolar disorder [21, 22] and aggression-prone adults [16]. A systematic review supported a link between general facial emotion recognition difficulties and ADHD and conduct problems in children, however, the emotion-specific evidence was inconsistent [23]. Further research is thus needed to determine how both general and emotion-specific recognition is related to distinct dimensions of psychopathology in youth.
Emotion recognition deficits among youth with symptoms of psychopathology may be reflected in atypical patterns of brain activity and connectivity. Regional brain activity can be indirectly measured with fMRI. A meta-analysis examined the links between whole brain activation patterns during emotion processing and mental disorders [24]. The analysis included mainly fMRI and positron emission tomography studies conducted with patient groups and healthy controls that underwent various emotion processing tasks. The results indicated a disorder-general pattern of altered brain activation during emotion processing, with hyperactivation in amygdala, hippocampal, and parahippocampal gyri and hypoactivation in medial and lateral prefrontal regions. The meta-analysis, however, mainly consisted of studies with adult samples and only a few small youth samples. It is nevertheless assumed that differences in brain activity patterns can also be found in adolescents that show emotion processing biases [17, 25, 26].
Beyond regional brain activation, functional connectivity measures indicate if activity of two regions are correlated and allow inferences to be made about functional neural circuits [27, 28]. Using data from the Philadelphia Neurodevelopmental Cohort (PNC), a large cross-sectional sample of participants 8–23 years old, Zhang et al. [3] analyzed fMRI data from an emotion recognition task and identified three functional modules that were associated with the identification of emotional facial expressions: the frontoparietal, the medial prefrontal-posterior cingulate, and the subcortical-posterior insular modules. This overall modular architecture was stable across the sampled age-span, but patterns of connectivity within modules were sensitive to distinct emotion categories.
These three task-based functional modules spatially overlap with the central executive, default mode, and salience resting-state networks that have previously been associated with psychopathology [29, 30]. Resting-state fMRI studies using the PNC sample have linked deviations from neurotypical connectivity patterns to both symptoms of psychopathology and cognition [4, 31]. Specifically, symptoms related to mood, psychosis, fear, and externalizing behavior were associated with lower functional segregation between both the central executive and salience network with the default mode network [4]. These findings suggest that lower connectivity between networks may be associated with better mental health. However, it remains unclear how brain activation and connectivity during emotion processing are linked to psychopathology in youth. Considering the spatial overlap of functional emotion processing modules with the central executive, default mode, and salience networks, it is plausible that potential circuit disruptions associated with psychopathology also contribute to emotion processing biases.
The aim of the present study was to examine to what extent neural activation and functional connectivity during emotion processing are associated with psychopathology in children and adolescents. Using a large sample from the PNC (N = 1221, 8–23 years), we tested whether emotion recognition performance, activation in, and connectivity within and between functional emotion modules were related to general and distinct dimensions of psychopathology. To examine potential differences in neural processing of emotional facial expressions related to psychopathology, we analyzed fMRI data during an emotion identification task and previously identified robust functional modules that are activated during this task [3]. We assessed functional connectivity using a generalized psycho-physiological interaction (gPPI) approach [32] and derived measures of distinct dimensions of psychopathology from an independent component analysis of self- and parent-reported questionnaire data [33]. We expected to find a negative association between emotion identification accuracy and general psychopathology [1, 17]. Based on previously reported associations between higher psychopathology and lower functional segregation between medial prefrontal-posterior cingulate and both frontoparietal and subcortical-posterior insular modules [4], we also expected general and distinct psychopathology to be associated with lower functional segregation between modules during processing of individual facial expressions. As specific emotions elicit distinct connectivity patterns [3] and their perception is biased in different forms of psychopathology, we predicted differences among the association between seven distinct psychopathology dimensions and functional connectivity during processing of different emotional facial expressions.
Methods
Participants and exclusion procedure
The sample consisted of children and adolescents from the PNC, a large cohort recruited at the Children’s Hospital of Philadelphia with genetic, clinical, cognitive, and neuroimaging data [34]. The exclusion criteria included serious medical or neurological conditions. The inclusion criteria included the capacity to provide informed consent (with parental consent obtained for participants under 18 years old), proficiency in the English language, and physical and cognitive capabilities necessary to engage in computerized clinical assessment and neurocognitive testing [35]. Following procedures of quality control (see below), the final sample for our analyses consisted of 1221 participants between the ages of 8 and 23 (M = 15.42, SD = 3.49, 53% female). Demographic information can be found in Table 1. The PNC study has been approved by the University of Pennsylvania and the Children’s Hospital of Philadelphia Institutional Review [34]. The present study was conducted in line with the Declaration of Helsinki and was approved by the Norwegian Regional Committee for Medical and Health Research Ethics.
Quality control
The initial sample included 1499 participants with task-based fMRI. Quality control procedures involved calculating total signal-to-noise-ratio (tSNR) and mean relative motion indicators [36]. Participants deviating two standard deviations on either of these metrics were excluded (N = 104). Next, we excluded 46 participants due to task performance (>2 SD non-responses, i.e., non-responses >11, or lower than above chance correct responses, i.e., total score < 18). Finally, we excluded participants due to incomplete demographic and clinical data (N = 101), or incorrect logfile format (N = 23), leaving the final sample at 1221 participants.
Assessment of psychopathology
Psychopathology was measured using 129 clinical symptom score items from a computerized structured interview, GOASSESS [37], a shortened and modified self-report version of the Kiddie-SADS [38]. Depending on the participant’s age, the GOASESS was administered by parents (ages 8–17) and/or the participant themselves (aged 11–23). An independent component analysis integrated in Icasso (version 1.21; MATLAB) [39] decomposed the item scores into seven independent components (IC; see Alnæs et al. [33] for details on the procedure). Follow-up and conditional items were excluded for the analysis. The seven resulting scores per participant indicated their loading on components mainly reflecting attention problems (IC1); anxiety (IC2); norm-violating behavior (IC3); psychosis positive and prodromal symptoms (IC4); depression, suicide, and psychosis negative symptoms (IC5); mania (IC6); and obsessive–compulsive symptoms (IC7). The mean participant weight on all seven components was used as a measure for general psychopathology. Distributions of psychopathology component loadings can be found in Fig. 1.
A Overview of psychopathology independent components (IC) obtained from an independent component analysis on clinical GOASSESS data. B Distribution of participant weights on each psychopathology component and general psychopathology. C Association between age and loading on each psychopathology component and generalized psychopathology. Age was negatively associated with participant’s loadings on attention problems (IC1; r = −0.10, p < 0.001) and positively correlated with norm-violating behavior (IC3; r = 0.19, p < 0.001), depressive and negative psychosis (IC5; r = 0.26, p < 0.001), manic (IC6; r = 0.16, p < 0.001), and obsessive–compulsive symptoms (IC7; r = 0.06, p = 0.040), as well as with loadings on general psychopathology (r = 0.22, p < 0.001). meanICs = generalized psychopathology; IC1 = attention problems; IC2 = anxiety; IC3 = norm-violating behavior; IC4 = positive and prodromal symptoms; IC5 = depression, suicide, and negative symptoms; IC6 = mania; IC7 = obsessive–compulsive symptoms.
MRI acquisition
All participants were scanned using the same scanner, a 3T Siemens TIM Trio Scanner with a 32-channel head coil. T1-weighted images were acquired using magnetization prepared rapid acquisition gradient echo (MPRAGE) sequence (TR = 1.81 s, TE = 3.5 ms, flip angle = 9°, FOV = 240 × 180 mm, slice thickness = 1 mm, slices = 160). The task-based BOLD scans were obtained using a single-shot, interleaved multi-slice, gradient echo, EPI sequence (TR = 3.0 s, TE = 32 ms, flip angle = 90°, FOV = 192 mm2, slice thickness = 3 mm). Further details on the scanning procedure are described elsewhere [35].
Emotion identification task
During scanning, a fast event-related emotion identification task with jittered inter-stimulus intervals (0.5–18.5 s) was used to elicit task-based BOLD signal changes [40, 41]. Participants were, in pseudorandomized order, presented with 60 photos of actors showing facial expressions of anger, fear, sadness, happiness, or neutral states for 5.5 s and asked to identify the emotion expressed in each photo by choosing one of the five emotion labels with a custom fiberoptic response pad. Each emotion was presented 12 times. A complex oval-shaped crosshair condition that matched facial features was displayed between each emotional stimulus. Each facial expression had a high identification accuracy (>80%). As measures of emotion recognition performance, we extracted the number of correct trial responses per emotion condition as a measure of accuracy and mean response time per condition. The total duration of the task was 10:36 min.
ROI creation
We used ROIs of three robust functional modules that have previously been identified [3] using PNC fMRI data during the emotion identification task as templates for our analyses. ROIs were created based on the coordinates used in Zhang et al. [3]. In short, Zhang et al. conducted a meta-analysis with Neurosynth [42] and the search term “emotion” yielded peaks of regions that are involved in emotion processing. The authors added single ROIs due to their association with emotion perception, leading to a total of 50 regions (as shown in Fig. 2). Using MarsBaR [43], we built 6 mm spheres around each peak. We additionally created one mask per module that included all its ROIs. An overview of the ROIs and their peak coordinate can be obtained from Supplementary Table 1.
A Emotion processing circuits. Distribution of emotion-related regions of interest (ROI) within each functional module. The frontoparietal (FP) module (beige) includes bilateral dorsal anterior insula (dAI), fusiform gyrus (FFG), dorsal ACC (dACC), inferior parietal lobule (IPL), superior parietal lobule (SPL), lateral orbitofrontal cortex (lOFC), presupplementary motor area (pre-SMA), ventrolateral PFC (vlPFC), and dorsolateral PFC (dlPFC). The medial prefrontal-posterior cingulate (mPFC/PCC) module (dark blue) consists of bilateral nucleus accumbens (NAc), subgenual ACC (sgACC), pregenual ACC (pgACC), posterior cingulate cortex (PCC), ventromedial PFC (vmPFC), dorsomedial PFC (dmPFC). The subcortical-posterior insular (SPI) module (light blue) encompasses bilateral basolateral amygdala (BLA), centromedial amygdala (CMA), ventral anterior insula (vAI), posterior insula (PI), bed nucleus stria terminalis (BNST), hippocampus (Hipp). The plot was created with brainconn [92]. B Overview of data processing steps and the analysis.
fMRI preprocessing
The fMRI preprocessing steps are described in detail elsewhere [44]. In short, we used FMRI Expert Analysis Tool (FEAT) [45] from the FMRIB Software Library (FSL) [46] that included motion correction with MCFLIRT [47], spatial smoothing (FWHM = 6 mm) and high-pass filtering (90 s). To deal with the typically high occurrence of motion artifacts in young samples [48], we ran ICA-AROMA [49, 50] to remove motion specific noise, followed by an additional noise reduction step using FSL FIX [51] with a threshold of 60. Using FLIRT [52], boundary-based registration [53], and FNIRT [54], T1-weighted data were employed to align fMRI images to standard MNI coordinate space.
Generalized psycho-physiological interaction analyses
The preprocessed fMRI data were imported to the CONN toolbox [55] to generate functional connectivity measures. All conditions (angry, sad, fear, happy, neutral) were modeled as 5.5 s boxcar functions. During CONN’s denoising step, temporal band-pass filtering removed temporal frequencies below 0.008 or above 0.09 Hz [56].
Task-based ROI-to-ROI connectivity was established using gPPI [32]. Each target ROI time series was estimated with a model that included one task regressor per condition, one time-series-regressor of the BOLD activity in the seed ROI, interaction terms between the time-series-regressor and each task regressor, and an error regressor. This resulted in two regressors of the interaction term of each ROI pairing: one when ROI 1 was used as the target region and ROI 2 as the seed region and vice versa. As we were interested in nondirectional average connectivity, we calculated the mean of the two interaction regressors per ROI pairing.
While gPPI is generally used to test whether the connectivity between two regions is modulated by a task condition, the directionality of the effect is disputed [57, 58]. A significant gPPI interaction might also indicate that the “relationship” between two regions A and B, e.g., buffers the effect of the task condition on activity within region A. Thus, we can only test whether there is a significant undirected correlation between connectivity of region A and B and the effect of a condition.
Statistical analyses
The main aim of our analyses was to identify associations between emotion type, psychopathology dimensions and their neural correlates. We ran one model per psychopathology dimension (general psychopathology, IC1, IC2, IC3, IC4, IC5, IC6, IC7) and each intra- and intermodule connectivity measure. To reduce the number of analyses, we used emotion types as a factor variable “emotion” with the levels “neutral”, “fear”, “angry”, “sad”, and “happy” in each model. For exploratory analyses we conducted the analyses with one model per emotion type (see Supplementary Material) to control for potential overlapping effects of emotions. In the following sections we use the term “psychopathology” to refer to the loadings of participants onto the respective psychopathology component.
Behavioral data
Statistical analyses were carried out using R version 4.2.0 [59]. As background analyses, to examine performance in the emotion identification fMRI task, we conducted two ANOVAs with labeling accuracy or response time as dependent variable and emotion, age, sex, and ethnicity as independent variables. To examine whether psychopathology and performance in the emotion identification task were significantly related, we conducted linear regression analyses for each psychopathology component and emotion, using emotion as the criterion and psychopathology component as our predictor while controlling for age, sex, and ethnicity. All results were corrected for multiple comparisons using the false discovery rate (FDR) method [60].
fMRI activation
To test for activation differences during emotion processing, we first performed individual level analyses in SPM12 [61] and modeled the following contrasts: angry vs crosshair, fear vs crosshair, happy vs crosshair, neutral vs crosshair, and sad vs crosshair. Nuisance variables were accounted for by including six motion parameters and a regressor coding for incorrect or missing trials. Only correct trials were included in each emotion regressor. We used a gray matter template as an explicit mask (MNI ICBM152) [62]. Out of the initial 1221 participants, 1217 were successfully analyzed on the individual level and thus used in further activity analyses. Next, we extracted the mean BOLD activity of each ROI per emotion. For analyses on a modular level, we calculated the mean BOLD activity of all ROIs within a module and used it as a measure for mean module activation per emotion. We employed general linear models (GLM) for the group level analysis with BOLD activity of the modules as the criterion and the predictors emotion, psychopathology, emotion × psychopathology, age, sex, six motion parameters, and three ethnicity dummy coded variables and FDR-corrected for multiple comparisons [60].
Functional connectivity analyses
To test for intra- and intermodule connectivity differences, we derived coefficients of each gPPI term (per emotion) per ROI–ROI connection, calculated their mean within and between each module, and performed linear mixed models using the lmer function [63]. In fitting the model, we entered either inter- or intramodule connectivity as the criterion, and emotion, psychopathology, emotion × psychopathology interactions, age, sex, ethnicity, and six head movement parameters as fixed effects, and subject as a random effect. Six regression models were carried out to test intra-module (FP, mPFC/PCC, SPI), and inter-module (FP–mPFC/PCC, FP–SPI, mPFC/PCC–SPI) connectivity per psychopathology dimension (general psychopathology, seven independent components) totaling 48 regression models. Our main interest were the regression coefficients of the psychopathology × emotion interaction, that indicated whether the relationship between emotion and functional connectivity was modulated by psychopathology. We used F-tests to determine the significance of the regression coefficients, i.e., investigate whether these coefficients are significantly different among emotions (see Zhang et al. [3]). Results were subsequently corrected for multiple comparisons using the FDR approach [60].
Exploratory analyses
Based on our results, we conducted exploratory analyses to examine emotion-specific relationships between connectivity within and between functional modules and psychopathology again using FDR-correction [60]. The methodological approach in our main analysis did not allow an examination of the relationship between psychopathology and connectivity patters on the regional level. We therefore applied an elastic net regularization technique as an exploratory analysis to test whether single ROI–ROI pairings within emotion circuits were associated with general psychopathology. Guided by post-hoc hypotheses, we additionally examined whether region-specific connectivity patterns during threat processing (angry, fearful faces) was associated with loadings on IC2 (anxiety), IC3 (norm-violating behavior), or IC5 (depressive symptoms). For exploratory purposes, we also conducted a Canonical Correlation Analysis (CCA) in combination with a tenfold cross-validation procedure and permutation testing to assess the robustness of our findings. Here we used either connectivity measures averaged across all emotions or emotion-separated connectivity as the domain “connectivity patterns” and the seven independent components to represent the domain “psychopathology”. These analyses were followed up by sensitivity analyses without including age, sex, and ethnicity as covariates. Finally, with the aim of conceptually replicating Zhang et al.’s [3] findings on age by using gPPI, we employed GLMs without the psychopathology regressor and tested for age and sex effects. Details and results of these exploratory analyses can be found in the Supplementary Material.
Results
Behavioral results
ANOVAs on behavioral data yielded a significant main effect of emotion on both emotion recognition accuracy (F(4,6073) = 254.58, p < 0.001) and response time (F(4,6073) = 281.45, p < 0.001). Happy facial expressions elicited the fastest responses (M = 2.04 s, SD = 0.39) and highest accuracy (M = 99%, SD = 1%), while participants were slowest (M = 2.56, SD = 0.48) and had the lowest accuracy (M = 87%, SD = 12%) in response to sad facial expressions (see Table 2 for descriptive statistics of all emotion variables).
Associations between psychopathology and emotion recognition performance
GLMs were used to assess the link between psychopathology and emotion recognition performance. On a behavioral level, higher loadings on depressive and negative psychosis symptoms (IC5) were associated with better recognition of sad (B = 0.06, p < 0.001), fearful (B = 0.03, p < 0.001), and angry (B = 0.06, p < 0.001) facial expressions. Moreover, loadings on psychosis positive and prodromal symptoms (IC4) were negatively linked to sadness recognition (B = −0.08, p < 0.001). Finally, higher loadings on manic symptoms were associated with faster responses to fearful (B = −0.06, p < 0.001) faces (see Fig. 3B, C).
Associations between age and emotion recognition accuracy (A) and response time (B) per emotion category. Age positively correlated with labeling accuracy and negatively with response time for all emotions. Regression coefficients indicating the relationship between psychopathology components and labeling accuracy (C), and response time (D) of each emotion category. Results were controlled for age, sex, and ethnicity and corrected for multiple comparisons using FDR-correction. meanICs = generalized psychopathology; IC1 = attention problems; IC2 = anxiety; IC3 = norm-violating behavior; IC4 = positive and prodromal symptoms; IC5 = depression, suicide, and negative symptoms; IC6 = mania; IC7 = obsessive–compulsive symptoms. *p < 0.05.
fMRI activity
Second-level GLMs tested the relationship between emotion and psychopathology on BOLD activity within each functional module and showed that emotion was associated with functional activity in all three modules (Table S2), while only loadings on depressive and negative psychosis symptoms (IC5) were positively related to activity in the mPFC/PCC module (B = 0.07, p = 0.008; Table S3), with higher loadings being associated with higher activity. The interaction of emotion and psychopathology components was not related to BOLD activity in any of the functional modules.
Functional connectivity: intra- and intermodule connectivity
The analyses yielded a significant main effect of emotion on functional connectivity patterns in all modules (for all psychopathology components; Table S4), while there was no main effect of psychopathology (Table S5). Results from our main interaction analyses are summarized in Table 3. We did not find any significant relationships between the interaction of emotion and general psychopathology and intra- and intermodule connectivity measures. Nevertheless, we found that intra-SPI connectivity and inter-mFPC–SPI connectivity were significantly associated with loadings on the norm-violating behavior component and their interaction with each emotion type. As illustrated by Fig. 4, the relationship between norm-violating behavior and intra-SPI and inter-mPFC/PCC–SPI connectivity depended on the emotion type the participants labeled. Regarding the interaction between emotion type and loadings onto psychopathology components for attention problems, anxiety, psychosis, depression, mania, and obsessive–compulsive symptoms, our analyses did not reveal any relationships between each interaction term and intra- and intermodule connectivity following FDR-correction.
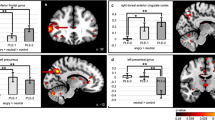
A Associations between loadings on the norm-violating behavior component (IC3) and mean inter- and intramodule connectivity changes. B Mean connectivity change per emotion within and between each module compared to a crosshair condition for groups with different tendencies toward norm-violating behavior. The groups were created by splitting the loadings on IC3 into quartiles. C Interaction effect of emotion and psychopathology on intra-SPI and inter-SPI–mPFC/PCC connectivity.
Discussion
The objective of the present study was to investigate the potential links between emotion recognition and brain activation and connectivity during facial emotion processing and dimensions of psychopathology in children and adolescents. The behavioral results showed that higher loadings on depression and negative psychosis symptoms were associated with better recognition of sadness, fear, and anger, while loadings on positive psychosis and prodromal symptoms were negatively associated with sadness recognition. Moreover, manic symptoms were linked to faster behavioral responses to fearful faces. We found evidence for a link between depressive symptoms and a general increased activity in the mPFC/PCC module during emotion processing, however BOLD activity did not differ between emotions. Further, our results indicate that functional connectivity within the SPI module, as well as between the SPI and mPFC/PCC modules distinguishes between emotions and the participant’s tendency toward norm-violating behavior. Finally, the data did not support the assumption that psychopathology is generally associated with connectivity patterns between the FP and the mPFC/PCC or SPI modules during emotion recognition. These findings contribute to our understanding of the brain bases of emotion processing difficulties in psychopathology of children and adolescents showing norm-violating behavior.
Our analyses revealed that the associations between norm-violating behavior and connectivity patterns within SPI and between SPI and mPFC/PCC modules varied between emotions. As shown in Fig. 3A, this effect was driven by lower connectivity during the presentation of angry faces with increasing symptoms of norm-violating behavior. Considering the spatial overlap of the modules with the salience and default mode network, our results indicate that the perception of angry facial expressions is related to lower within connectivity of the salience network and increased segregation (i.e., lower connectivity) of the salience and default mode network in participants with tendencies toward norm-violating behavior.
These findings are in line with multiple studies showing associations between aberrant functional connectivity of default mode network and limbic structures and aggressive tendencies. Generally, both resting state and task fMRI studies with children and adolescents have linked norm-violating behavior to diminished functional connectivity between the amygdala and vmPFC and regions of both the default mode network and salience network [64,65,66,67,68]. It should however be noted that these resting-state patterns might differ among subtypes of aggression, such as reactive, proactive, and callous–unemotional [69, 70].
Aggressive children show tendencies toward under-arousal [71], e.g., a low resting heart rate. It has been argued that emotion recognition deficits in youth with norm-violating behavior are based on these arousal characteristics [72]. At the same time, distinct connectivity patterns are dependent on arousal [73, 74] and can be modulated through anger provocation [75]. Increased arousal has been linked to cohesion of the salience network [74], while aggressive behavior in children is associated with global connectivity of the amygdala and strengthened connectivity between amygdala and regions outside the salience network [68]. Thus, weaker intra-SPI connectivity in children with tendencies toward norm-violating behavior could derive from disrupted arousal-linked circuitry in the salience network.
Despite previous work linking functional segregation between the default mode network and both salience and central executive network to overall psychopathology [4], we did not observe this general association. This might indicate a discrepancy between resting-state and task-based connectivity. While general and distinct dimensions of psychopathology seem to be associated with specific resting-state connectivity patterns [4, 76, 77], they might not be linked to task-based connectivity patterns. Interestingly, depressive symptoms were positively associated with BOLD activation in regions overlapping with the default mode network, yet not to their functional connectivity patterns. Yet, both aberrant default mode network activity and connectivity have been linked to emotion processing and depression [78, 79]. On the other hand, we found evidence for a negativity bias of emotion recognition in association with depression and negative psychosis loadings on a behavioral level, yet our data did not mirror this effect on a neural level as anger, fear, and sadness did not differ from processing of neutral and happy facial expressions.
These observations highlight the potential relevance of task fMRI paradigms and methodologies to examine the link between different behavioral characteristics of psychopathology dimensions [80]. One important aspect to mention is that we used an oval-shaped crosshair condition between the stimuli as an implicit baseline and thus acts as our control condition. While it is common to use neutral faces as control conditions, we decided to treat neutral faces as a distinct emotion based on Zhang et al.’s [3] finding that processing of neutral faces followed the same pattern as valanced emotions, and its role in psychopathology [81]. This might partly explain inconsistencies with previous studies reporting links between neural functional abnormalities and psychopathology [24, 82]. It should be noted that varying intensities of emotional expressions can offer additional insights on the link between emotion-specific recognition sensitivity and psychopathology [83] and on neural processing of emotions in adolescents [84]. Thus, emotion recognition biases in mental disorders might stem from mechanisms that are not detectable with our current methodological approach.
The task used in the present study was based on photographs of facial expressions with high labeling accuracy. The high accuracy may impede conclusions that can be drawn about emotion recognition sensitivities in psychopathology. Further, it does not allow an interpretation of functional connectivity during incorrect trials, as we cannot rule out that inattention or other processes may have led to the incorrect labeling. Distinct functional connectivity patterns during errors in emotion recognition might however be an important determinator for the link between biases in emotion recognition and psychopathology. Furthermore, previous literature shows that task characteristics, such as instructions (e.g., labeling, passive viewing) and stimuli (e.g., photographs of adults or children) affect emotion processing on both a behavioral and neural level [85,86,87]. Thus, studies with different task characteristics (e.g., ambiguous stimuli, passive viewing of emotional scenes) might reveal different aspects of potential neural links underlying emotion processing differences among dimensions of psychopathology.
The current study has several strengths. First, we used a large sample and sensitive analyses to investigate associations between functional connectivity patterns during emotion processing and psychopathology in children and adolescents. Further, we used a task-based connectivity approach, which has the potential to yield behaviorally more meaningful patterns than resting-state connectivity [80]. Additionally, the data-driven approach to clinical symptoms allowed us to examine associations between functional connectivity patterns during an emotion recognition task and distinct dimensions of psychopathology in youth.
It can be argued that the study lacks ecological validity, since we can merely infer from associations based on perception of emotional facial expressions, rather than real-world emotion perception [88], nor does the task and sample allow us to study associations between subtle emotion recognition biases in youth with confirmed diagnoses. Using stimulus sets with dynamic facial expressions of children and adolescents has the potential to explain aspects of emotion recognition development and their link to psychopathology with greater ecologically validity [89]. Considering the role of valence, intensity, and arousal in emotion perception [72, 83], future studies could use stimuli with varying emotion intensities, ecologically valid stimuli (e.g., dynamic facial expressions), add arousal measurements, such as heart-rate variability, and specifically focus on emotion recognition biases in clinical samples. Another limitation includes the potential impact of pubertal development on the processing of emotions [90], which we did not account for in our study. Finally, future longitudinal studies should examine the developmental relationships between functional emotion circuits and psychopathology in youth and aim to disentangle the interplay between emotion and cognitive processing, i.e., emotion regulation and working memory, to fully understand the roots and consequences of emotion processing biases. Given the relevance and availability of psychosocial interventions, it is particularly important to identify environmental contributors of emotion recognition biases and their subsequent impact on interpersonal relationships [91]. Specifically, in the case of emotion recognition biases in youth with norm-violating behavior, more research and innovation on emotion recognition and regulation trainings is needed to potentially compensate biased neural emotion processing.
Using a large-scale developmental cohort, we assessed whether individual differences in functional brain activation and connectivity patterns within and between frontoparietal, medial prefrontal-posterior cingulate and subcortical-posterior insular modules during emotion processing were associated with distinct dimensions of psychopathology in youth. Our analysis suggested a negativity bias in emotion recognition in individuals with depressive symptoms and poor sadness recognition with increasing positive psychosis symptoms on a behavioral level, yet the fMRI analysis did not reveal evidence for a neural mechanism of this negativity bias on the functional activation or connectivity level. Nevertheless, the results indicate a relationship between depressive symptoms and activation within the default mode network. Furthermore, higher tendencies toward norm-violating behavior were associated with emotion-specific reduced functional connectivity within the salience network and between the salience and default mode networks. Future studies using various intensities of emotional stimuli, additional arousal measurements, and large-scale network-focused multimodal imaging approaches can provide further insights on the link between psychopathology and emotion-specific biases.
Data availability
Data from the Philadelphia Developmental Cohort are available through the Database of Genotypes and Phenotypes (dbGaP): https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000607.v3.p2.
References
Collin L, Bindra J, Raju M, Gillberg C, Minnis H. Facial emotion recognition in child psychiatry: a systematic review. Res Dev Disabil. 2013;34:1505–20.
Dalsgaard S, Thorsteinsson E, Trabjerg BB, Schullehner J, Plana-Ripoll O, Brikell I, et al. Incidence rates and cumulative incidences of the full spectrum of diagnosed mental disorders in childhood and adolescence. JAMA Psychiatry. 2020;77:155.
Zhang Y, Padmanabhan A, Gross JJ, Menon V. Development of human emotion circuits investigated using a big-data analytic approach: stability, reliability, and robustness. J Neurosci. 2019;39:7155–72.
Xia CH, Ma Z, Ciric R, Gu S, Betzel RF, Kaczkurkin AN, et al. Linked dimensions of psychopathology and connectivity in functional brain networks. Nat Commun. 2018;9:3003.
Batty M, Taylor MJ. The development of emotional face processing during childhood. Dev Sci. 2006;9:207–20.
Rodger H, Vizioli L, Ouyang X, Caldara R. Mapping the development of facial expression recognition. Dev Sci. 2015;18:926–39.
Somerville LH, Fani N, McClure-Tone EB. Behavioral and neural representation of emotional facial expressions across the lifespan. Dev Neuropsychol. 2011;36:408–28.
Widen SC. Children’s interpretation of facial expressions: the long path from valence-based to specific discrete categories. Emot Rev. 2013;5:72–77.
Thomas LA, De Bellis MD, Graham R, LaBar KS. Development of emotional facial recognition in late childhood and adolescence. Dev Sci. 2007;10:547–58.
Kilford EJ, Garrett E, Blakemore S-J. The development of social cognition in adolescence: an integrated perspective. Neurosci Biobehav Rev. 2016;70:106–20.
Nelson EE, Jarcho JM, Guyer AE. Social re-orientation and brain development: an expanded and updated view. Dev Cogn Neurosci. 2016;17:118–27.
Dalili MN, Penton-Voak IS, Harmer CJ, Munafò MR. Meta-analysis of emotion recognition deficits in major depressive disorder. Psychol Med. 2015;45:1135–44.
Mathews A, Mackintosh B. Induced emotional interpretation bias and anxiety. J Abnorm Psychol. 2000;109:602–15.
Chan RCK, Li H, Cheung EFC, Gong Q. Impaired facial emotion perception in schizophrenia: a meta-analysis. Psychiatry Res. 2010;178:381–90.
Neves M, de CL, Albuquerque MR, Malloy-Diniz L, Nicolato R, Silva Neves F, et al. A voxel-based morphometry study of gray matter correlates of facial emotion recognition in bipolar disorder. Psychiatry Res Neuroimaging. 2015;233:158–64.
Mellentin AI, Dervisevic A, Stenager E, Pilegaard M, Kirk U. Seeing enemies? A systematic review of anger bias in the perception of facial expressions among anger-prone and aggressive populations. Aggression Violent Behav. 2015;25:373–83.
Simcock G, McLoughlin LT, De Regt T, Broadhouse KM, Beaudequin D, Lagopoulos J, et al. Associations between facial emotion recognition and mental health in early adolescence. Int J Environ Res Public Health. 2020;17:330.
Nyquist AC, Luebbe AM. An emotion recognition–awareness vulnerability hypothesis for depression in adolescence: a systematic review. Clin Child Fam Psychol Rev. 2020;23:27–53.
Jarros RB, Salum GA, Belem da Silva CT, Toazza R, de Abreu Costa M, Fumagalli de Salles J, et al. Anxiety disorders in adolescence are associated with impaired facial expression recognition to negative valence. J Psychiatr Res. 2012;46:147–51.
Vidal-Ribas P, Brotman MA, Salum GA, Kaiser A, Meffert L, Pine DS, et al. Deficits in emotion recognition are associated with depressive symptoms in youth with disruptive mood dysregulation disorder. Depress Anxiety. 2018;35:1207–17.
Brotman MA, Rich BA, Schmajuk M, Reising M, Monk CS, Dickstein DP, et al. Attention bias to threat faces in children with bipolar disorder and comorbid lifetime anxiety disorders. Biol Psychiatry. 2007;61:819–21.
McClure EB, Pope K, Hoberman AJ, Pine DS, Leibenluft E. Facial expression recognition in adolescents with mood and anxiety disorders. Am J Psychiatry. 2003;160:1172–4.
Cooper S, Hobson CW, van Goozen SH. Facial emotion recognition in children with externalising behaviours: a systematic review. Clin Child Psychol Psychiatry. 2020;25:1068–85.
McTeague LM, Rosenberg BM, Lopez JW, Carreon DM, Huemer J, Jiang Y, et al. Identification of common neural circuit disruptions in emotional processing across psychiatric disorders. Am J Psychiatry. 2020;177:411–21.
Hall LMJ, Klimes-Dougan B, H. Hunt R, M. Thomas K, Houri A, Noack E, et al. An fMRI study of emotional face processing in adolescent major depression. J Affect Disord. 2014;168:44–50.
Li X, Wang J. Abnormal neural activities in adults and youths with major depressive disorder during emotional processing: a meta-analysis. Brain Imaging Behav. 2021;15:1134–54.
Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. NeuroImage. 2010;52:1059–69.
Tau GZ, Peterson BS. Normal development of brain circuits. Neuropsychopharmacology. 2010;35:147–68.
Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483–506.
Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–56.
Zhu J, Qiu A. Interindividual variability in functional connectivity discovers differential development of cognition and transdiagnostic dimensions of psychopathology in youth. NeuroImage. 2022;260:119482.
McLaren DG, Ries ML, Xu G, Johnson SC. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. NeuroImage. 2012;61:1277–86.
Alnæs D, Kaufmann T, Doan NT, Córdova-Palomera A, Wang Y, Bettella F, et al. Association of heritable cognitive ability and psychopathology with white matter properties in children and adolescents. JAMA Psychiatry. 2018;75:287.
Satterthwaite TD, Connolly JJ, Ruparel K, Calkins ME, Jackson C, Elliott MA, et al. The Philadelphia Neurodevelopmental Cohort: a publicly available resource for the study of normal and abnormal brain development in youth. NeuroImage. 2016;124:1115–9.
Satterthwaite TD, Elliott MA, Ruparel K, Loughead J, Prabhakaran K, Calkins ME, et al. Neuroimaging of the Philadelphia Neurodevelopmental Cohort. NeuroImage. 2014;86:544–53.
Roalf DR, Quarmley M, Elliott MA, Satterthwaite TD, Vandekar SN, Ruparel K, et al. The impact of quality assurance assessment on diffusion tensor imaging outcomes in a large-scale population-based cohort. NeuroImage. 2016;125:903–19.
Calkins ME, Merikangas KR, Moore TM, Burstein M, Behr MA, Satterthwaite TD, et al. The Philadelphia Neurodevelopmental Cohort: constructing a deep phenotyping collaborative. J Child Psychol Psychiatry. 2015;56:1356–69.
Merikangas KR, Avenevoli S, Costello EJ, Koretz D, Kessler RC. National comorbidity survey replication adolescent supplement (NCS-A): I. Background and measures. J Am Acad Child Adolesc Psychiatry. 2009;48:367–79.
Himberg J, Hyvärinen A, Esposito F. Validating the independent components of neuroimaging time series via clustering and visualization. NeuroImage. 2004;22:1214–22.
Gur RC, Sara R, Hagendoorn M, Marom O, Hughett P, Macy L, et al. A method for obtaining 3-dimensional facial expressions and its standardization for use in neurocognitive studies. J Neurosci Methods. 2002;115:137–43.
Gur RC, Richard J, Hughett P, Calkins ME, Macy L, Bilker WB, et al. A cognitive neuroscience-based computerized battery for efficient measurement of individual differences: standardization and initial construct validation. J Neurosci Methods. 2010;187:254–62.
Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011;8:665–70.
Brett M, Anton J-L, Valabregue R, Poline J-B. MarsBaR. Region of interest analysis using the MarsBar toolbox for SPM 99. Neuroimage. 2002;16:S497.
Kaufmann T, Alnæs D, Doan NT, Brandt CL, Andreassen OA, Westlye LT. Delayed stabilization and individualization in connectome development are related to psychiatric disorders. Nat Neurosci. 2017;20:513–5.
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23:S208–S219.
Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. NeuroImage. 2012;62:782–90.
Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–41.
Afacan O, Erem B, Roby DP, Roth N, Roth A, Prabhu SP, et al. Evaluation of motion and its effect on brain magnetic resonance image quality in children. Pediatr Radiol. 2016;46:1728–35.
Pruim RHR, Mennes M, Buitelaar JK, Beckmann CF. Evaluation of ICA-AROMA and alternative strategies for motion artifact removal in resting state fMRI. NeuroImage. 2015;112:278–87.
Pruim RHR, Mennes M, van Rooij D, Llera A, Buitelaar JK, Beckmann CF. ICA-AROMA: a robust ICA-based strategy for removing motion artifacts from fMRI data. NeuroImage. 2015;112:267–77.
Salimi-Khorshidi G, Douaud G, Beckmann CF, Glasser MF, Griffanti L, Smith SM. Automatic denoising of functional MRI data: combining independent component analysis and hierarchical fusion of classifiers. NeuroImage. 2014;90:449–68.
Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–56.
Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. NeuroImage. 2009;48:63–72.
Andersson JLR, Jenkinson M, Smith S. Non-linear registration, aka spatial normalisation. 2010.https://www.fmrib.ox.ac.uk/datasets/techrep/.
Nieto-Castanon A, Whitfield-Gabrieli S. CONN functional connectivity toolbox: RRID SCR_009550, release 22. 22nd ed. Hilbert Press, 2022. https://doi.org/10.56441/hilbertpress.2246.5840.
Hallquist MN, Hwang K, Luna B. The nuisance of nuisance regression: spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. NeuroImage. 2013;82:208–25.
Cisler JM, Bush K, Steele JS. A comparison of statistical methods for detecting context-modulated functional connectivity in fMRI. NeuroImage. 2014;84:1042–52.
Gerchen MF, Bernal‐Casas D, Kirsch P. Analyzing task‐dependent brain network changes by whole‐brain psychophysiological interactions: a comparison to conventional analysis. Hum Brain Mapp. 2014;35:5071–82.
R Core Team. R: a language and environment for statistical computing. 2022. https://www.R-project.org/.
Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 1995;57:289–300.
Ashburner J. Computational anatomy with the SPM software. Magn Reson Imaging. 2009;27:1163–74.
Fonov V, Evans AC, Botteron K, Almli CR, McKinstry RC, Collins DL. Unbiased average age-appropriate atlases for pediatric studies. NeuroImage. 2011;54:313–27.
Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48.
Finger EC, Marsh A, Blair KS, Majestic C, Evangelou I, Gupta K, et al. Impaired functional but preserved structural connectivity in limbic white matter tracts in youth with conduct disorder or oppositional defiant disorder plus psychopathic traits. Psychiatry Res Neuroimaging. 2012;202:239–44.
Ibrahim K, Noble S, He G, Lacadie C, Crowley MJ, McCarthy G, et al. Large-scale functional brain networks of maladaptive childhood aggression identified by connectome-based predictive modeling. Mol Psychiatry. 2022;27:985–99.
Park AT, Leonard JA, Saxler PK, Cyr AB, Gabrieli JDE, Mackey AP. Amygdala–medial prefrontal cortex connectivity relates to stress and mental health in early childhood. Soc Cogn Affect Neurosci. 2018;13:430–9.
Romero-Martínez Á, González M, Lila M, Gracia E, Martí-Bonmatí L, Alberich-Bayarri Á, et al. The brain resting-state functional connectivity underlying violence proneness: is it a reliable marker for neurocriminology? A systematic review. Behav Sci. 2019;9:11.
Sukhodolsky DG, Ibrahim K, Kalvin CB, Jordan RP, Eilbott J, Hampson M. Increased amygdala and decreased frontolimbic resting-state functional connectivity in children with aggressive behavior. Soc Cogn Affect Neurosci. 2022;17:634–44.
Werhahn JE, Mohl S, Willinger D, Smigielski L, Roth A, Hofstetter C, et al. Aggression subtypes relate to distinct resting state functional connectivity in children and adolescents with disruptive behavior. Eur Child Adolesc Psychiatry. 2021;30:1237–49.
Zhu W, Zhou X, Xia L-X. Brain structures and functional connectivity associated with individual differences in trait proactive aggression. Sci Rep. 2019;9:7731.
Portnoy J, Farrington DP. Resting heart rate and antisocial behavior: an updated systematic review and meta-analysis. Aggression Violent Behav. 2015;22:33–45.
Souroulla AV, Panteli M, Robinson JD, Panayiotou G. Valence, arousal or both? Shared emotional deficits associated with attention deficit and hyperactivity disorder and oppositional/defiant-conduct disorder symptoms in school-aged youth. Biol Psychol. 2019;140:131–40.
Nummenmaa L, Glerean E, Hari R, Hietanen JK. Bodily maps of emotions. Proc Natl Acad Sci USA. 2014;111:646–51.
Young CB, Raz G, Everaerd D, Beckmann CF, Tendolkar I, Hendler T, et al. Dynamic shifts in large-scale brain network balance as a function of arousal. J Neurosci. 2017;37:281–90.
Siep N, Tonnaer F, van de Ven V, Arntz A, Raine A, Cima M. Anger provocation increases limbic and decreases medial prefrontal cortex connectivity with the left amygdala in reactive aggressive violent offenders. Brain Imaging Behav. 2019;13:1311–23.
Lees B, Squeglia LM, McTeague LM, Forbes MK, Krueger RF, Sunderland M, et al. Altered neurocognitive functional connectivity and activation patterns underlie psychopathology in preadolescence. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021;6:387–98.
Voldsbekk I, Kjelkenes R, Dahl A, Holm MC, Lund MJ, Kaufmann T, et al. Delineating disorder-general and disorder-specific dimensions of psychopathology from functional brain networks in a developmental clinical sample. Dev Cogn Neurosci. 2023;62:101271.
Grimm S, Boesiger P, Beck J, Schuepbach D, Bermpohl F, Walter M, et al. Altered negative BOLD responses in the default-mode network during emotion processing in depressed subjects. Neuropsychopharmacology. 2009;34:932–43.
Ho TC, Yang G, Wu J, Cassey P, Brown SD, Hoang N, et al. Functional connectivity of negative emotional processing in adolescent depression. J Affect Disord. 2014;155:65–74.
Zhao W, Makowski C, Hagler DJ, Garavan HP, Thompson WK, Greene DJ, et al. Task fMRI paradigms may capture more behaviorally relevant information than resting-state functional connectivity. NeuroImage. 2023;270:119946.
Filkowski MM, Haas BW. Rethinking the use of neutral faces as a baseline in fMRI neuroimaging studies of axis-I psychiatric disorders. J Neuroimaging. 2017;27:281–91.
Groenewold NA, Opmeer EM, de Jonge P, Aleman A, Costafreda SG. Emotional valence modulates brain functional abnormalities in depression: evidence from a meta-analysis of fMRI studies. Neurosci Biobehav Rev. 2013;37:152–63.
Rutter LA, Dodell-Feder D, Vahia IV, Forester BP, Ressler KJ, Wilmer JB, et al. Emotion sensitivity across the lifespan: mapping clinical risk periods to sensitivity to facial emotion intensity. J Exp Psychol Gen. 2019;148:1993–2005.
Wiggins JL, Adleman NE, Kim P, Oakes AH, Hsu D, Reynolds RC, et al. Developmental differences in the neural mechanisms of facial emotion labeling. Soc Cogn Affect Neurosci. 2016;11:172–81.
Calvo MG, Nummenmaa L. Perceptual and affective mechanisms in facial expression recognition: an integrative review. Cogn Emot. 2016;30:1081–106.
Lange K, Williams LM, Young AW, Bullmore ET, Brammer MJ, Williams SCR, et al. Task instructions modulate neural responses to fearful facial expressions. Biol Psychiatry. 2003;53:226–32.
Marusak HA, Carré JM, Thomason ME. The stimuli drive the response: an fMRI study of youth processing adult or child emotional face stimuli. NeuroImage. 2013;83:679–89.
Camacho MC, Nielsen AN, Balser D, Furtado E, Steinberger DC, Fruchtman L, et al. Large-scale encoding of emotion concepts becomes increasingly similar between individuals from childhood to adolescence. Nat Neurosci. 2023. https://doi.org/10.1038/s41593-023-01358-9.
Giuliani NR, Flournoy JC, Ivie EJ, Von Hippel A, Pfeifer JH. Presentation and validation of the DuckEES child and adolescent dynamic facial expressions stimulus set. Int J Methods Psychiatr Res. 2017;26:e1553.
Motta-Mena NV, Scherf KS. Pubertal development shapes perception of complex facial expressions. Dev Sci. 2017;20:e12451.
Assed MM, Khafif TC, Belizario GO, Fatorelli R, Rocca CC, de A, et al. Facial emotion recognition in maltreated children: a systematic review. J Child Fam Stud. 2020;29:1493–509.
Orchard ER, Ward PGD, Chopra S, Storey E, Egan GF, Jamadar SD. Neuroprotective effects of motherhood on brain function in late life: a resting-state fMRI study. Cereb Cortex. 2021;31:1270–83.
Acknowledgements
The current work was supported by the Research Council of Norway (#223273, #288083, #323951) and the South-Eastern Norway Regional Health Authority (#2019069, #2019107, #2020086, #2021070, #2023012, #500189), the European Research Council under the European Union’s Horizon 2020 Research and Innovation Program (802998). Support for the collection of the Philadelphia Neurodevelopment Cohort (PNC) was provided by Grant RC2MH089983 to Raquel Gur, and RC2MH089924 to Hakon Hakonarson. The participants were recruited through the Center for Applied Genomics at the Children’s Hospital of Philadelphia. Phenotypic data collection occurred at the CAG/CHOP and at the Brain Behavior Laboratory, University of Pennsylvania.
Author information
Authors and Affiliations
Contributions
VK, HE, DB, and CKT conceived and designed the study with support from CDL and LBN. VK, HE, and DA performed statistical analyses. OAA, LTW, and CKT were involved in funding acquisition and data curation. VK wrote the original manuscript. All authors reviewed, wrote, and edited the manuscript. CKT supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The present study was conducted in line with the Declaration of Helsinki and was approved by the Norwegian Regional Committee for Medical and Health Research Ethics. The PNC study has been approved by the institutional review boards of the University of Pennsylvania and the Children’s Hospital of Philadelphia.
Informed consent
Signed informed consent was obtained for all participants (for participants under age 18 signed informed parental consent was provided).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Karl, V., Engen, H., Beck, D. et al. The role of functional emotion circuits in distinct dimensions of psychopathology in youth. Transl Psychiatry 14, 317 (2024). https://doi.org/10.1038/s41398-024-03036-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-024-03036-1
- Springer Nature Limited