Abstract
There are only a few studies reporting on the immunological profiles of methamphetamine (MA) use, MA dependency, or MA-induced psychosis (MAP). This study measured M1 macrophage, T helper (Th)-1, Th-2, growth factor, and chemokine profiles, as well as the immune inflammatory response system (IRS) and compensatory immunoregulatory system (CIRS) in peripheral blood samples from patients with MA use (n = 51), MA dependence (n = 47), and MAP (n = 43) in comparison with controls (n = 32). We discovered that persistent MA use had a robust immunosuppressive impact on all immunological profiles. The most reliable biomarker profile of MA use is the combination of substantial CIRS suppression and a rise in selected pro-inflammatory cytokines, namely CCL27 (CTACK), CCL11 (eotaxin), and interleukin (IL)-1α. In addition, MA dependency is associated with increased immunosuppression, as demonstrated by lower stem cell factor levels and higher IL-10 levels. MAP is related to a significant decrease in all immunological profiles, particularly CIRS, and an increase in CCL5 (RANTES), IL-1α, and IL-12p70 signaling. In conclusion, long-term MA use and dependency severely undermine immune homeostasis, whereas MAP may be the consequence of increased IL-1α – CCL5 signaling superimposed on strongly depleted CIRS and Th-1 functions. The widespread immunosuppression established in longstanding MA use may increase the likelihood of infectious and immune illness or exacerbate disorders such as hepatitis and AIDS. Furthermore, elevated levels of CCL5, CCL11, CCL27, IL-1α, and/or IL-12p70 may play a role in the peripheral (atherosclerosis, cutaneous inflammation, immune aberrations, hypospermatogenesis) and central (neuroinflammation, neurotoxic, neurodegenerative, depression, anxiety, and psychosis) side effects of MA use.
Similar content being viewed by others
Introduction
Methamphetamine (MA), a psychostimulant, is the second most frequently used illegal substance worldwide [1, 2]. Between 2009 and 2018, the number of people who used MA increased from 210 million to 265 million worldwide [3]. In Southeast Asia, particularly Thailand, one of the most popular drugs is MA speed tablets, which combine MA and caffeine [4, 5]. In recent years, crystal meth (ice), a crystalline and pure version of MA, has gained popularity and was introduced as a chemsex substance, including among those who inject drugs [6, 7].
An increase in dopamine, norepinephrine, and serotonin turnover causes the stimulant effects of MA on the central nervous system [8]. In addition, the use of MA causes significant neurological and physical consequences, such as sleep disturbances, aggressive behavior, anxiety, sadness, depression, and psychosis, including delusions and hallucinations [1, 9]. MA-induced psychosis (MAP) is characterized by persecuting delusions, hallucinations, and conceptual disorganization [10,11,12]. Around 3–23% of individuals who frequently use MA in a population sample with MA use reported MAP or psychotic symptoms [13]. Around 40% of people with MA dependency in a hospital-based research group may have psychotic symptoms, such as paranoia, throughout their lifetime use of MA [14]. MAP occurs over the course of MA use, including acute intoxication and chronic use [15]. Typically, MAP may be present for a brief period, a few days to one month after the cessation of MA use; nevertheless, 38.8% of patients have a continuous course of psychosis, suggesting an association between MAP and primary psychotic disorders, such as schizophrenia [16].
A number of studies demonstrated that MA use may disrupt immunological homeostasis and suppress immune functions [17], including suppressing the production of T-helper (Th)-1 cytokines [18], T-cell proliferation [19], and the number of lymphocytes and immune cells [20, 21]. Nitrostyrene, an amphetamine derivative, decreases interleukin (IL)-12 and IL-6 concentrations [22]. Earlier research showed that MAP is associated with symptoms of immunological activation and inflammation, as well as neuroinflammation, as evidenced by elevated IL-6 and IL-8 [23], as well as elevated oxidative stress and decreased antioxidant defenses [12]. MA may affect antigen-presenting cells (APCs) in the brain, leading to an increase in pro-inflammatory cytokines such as IL-1β, IL-6, IL-8, interferons (IFN), and tumor necrosis factor (TNF)-α [24]. A few studies have reported changes in immunological profiles in individuals who use MA or have MA dependence [25,26,27]. Nevertheless, the immune profile of MAP has remained elusive.
MAP is often used and investigated as a model of schizophrenia because of its overlapping symptoms and biomarker characteristics, such as elevated dopamine turnover [28]. There is now evidence that schizophrenia, and particularly its more severe phenotype deficit schizophrenia, is a neuro-immune and neuro-oxidative disorder [29,30,31], with elevated levels of pro-inflammatory cytokines such as IL-1β, IL-6, IL-12p70, and TNF-α [32, 33], activation of macrophage M1, Th-1, Th-2, Th-17, and T regulatory (Treg) phenotypes, as well as activation of the immune-inflammatory response system (IRS) and the compensatory immune-regulatory systems (CIRS), which tend to downregulate the IRS and prevent hyperinflammation [31]. However, no research has studied the comprehensive immunological profiles of MA use, MA dependence, and MAP, including M1, Th-1, Th-2, Th-17, IRS, CIRS, chemokine, and growth factor profiles, to determine whether these conditions are characterized by immune activation or suppression.
Hence, we explored peripheral blood immune profiles in MA use, MA dependence, and MAP as compared with controls to examine whether these conditions are accompanied by immune activation or immunosuppression and which immune profiles and cytokines are specifically involved in the three conditions. More specifically, the a priori hypothesis was to detect increased levels of pro-inflammatory cytokines such as IL-1β, IL-6, IL-8, IFN-γ, and TNF-α in MAP, and a moderate immunosuppression in MA dependency.
Methods
Participants
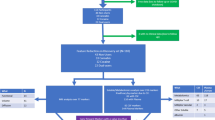
This research recruited 173 participants, 32 controls, and 141 patients with MA use from the Princess Mother National Institute on Drug Abuse Treatment (PMNIDAT), Thailand, including those with no MA dependency (n = 51), MA dependence (n = 47), and MAP (n = 43). A verbal notice was made at King Chulalongkorn Memorial Hospital to recruit controls (n = 32) who had never taken MA in their lives and did not have any drug dependency except for a tobacco use disorder. By age and gender, the controls were matched with the MA group. All participants were between the ages of 18 and 65. Participants with severe neuropsychiatric disorders, such as schizophrenia, bipolar disorder, psycho-organic disorders, and schizo-affective disorder, as well as subjects with (auto)immune disorders, such as psoriasis, inflammatory bowel disease, rheumatoid arthritis, and lupus erythematosus, were excluded from the study. We also omitted women who were pregnant or nursing. The data were collected between March - August 2022, and the project was approved by the Institutional Review Board of the Faculty of Medicine of Chulalongkorn University and the PMNIDAT (No. 28/2565).
Clinical assessment
Using the Thai version of the Semi-Structured Assessment of Drug Dependency and Alcoholism (SSADDA), demographics, drug use data including tobacco, alcohol, cannabis, and MA, and diagnoses were acquired [14, 34]. Based on the Fourth Version of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV), the SSADDA is a thorough interview to identify drug dependency and associated mental illnesses [35]. The MA dependency group comprised patients who complied with 6-7 criteria on the DSM-IV MA dependent scale (utilizing the SSADDA), whereas patients with 0-1 criteria were recruited for the study group with MA use. Persons having a history of psychotic symptoms while taking MA, including hallucinations and/or delusions, were selected for the MAP group if they answered “yes” to any of the psychosis section of the SSADDA questions (i.e., probing code = yes, always due to drugs (MA)). Using the SSADDA, we also determined the length of heavy MA use (in months), the daily quantity of MA (in milligrams; mg), and the forms of MA, such as crystal meth, speed pills, or both. We also evaluated lifetime and present alcohol and illicit substance use. We estimated the maximum number of alcoholic drinks per day; one standard alcoholic drink is equivalent to 10 g of pure alcohol. The interviewers were two psychologists with more than five years of training and experience in performing SSADDA interviews. Before finishing the interview data, all interviews were cross-checked by a second interviewer.
Measurement of cytokines
Early in the morning (between 8:00 and 9:00 a.m.), 5 ml of fasting venous blood was drawn from each participant, using a disposable syringe. Blood was stored at −80 °C until thawed for biomarker assays. The samples were placed at room temperature for 1 h, then aliquots 25 µl. whole blood were diluted 1:4 (sample: diluent) after that, the standard dilutions were prepared. The concentrations of 48 cytokines, chemokines, and growth factors were measured in duplicate using Bio-Plex Multiplex Immunoassays (Bio-Rad Laboratories Inc., USA). 50 µl of the diluted samples were combined with 50 µl of the microparticle cocktail (containing cytokines/chemokines per well of a 96-well plate) and incubated for 1 h at room temperature while shaking at 850 rpm. The plates were rinsed three times, then 50 µl of diluted Streptavidin-PE was added to each well, and it was incubated for 10 min at room temperature. 125 µl of assay buffer was added, and the wells were shaken for 30 s at room temperature (850 rpm) prior to being read with the Bio-Plex® 200 System (Bio-Rad Laboratories, Inc.). In the present study, we employed the concentrations of the different cytokines/chemokine/growth factors in the statistical analyses. Concentrations that were not detectable (i.e., the lower limit out of range values) were censored and we imputed these censored data with the sensitivity values of each assay. Cytokines/chemokines/growth factors with concentration levels that were >45% out of range were excluded from the analysis concerning the solitary analytes. Nevertheless, we also computed different immune profiles (see Introduction), including M1, Th-1, Th-2, IRS, and CIRS profiles, and all analytes (even those with <45% detectable concentrations) may be used toward that end [30, 36], except those with <7% detectable concentrations (GM-CSF, IL-2, IL-3, IL-5, IL-15, and β-NGF). The reason is that even a smaller number of measurable concentrations (> more than 12) may contribute to the immune profiles. Electronic Supplementary File (ESF), Table 1 lists all cytokine/chemokines/growth factors measured here, as well as their names, gene IDs and % measurable analytes (> lower limit of out-of-range concentration). ESF, Table 2 shows how we constructed M1, Th-1, Th-2, IRS, and CIRS immune profiles, based on our previous publications [30, 36, 37] Unfortunately, we were unable to construct the Th-17 profile because its major players, IL-6 and IL-17, were not measurable in many blood samples. We also computed the ratio z transformation of IL-12p70 - z IL-12p40, because IL-12 signaling via IL-12p70 may be inhibited via IL-12p40 [38].
Statistical analysis
Using Pearson’s product-moment correlation coefficients, correlations between variables were analyzed. The analysis of contingency tables was used to compare variables depending on their categories (Chi-square tests). Analysis of variance was employed to examine the differences in continuous variables across the 4 study groups (controls (C), MA use, MA dependence, and MAP). We used univariate generalized linear models (considering the impacts of gender, age, BMI, current tobacco use, and alcohol use disorder) to examine the associations among these groups and cytokines and immune profiles. The Levene test for equality of variances was checked to ascertain that the variances of the study samples are equal. At a significance level of p < 0.05, pairwise comparisons of group means were conducted to detect differences between the four study groups. We did not use p-corrections for false discovery rate, because it is well established that the cytokines/chemokines/growth factors measured here act in a tight network and show strongly integrated responses [30]. Binary logistic regression analysis was performed to delineate the most important predictors of MAP versus MA use + MA dependence, and MA dependence versus MA use. Using the overfit criterion as entry and/or removal criterion (maximum effects number set at 5) we performed forward stepwise automatic linear modeling analyses (allowing for the confounders) and consequently examined the model using a manual regression analysis. We checked the residual distributions of the final model, the variance inflation factor and tolerance to identify any collinearity or multicollinearity concerns, and heteroskedasticity using the White and modified Breusch-Pagan homoscedasticity tests. We calculated the partial regression analysis of immune data on clinical data, model statistics (F, df, and p values, total variance explained, which was used as effect size), and the standardized β coefficients, t statistics (with exact p-value) for each predictor. The significance threshold of all statistical analyses was calculated using two-tailed tests with a value of 0.05. Where needed, the cytokines/chemokines/growth factors were introduced in the statistical analyses after transformations, including log10, fractional rank-based normal transformations, and Winsorization. We used principal component analysis (PCA) as a feature reduction method, and the first PC was considered adequate when the variance explained (VE) in the variables was >50%, and all have a loading >0.66, the factoriability of the correlation matrix using the Kaiser-Meyer-Olkin (KMO) Measure of Sampling Adequacy, the Bartlett’s sphericity and the anti-image correlation matrix were accurate. All the statistical studies were conducted using IBM, SPSS windows version 28. A priori sample size calculation was conducted using G*Power 3.1.9.4; for an analysis of covariance with 5 covariates, effect size of 0.27, alpha = 0.05 (two tailed), power = 0.8, and 4 groups the sample size should be at least 154.
Results
Demographics and clinical data in the four study groups
To examine differences in demographic data between the study groups we used analysis of variance and analysis of contingency tables. Table 1 shows the sociodemographic and clinical data of the 4 study groups. There were no significant differences in the sex ratio among groups, although there were some differences in age, BMI, and marital status between the groups. Duration of heaviest MA use was significantly greater in MA dependence than in MAP and MA use. The MA daily amount of heaviest MA use was significantly different between the three groups are increased from MA use to MA dependence to MAP. The DSM criteria number and MA dependence severity were significantly higher in the MAP and MA dependence groups than in the MA use group. The severity of MA dependence and the DSM-IV number of dependence criteria were significantly higher in MA dependence and MAP than in those with MA use. There were significant differences in lifetime, but not current, tobacco and cannabis use among the four study groups.
Lowered immune profiles in MA use, MA dependence, and MAP
We performed PCA, which is a feature reduction method, to reduce the various immune profiles into one construct, which reflects a generalized index of immune function. Using PCA, we were able to extract one PC from M1, Th-1, Th-2, IRS, CIRS, chemokine and growth factor profiles (VE = 84.7%, all loadings >0.878, KMO = 0.896, Bartlett’s test: χ2 = 1652.808, df = 21, p < 0.001), labeled PC_immune. The latter is, therefore, an overall index of immune functions. These results further show that no p-correction for false discovery rate may be used as all immune responses are strongly interrelated [30].
To examine differences in the immune profiles between the study groups we used analysis of variance. Table 2 shows the immune profiles of the four study groups. All the cytokine profiles, including PC_immune, were significantly different between the study groups (p < 0.001). The MAP group had the lowest M1, Th-1, Th-2, IRS, CIRS, chemokine, growth factor, and PC_immune scores but higher IL1270 - IL2040 scores as compared with the three other groups. In addition, MA dependence and/or MA use had lower z-scores of M1, Th-1, Th-2, IRS, CIRS, chemokine, growth factor, and PC_immune scores than controls. The PC_immune score was lower in MAP than in all other subjects. The IL1270 - IL2040 scores were higher in patients than controls. A post-hoc analysis to compute the achieved power for the least significant comparison in Table 2 (IRS/CIRS), showed that the achieved power (at α = 0.05, 4 groups, 5 covariates, n = 174) was 0.987. The achieved power for all other comparisons was 1.0. ESF, Figures S1–S36 show the levels of the measurable cytokines/chemokines/growth factors in controls, MA-use, MA dependence, and MAP.
All in all, the results show significant differences in immune profiles between patients who use MA and controls, and significant decreases in the generalized immune function index in patients who use MA and especially in those with MA.
Intercorrelation matrix
To examine the associations between MA use features and immune profiles we computed the correlation matrix (based on Pearson’s product moment correlation coefficients) among both sets of data. Table 3 shows the associations between MA use and dependence features and immune profiles. The MA dose was significantly and negatively correlated with the M1, Th-1, Th-2, IRS, CIRS, chemokine, growth factor, and PC_immune profiles, and positively with IL1270 - IL2040 and the IRS/CIRS ratio. The duration of MA use showed an inverse association with the growth factor profile. The severity of MA dependence was significantly associated with M1, Th-1, Th-2, IRS, CIRS, chemokine, growth factor, and PC_immune profiles, with a positive correlation with IL1270 - IL2040 and the IRS/CIRS ratio.
All in all, the results show that MA amount of use and dependency severity are associated with all immune profiles, including the generalized immune function index.
The general immune profile is predicted by MA dose and dependency criteria
To examine the associations between PC_immune and important clinical data, including MA dose and dependency criteria, we used multiple regression analysis with PC_immune as the dependent variable and the clinical data as explanatory (independent) variables). Table 4 shows two different regression models predicting PC_immune. The first model was performed using MA features only and shows that PC_immune is significantly and inversely predicted by the number of dependency criteria and MA dose. Figure 1 shows the partial regression of PC_immune on MA dose. In addition, we checked whether the association between PC_immune and the 4 diagnostic groups (controls and the three MA groups) was affected by alcohol dependence or cannabis features. Regression #2 shows that PC_immune is associated with the 4 diagnostic groups and alcohol dependence, indicating that the groups and alcohol dependence have independent effects. Cannabis features did not have any effects on PC_immune.
All in all, we found that the number of dependency criteria and MA dose were associated with suppressed immune functions.
MAP and MA dependence are predicted by immune data
To delineate the most important immune profiles or cytokine/chemokines/growth factors that are associated with MA use, MA dependence, and MAP, we performed binary regression analyses with these groups (present versus not present) as dependent variables and the immune data as explanatory variables (while allowing for the effects of age, sex, etc). Table 5 shows the results of these logistic binary regression analyses with MAP, MA dependence, and MA use as dependent variables. Binary logistic regression #1 shows that MAP (reference group = MA use + MA dependence) is associated with lowered CIRS, increased MA dose, number of DSM-IV MA dependence criteria, and alcohol dependence (χ2 = 60.69, df = 2, p < 0.001, correctly classified subjects: 82.5%, Nagelkerke = 0.492). Regression #2 shows that MAP (reference group = MA use + MA dependence) was strongly associated with Th-1 and CIRS profiles (inversely) and CCL5 and IL-1α (positively) (χ2 = 96.41, df = 4, p < 0.001, correctly classified subjects: 87.3%, Nagelkerke=0.698). Regression #3 shows an alternative prediction of MAP and that CIRS (inversely), IL1270 - IL2040 and IL-1α (positively) are significant explanatory variables (χ2 = 103.4, df = 3, p < 0.001, correctly classified cases: 86.7%, Nagelkerke = 0.667). Regression #4 examined the prediction of MAP versus all other patients and controls and found that the same variables as shown in regression #2 predicted MAP (χ2 = 116.812, df = 4, p < 0.001, correctly classified cases: 89.8%, Nagelkerke = 0.726). Regression #5 shows that IRS/CIRS was the single best predictor of MAP (versus MA dependence) (positive correlation). Binary logistic regression #6 examines the prediction of MA dependence (MA use as reference group) and shows that MA dependence was associated with SCF (inversely) and IL-10 (positively) (χ2 = 107.362, df = 2, p < 0.001, correctly classified cases: 94.1%, Nagelkerke = 0.888). The last regression (#7) shows that the best discrimination of MA patients versus controls was obtained using CIRS (inversely) and CTACK + CCL11 + IL-1α (positively) as predictors (χ2 = 92.793, df = 2, p < 0.001, correctly classified cases: 93.1%, Nagelkerke = 0.672).
All in all, the results show that MA use, MA dependence and MAP are predicted by different immune profiles and selected cytokines.
Discussion
Immune profiles in MA use and dependence
The first major finding is that chronic MA use reduces the first factor extracted from all immunological profiles (M1, Th-1, Th-2, IRS, CIRS, chemokine, and growth factors), indicating widespread immunosuppression. These findings expand upon those of recent papers demonstrating the many immunosuppressive effects of MA. MA at pharmacological doses exerts immunosuppressive effects on antigen-presenting cells, including macrophages and dendritic cells, reduces T cell proliferative activity, and inhibits receptor-mediated phagocytosis of antibody particles and MHC class II antigen presentation by T cells [19, 39]. In addition, MA treatment of splenocytes has a substantial influence on antigen-induced proliferation and macrophage phagocytosis [40]. Two weeks of MA treatment inhibits stimulated lymphoproliferative responses in mice [20]. In rodent models, injection of MA reduces the number of T lymphocytes in the spleen, including CD4 and CD8 cells, and the number of macrophages, dendritic cells, and natural killer cells [21]. Furthermore, treatment of MA lowers the production of Th-1 cytokines, including IFN-γ, and IL-2, but has no effect on the production of IL-6 and IL-4 [18].
Importantly, the immunosuppression in individuals with MA use is strongly associated with increasing doses of MA (but not the duration of the heaviest MA use) and with the severity of MA dependence. This indicates that MA has a strong suppressant effect on IRS and CIRS products. Overall, our data on M1 and Th-1 profiles are consistent with animal studies showing decreased M1 and Th-1 activity following MA exposure. Despite this, our findings indicate that chronic MA use additionally suppresses Th-2 and CIRS profiles, as well as the chemokine and growth factor subnetworks. This is important, as the latter subnetwork is intimately connected with the cytokine-chemokine network [41]. In addition, we discovered that prolonged MA use lowers the levels of several cytokines/chemokines/growth factors, such as FGF, GRO, IL-1β, sIL-1RA, sIL-2R, IL-4, IL-8, IL-12p40, IL-13, IL-18, LIF, CCL7 (MCP3), CSF, MIF, CXCL9 (MIG), SCF, and TRAIL.
Obviously, such a general decrease in immune processes may be accompanied by dysfunctions in host immunity, as observed in persons who used MA, resulting in greater susceptibility to acquire new infections and a worsening of infectious diseases, including hepatitis and AIDS [42].
Factors explaining MA-induced immunosuppression
Several variables may account for the significant immunosuppression generated by persistent MA use. First, MA may interfere with the cell cycle machinery, thereby preventing T cell proliferation and a proper adaptive immune response [19]. MA may reduce T cell cycle gene expression, including cyclin E, CDK2, and E2F, resulting in a prolonged G1/S phase transition [19]. Second, injection of MA causes apoptosis in macrophages and T cells [43, 44]. Third, MA-stimulated catecholamine turnover may suppress the production of IL-6, TNF-α, and sIL-1RA, therefore influencing the IRS and CIRS [45]. Fourth, exposure to MA, a weak base, may interfere with the pH maintenance of the more acidic organelles, which govern protein breakdown and surface receptor expression [39, 40]. The effects of MA on the pH gradient may impair acidic organelles inside immune cells, MHC class II antigen processing, antigen presentation by dendritic cells to T cells, and ultimately the immunological response [39, 40]. Fifth, MA exposure may induce T cell mRNA expression of the trace amine-associated receptor 1 (TAAR1) [46], which is involved in rheostasis, homeostasis, and cAMP signaling and interacts with monoamine turnover [47, 48]. This indicates that MA-induced TAAR1 expression may downregulate Th-1-like cytokines such as IL-2 [46].
Signs of immune activation in MA use and dependence
Our second major observation is that persistent MA use is also associated with indicators of immunological activation. Various analytes were significantly elevated, such as CCL27 (CTACK) in subjects who used MA, and IL-1α, IL-12p70, and CCL5 (RANTES) in subjects with MA dependence. In addition, not all analytes were lowered in persons who used MA, including CCL11 (eotaxin), IL-9, IL-16, CXCL10 (IP10), CCL3 (MIP1α), CCL4 (MIP1β), TNF-α and TNF-β (in both MA use and dependence), and G-CSF, HGF, CCL2 (MCP1), PDGF-BB, CXCL12 (SDF-1α), IL-9, and IL-10 (in MA dependence). These findings expand on the findings of prior investigations. For example, administration of MA to microglia markedly elevates IL-12p70 [49], and MA exposure substantially increases IL-12 levels in the kidney and liver of mice [50]. Moreover, MA induces T cell proliferation in the brain via upregulating IL-15 in astrocytes [51]. Macaques afflicted with Simian Immunodeficiency Virus may produce more CCL5 if they are treated with MA [52]. MA exposure elevates TNF-α levels in selected mouse brain areas, which are partially mediated by redox processes [53]. Another research paper showed that MA exposure may considerably boost TNF-α production even in the presence of Th-1 suppression [18].
Considering that people who use MA have a suppressed immune system, the significant or relative increase in some cytokines/growth factors may be relevant. This is further supported by a higher IRS/CIRS ratio in individuals who use MA compared to controls. Hence, despite the overall immunosuppression, there is a relative rise in pro-inflammatory cytokines/chemokines, as well as growth factors (G-CSF, PDGF-BB, and CXCL12 or SDF-1α) that may fuel the synthesis of these cytokines/chemokines. In fact, a combination of a decreased CIRS profile and an increase in pro-inflammatory cytokines/chemokines (CCL27 + CCL11 + IL-1α) is the best biomarker profile of MA use. It is noteworthy to note that CD4+ cells, which are inhibited during MA use, also exhibit indicators of activation, such as increased expression of CD150 and CD226 [21].
All in all, not only generalized immunosuppression but also signs of immune activation are important to understand the pathophysiology of MA use. For example, CCL27 (CTACK or cutaneous T cell-attracting chemokine) is a cutaneous chemokine that attracts lymphocyte-associated antigen (CLA)+ memory cells, which are implicated in cutaneous inflammatory lesions [54]. As such, increased CCL27 may perhaps play a role in MA-associated skin damage, including itching (due to meth mites), lesions, excoriations, and ulcers [55]. IL-1α, IL-12p70, and CCL5 are pro-inflammatory cytokines/chemokines that may contribute to the inflammatory pathways and the accompanying brain dysfunctions [42] as well as peripheral inflammation-linked disorders, including cardiovascular diseases, which frequently occur in persons who use MA [12]. CCL11 levels may be associated with greater anxiety, depression, and cognitive deficits among individuals who use MA [26].
Moreover, the transition from moderate MA use to severe MA dependency (greater dosage and longer duration of MA use) is characterized by decreased SCF (stem cell factor) and increased IL-10 levels. SCF is a cytokine that plays a major role in hematopoiesis and spermatogenesis; consequently, decreased SCF may contribute to the decreased lymphoproliferative responses to MA (see discussion above) and the decreased sperm count, motility, and morphology in persons who use MA [56]. In response to MA exposure, IL-10 levels are elevated in the plasma of both mice and humans [50, 57]. Such a non-protective immunological response to IL-10 may restrict T cell proliferation even further.
The immune profile of MAP
The third major finding of the study is that MAP is characterized by a) extremely reduced levels of M1, Th-1, Th-2, IRS, chemokine, and growth factor profiles, but especially the CIRS profile; b) an increase in a few selected cytokines/chemokines with systemic effects, including CCL5 and IL-1α, and IL-12p70 signaling; and c) an increased IRS/CIRS ratio.
IL-1α produced from peripheral blood may cross the BBB and get access to cortical brain cells [58]. The production of IL-1α is mediated by elevated levels of damage-associated molecular patterns, necrosis, and necroptotic stimuli during sterile inflammation [59, 60]. Moreover, MA exposure may enhance the production of HMGB1, a significant DAMP, that contributes to neuroinflammation [61] and may promote necrosis/necroptosis via dopaminergic, oxidative stress, and AGE-RAGE pathways [62, 63]. Additionally, peripheral and central IL-1α elevations play a significant role in CNS inflammation [60]. Interestingly, IL-1α may generate CCL5, another cytokine related with MAP [60]. As the levels of IL-1RA (which regulates IL-1-signaling; [64] are significantly reduced in MAP, the effects of IL-1α may become more prominent. CCL5 (RANTES) is a chemokine that may cross the BBB and reach the brain parenchyma, and CCL5 and its receptor are expressed in glia, whilst microglia and astrocytes are capable of producing CCL5 [65,66,67,68]. Elevated CCL5 expression in the central nervous system is associated with increased neuroinflammation, cortical synaptic excitability, and hyperalgesia, and is implicated in neuroinflammatory and neurodegenerative disease [65, 69, 70]. In addition, enhanced IL-12 signaling contributes to neuroinflammation and neurodegeneration [71,72,73], and IL-12 [73] is, as CCL27, implicated in inflammatory skin lesions.
Despite the fact that it is now well established that schizophrenia is an immunological condition and that first-episode schizophrenia and deficit schizophrenia are associated with IRS and CIRS activation [29, 74, 75], there are relatively few reports on the cytokine network in MAP. Yang et al. (2020) found that IL-6 and IL-8 were elevated in MAP patients and that sIL-2R was negatively correlated with positive symptoms [23]. Psychosis in amphetamine-dependent women during early cessation is associated with increases in IL-10, TNF-α, and IL-5 (the latter cytokine was not measurable in our research) [76]. MAP is also accompanied by neuroinflammatory aberrations in the AAC-thalamus circuits [77].
Limitations
The study had several limitations that should be addressed. First, because the study is a case-control study, it is not possible to draw a firm conclusion about the causality of the associations. Second, self-reported drug use amounts are more difficult to interpret, and therefore we cannot conclude that MA may have “dose-dependent immunosuppressant effects.” Third, our MA dependence group included patients with high levels of dependence, reflecting a severe phenotype. Fourth, while we examined the effects of alcohol and cannabis use, no patients with other drug use were included, such as people who use opioids. Fifth, our results on the immune profiles in MA use, dependence, and MAP, deserve cross-validation in other countries and cultures. Sixth, the BioRad BioPlex kit is designed for use with plasma or serum, while we used whole blood. This is a deviation from the manufacturer’s recommended protocol. Nevertheless, in our hands, no major differences could be established between serum, plasma, and whole blood concentrations of cytokines. It should be noted that our observation that active MA use is accompanied by immunosuppression and increased levels of selected pro-inflammatory cytokines was made in peripheral blood. CNS samples can show more pro-inflammatory effects of MA during active use as well as in early abstinence.
Another question is whether immune markers associated with different stages of MA use (use, dependence, and MAP) could serve as diagnostic or prognostic indicators. Nevertheless, we would never recommend using cytokine/chemokines/growth factors as diagnostic biomarkers for MA use or MAP, because Elisa and Multiplex analyses have a large between-run analytical error especially at the low concentration levels as those detected in our study. Another clinical question is what types of interventions would be needed for people with MAP and those with MA dependency. One could of course argue that treatments which augment immune functions could counteract the immunosuppressive effects of MA. However, because immune activation may induce psychosis, it may not be the best strategy to activate the immune system. Future research should first examine if the MA-induced immunosuppression improves over time and additionally, how long it would take exactly before the immune system normalizes or improves.
Conclusions
Our results are summarized in Fig. 2. Long-term MA usage alters the immune system’s balance, leading to extensive immunosuppression (including increased IL-10 levels and decreased SCF levels) and the activation of certain cytokines, chemokines, and growth factors (including CCL27, IL-1α, and CCL11). Increases in IL-1α - CCL5 signaling, the IRS/CIRS ratio, and the severity of MA dependency all appear to work in concert to determine the onset of MAP. The results demonstrate that, despite the pervasive immunosuppression, elevated IL-1α - CCL5 signaling and maybe IL-12p70 signaling may be responsible for MAP.
This figure shows the transitions from healthy controls to MA use, from MA use to MA dependence, and from the latter to MAP that are associated with changes in immune factors. Immune markers above the arrow are increased, under the arrow: decreased. HC healthy controls, MA methamphetamine, MAP MA-induced psychosis, IRS immune-inflammatory responses system, CIRS compensatory immunoregulatory system, IL interleukin, Th T helper, SCF stem cell factor.
Data availability
The corresponding author (MM) will make the files used in the current study available upon receipt of an appropriate request.
References
Paulus MP, Stewart JL. Neurobiology, clinical presentation, and treatment of methamphetamine use disorder: a review. JAMA psychiatry. 2020;77:959–66.
Stoneberg DM, Shukla RK, Magness MB. Global methamphetamine trends: an evolving problem. Int Crim Justice Rev. 2018;28:136–61.
UNODC. World Drug Report (2022) (Sales No. E.22.XI.8): Retrieved from United Nations publication; 2022.
UNODC. World Drug Report (2021) (Sales No. E.21.XI.8): Retrieved from United Nations publication; 2021.
Kalayasiri R. Addiction in Thailand. Neuropathology of drug addictions and substance misuse. 2: London: Academic Press; 2016. p. 1094–100.
Giorgetti R, Tagliabracci A, Schifano F, Zaami S, Marinelli E, Busardò FP. When “chems” meet sex: a rising phenomenon called “chemsex”. Curr Neuropharmacol. 2017;15:762–70.
Werb D, Hayashi K, Fairbairn N, Kaplan K, Suwannawong P, Lai C, et al. Drug use patterns among Thai illicit drug injectors amidst increased police presence. Subst Abus Treat Prev Policy. 2009;4:1–5.
Cruickshank CC, Dyer KR. A review of the clinical pharmacology of methamphetamine. Addiction 2009;104:1085–99.
Zeng L, Tao Y, Hou W, Zong L, Yu L. Electro-acupuncture improves psychiatric symptoms, anxiety and depression in methamphetamine addicts during abstinence: a randomized controlled trial. Medicine. 2018;97:e11905.
Chiang M, Lombardi D, Du J, Makrum U, Sitthichai R, Harrington A, et al. Methamphetamine‐associated psychosis: clinical presentation, biological basis, and treatment options. Hum Psychopharmacol: Clin Exp. 2019;34:e2710.
Voce A, Calabria B, Burns R, Castle D, McKetin R. A systematic review of the symptom profile and course of methamphetamine-associated psychosis: substance use and misuse. Subst Use Misuse. 2019;54:549–59.
Al-Hakeim HK, Altufaili MF, Almulla AF, Moustafa SR, Maes M. Increased lipid peroxidation and lowered antioxidant defenses predict methamphetamine induced psychosis. Cells 2022;11:3694.
McKetin R, McLaren J, Lubman DI, Hides L. The prevalence of psychotic symptoms among methamphetamine users. Addiction 2006;101:1473–8.
Kalayasiri R, Verachai V, Gelernter J, Mutirangura A, Malison RT. Clinical features of methamphetamine‐induced paranoia and preliminary genetic association with DBH‐1021 C→ T in a T hai treatment cohort. Addiction 2014;109:965–76.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders, fifth edition (DSM-5). Arlington, VA: American Psychiatric Publishing; 2013.
Kittirattanapaiboon P, Mahatnirunkul S, Booncharoen H, Thummawomg P, Dumrongchai U, Chutha W. Long‐term outcomes in methamphetamine psychosis patients after first hospitalisation. Drug Alcohol Rev. 2010;29:456–61.
Shi S, Chen T, Zhao M. The crosstalk between neurons and glia in methamphetamine-induced neuroinflammation. Neurochem Res. 2022;47:872–84.
Yu Q, Zhang D, Walston M, Zhang J, Liu Y, Watson RR. Chronic methamphetamine exposure alters immune function in normal and retrovirus-infected mice. Int Immunopharmacol. 2002;2:951–62.
Potula R, Haldar B, Cenna JM, Sriram U, Fan S. Methamphetamine alters T cell cycle entry and progression: role in immune dysfunction. Cell Death Discov. 2018;4:44.
In S-W, Son E-W, Rhee D-K, Pyo S. Methamphetamine administration produces immunomodulation in mice. J Toxicol Environ Health, Part A. 2005;68:2133–45.
Harms R, Morsey B, Boyer CW, Fox HS, Sarvetnick N. Methamphetamine administration targets multiple immune subsets and induces phenotypic alterations suggestive of immunosuppression. PLoS ONE. 2012;7:e49897.
Carter K, Finnon Y, Daeid NN, Robson D, Waddell R. The effect of nitrostyrene on cell proliferation and macrophage immune responses. Immunopharmacol Immunotoxicol. 2002;24:187–97.
Yang X, Zhao H, Liu X, Xie Q, Zhou X, Deng Q, et al. The relationship between serum cytokine levels and the degree of psychosis and cognitive impairment in patients with methamphetamine-associated psychosis in Chinese patients. Front Psychiatry. 2020;11:594766.
Prakash MD, Tangalakis K, Antonipillai J, Stojanovska L, Nurgali K, Apostolopoulos V. Methamphetamine: effects on the brain, gut and immune system. Pharmacol Res. 2017;120:60–7.
Shi WL, Zhao X, Liu ZM, Zhang M, Zhou BY, Pu XP. Serum proteomics of methamphetamine addicts and up-regulation of complement factor H related to methamphetamine addiction. Neurosci Lett. 2012;525:23–8.
Huckans M, Fuller BE, Chalker AL, Adams M, Loftis JM. Plasma inflammatory factors are associated with anxiety, depression, and cognitive problems in adults with and without methamphetamine dependence: an exploratory protein array study. Front Psychiatry. 2015;6:178.
Pottiez G, Jagadish T, Yu F, Letendre S, Ellis R, Duarte NA, et al. Plasma proteomic profiling in HIV-1 infected methamphetamine abusers. PLoS One. 2012;7:e31031.
Ikeda M, Okahisa Y, Aleksic B, Won M, Kondo N, Naruse N, et al. Evidence for shared genetic risk between methamphetamine-induced psychosis and schizophrenia. Neuropsychopharmacology 2013;38:1864–70.
Maes M, Meltzer H, Bosmans E. Immune‐inflammatory markers in schizophrenia: comparison to normal controls and effects of clozapine. Acta Psychiatr Scandinavica. 1994;89:346–51.
Maes M, Plaimas K, Suratanee A, Noto C, Kanchanatawan B. First episode psychosis and schizophrenia are systemic neuro-immune disorders triggered by a biotic stimulus in individuals with reduced immune regulation and neuroprotection. Cells 2021;10:2929.
Roomruangwong C, Noto C, Kanchanatawan B, Anderson G, Kubera M, Carvalho AF, et al. The role of aberrations in the immune-inflammatory response system (IRS) and the compensatory immune-regulatory reflex system (CIRS) in different phenotypes of schizophrenia: the IRS-CIRS theory of schizophrenia. Mol Neurobiol. 2020;57:778–97.
Song X-Q, Lv L-X, Li W-Q, Hao Y-H, Zhao J-P. The interaction of nuclear factor-kappa B and cytokines is associated with schizophrenia. Biol Psychiatry. 2009;65:481–8.
Monji A, Kato T, Kanba S. Cytokines and schizophrenia: microglia hypothesis of schizophrenia. Psychiatry Clin Neurosci. 2009;63:257–65.
Malison RT, Kalayasiri R, Sanichwankul K, Sughondhabirom A, Mutirangura A, Pittman B, et al. Inter-rater reliability and concurrent validity of DSM-IV opioid dependence in a Hmong isolate using the Thai version of the Semi-Structured Assessment for Drug Dependence and Alcoholism (SSADDA). Addict Behav. 2011;36:156–60.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, D.C.: American Psychiatric Association; 2000.
Thisayakorn P, Thipakorn Y, Tantavisut S, Sirivichayakul S, Maes M. Delirium due to hip fracture is associated with activated immune-inflammatory pathways and a reduction in negative immunoregulatory mechanisms. BMC Psychiatry. 2022;22:369.
Maes M, Carvalho AF. The compensatory immune-regulatory reflex system (CIRS) in depression and bipolar disorder. Mol Neurobiol. 2018;55:8885–903.
Zagozdzon R, Lasek W. Biology of IL-12. In: Lasek W, Zagozdzon R, editors. Interleukin 12: antitumor activity and immunotherapeutic potential in oncology. Cham: Springer International Publishing; 2016. p. 1–19.
Tallóczy Z, Martinez J, Joset D, Ray Y, Gácser A, Toussi S, et al. Methamphetamine inhibits antigen processing, presentation, and phagocytosis. PLoS Pathog. 2008;4:e28.
Martinez LR, Mihu MR, Gácser A, Santambrogio L, Nosanchuk JD. Methamphetamine enhances histoplasmosis by immunosuppression of the host. J Infect Dis. 2009;200:131–41.
Maes M, Rachayon M, Jirakran K, Sodsai P, Klinchanhom S, Debnath M, et al. Adverse childhood experiences predict the phenome of affective disorders and these effects are mediated by staging, neuroimmunotoxic and growth factor profiles. Cells 2022;11:1564.
Salamanca SA, Sorrentino EE, Nosanchuk JD, Martinez LR. Impact of methamphetamine on infection and immunity. Front Neurosci. 2015;8:445.
Potula R, Hawkins BJ, Cenna JM, Fan S, Dykstra H, Ramirez SH, et al. Methamphetamine causes mitrochondrial oxidative damage in human T lymphocytes leading to functional impairment. J Immunol. 2010;185:2867–76.
Aslanyan L, Ekhar VV, DeLeon-Rodriguez CM, Martinez LR. Capsular specific IgM enhances complement-mediated phagocytosis and killing of Cryptococcus neoformans by methamphetamine-treated J774. 16 macrophage-like cells. Int Immunopharmacol. 2017;49:77–84.
Maes M, Lin A, Kenis G, Egyed B, Bosmans E. The effects of noradrenaline and alpha-2 adrenoceptor agents on the production of monocytic products. Psychiatry Res. 2000;96:245–53.
Sriram U, Cenna JM, Haldar B, Fernandes NC, Razmpour R, Fan S, et al. Methamphetamine induces trace amine‐associated receptor 1 (TAAR1) expression in human T lymphocytes: role in immunomodulation. J Leukoc Biol. 2016;99:213–23.
Dodd S, Carvalho AF, Puri BK, Maes M, Bortolasci CC, Morris G, et al. Trace amine-associated receptor 1 (TAAR1): a new drug target for psychiatry? Neurosci Biobehav Rev. 2021;120:537–41.
Maes M, Kenis G, Kubera M, De Baets M, Steinbusch H, Bosmans E. The negative immunoregulatory effects of fluoxetine in relation to the cAMP-dependent PKA pathway. Int Immunopharmacol. 2005;5:609–18.
Vargas AM, Rivera-Rodriguez DE, Martinez LR. Methamphetamine alters the TLR4 signaling pathway, NF-κB activation, and pro-inflammatory cytokine production in LPS-challenged NR-9460 microglia-like cells. Mol Immunol. 2020;121:159–66.
Peerzada H, Gandhi JA, Guimaraes AJ, Nosanchuk JD, Martinez LR. Methamphetamine administration modifies leukocyte proliferation and cytokine production in murine tissues. Immunobiology 2013;218:1063–8.
Bortell N, Basova L, Semenova S, Fox HS, Ravasi T, Marcondes MCG. Astrocyte-specific overexpressed gene signatures in response to methamphetamine exposure in vitro. J Neuroinflammation. 2017;14:1–20.
Najera JA, Bustamante EA, Bortell N, Morsey B, Fox HS, Ravasi T, et al. Methamphetamine abuse affects gene expression in brain-derived microglia of SIV-infected macaques to enhance inflammation and promote virus targets. BMC Immunol. 2016;17:1–19.
Flora G, Lee YW, Nath A, Maragos W, Hennig B, Toborek M. Methamphetamine-induced TNF-α gene expression and activation of AP-1 in discrete regions of mouse brain: potential role of reactive oxygen intermediates and lipid peroxidation. Neuromolecular Med. 2002;2:71–85.
Morales J, Homey B, Vicari AP, Hudak S, Oldham E, Hedrick J, et al. CTACK, a skin-associated chemokine that preferentially attracts skin-homing memory T cells. Proc Natl Acad Sci USA. 1999;96:14470–5.
Banyan Treatment Centers. Taking care of your skin after meth addiction. Chicago 2021. Available from: https://www.banyantreatmentcenter.com.
Allaeian Jahromi Z, Meshkibaf MH, Naghdi M, Vahdati A, Makoolati Z. Methamphetamine downregulates the sperm-specific calcium channels involved in sperm motility in rats. ACS omega. 2022;7:5190–6.
Loftis JM, Choi D, Hoffman W, Huckans MS. Methamphetamine causes persistent immune dysregulation: a cross-species, translational report. Neurotox Res. 2011;20:59–68.
Banks WA, Ortiz L, Plotkin S, Kastin A. Human interleukin (IL) 1 alpha, murine IL-1 alpha and murine IL-1 beta are transported from blood to brain in the mouse by a shared saturable mechanism. J Pharmacol Exp Ther. 1991;259:988–96.
Banks WA, Kastin AJ, Gutierrez EG. Interleukin-1α in blood has direct access to cortical brain cells. Neurosci Lett. 1993;163:41–4.
Brough D, Denes A. Interleukin‐1α and brain inflammation. IUBMB Life. 2015;67:323–30.
Frank MG, Adhikary S, Sobesky JL, Weber MD, Watkins LR, Maier SF. The danger-associated molecular pattern HMGB1 mediates the neuroinflammatory effects of methamphetamine. Brain Behav Immun. 2016;51:99–108.
Al-Hakeim H, Altufaili M, Alhaideri A, Almulla AF, Moustafa S, Maes M. Increased AGE-RAGE axis stress in methamphetamine (MA) abuse and MA-induced psychosis: associations with oxidative stress and increased atherogenicity. Addict Biol. 2023;28:e13333.
Davidson C, Gow AJ, Lee TH, Ellinwood EH. Methamphetamine neurotoxicity: necrotic and apoptotic mechanisms and relevance to human abuse and treatment. Brain Res Rev. 2001;36:1–22.
Maes M, Song C, Yirmiya R. Targeting IL-1 in depression. Expert Opin Ther Targets. 2012;16:1097–112.
Bajetto A, Bonavia R, Barbero S, Schettini G. Characterization of chemokines and their receptors in the central nervous system: physiopathological implications. J Neurochemistry. 2002;82:1311–29.
Dorf ME, Berman MA, Tanabe S, Heesen M, Luo Y. Astrocytes express functional chemokine receptors. J Neuroimmunol. 2000;111:109–21.
Guo H, Jin YX, Ishikawa M, Huang YM, van der Meide PH, Link H, et al. Regulation of beta-chemokine mRNA expression in adult rat astrocytes by lipopolysaccharide, proinflammatory and immunoregulatory cytokines. Scand J Immunol. 1998;48:502–8.
Quaranta DV, Weaver RR, Baumann KK, Fujimoto T, Williams LM, Kim HC, et al. Transport of the proinflammatory chemokines CC motif chemokine ligand 2 (MCP-1) and CC motif chemokine ligand 5 (RANTES) across the intact mouse blood-brain barrier is inhibited by heparin and eprodisate and increased with systemic inflammation. J Pharmacol Exp Ther. 2023;384:205–23.
Benamar K, Geller EB, Adler MW. Elevated level of the proinflammatory chemokine, RANTES/CCL5, in the periaqueductal grey causes hyperalgesia in rats. Eur J Pharmacol. 2008;592:93–5.
Mori F, Nisticò R, Nicoletti CG, Zagaglia S, Mandolesi G, Piccinin S, et al. RANTES correlates with inflammatory activity and synaptic excitability in multiple sclerosis. Multiple Sclerosis. Journal 2016;22:1405–12.
Schneeberger S, Kim S, Eede P, Boltengagen A, Braeuning C, Andreadou M, et al. The neuroinflammatory interleukin-12 signaling pathway drives Alzheimer’s disease-like pathology by perturbing oligodendrocyte survival and neuronal homeostasis. bioRxiv. 2021. https://doi.org/10.1101/2021.04.25.441313.
Vom Berg J, Prokop S, Miller KR, Obst J, Kälin RE, Lopategui-Cabezas I, et al. Inhibition of IL-12/IL-23 signaling reduces Alzheimer’s disease-like pathology and cognitive decline. Nat Med. 2012;18:1812–9.
Turka LA, Goodman RE, Rutkowski JL, Sima AAF, Merry A, Mitra RS, et al. Interleukin 12: a potential link between nerve cells and the immune response in inflammatory disorders. Mol Med. 1995;1:690–9.
Maes M. Major neurocognitive psychosis: a novel schizophrenia endophenotype class that is based on machine learning and resembles Kraepelin’s and Bleuler’s conceptions. Acta Neuropsychiatrica. 2023;35:123–37.
Noto MN, Maes M, Nunes SOV, Ota VK, Rossaneis AC, Verri WA, et al. Activation of the immune-inflammatory response system and the compensatory immune-regulatory system in antipsychotic naive first episode psychosis. Eur Neuropsychopharmacol. 2019;29:416–31.
Kuo SC, Yeh YW, Chen CY, Huang CC, Ho PS, Liang CS, et al. Differential effect of the DRD3 genotype on inflammatory cytokine responses during abstinence in amphetamine-dependent women. Psychoneuroendocrinology 2018;97:37–46.
Burger A, Lindner M, Robertson F, Blake L, Williams K, Naude P, et al. Comparison of schizophrenia and methamphetamine-induced psychosis: a proton magnetic resonance spectroscopy and cytokine study. Authorea. 2023. https://doi.org/10.22541/au.167748329.98247006/v1.
Acknowledgements
The study is supported by the Centre for Addiction Studies, Department of Psychiatry, Faculty of Medicine, Chulalongkorn University, and the Thai Health Promotion Foundation. We would like to thank Thitima Duangsanit and Somruk Kongchai for facilitate the process of data recruitment.
Funding
The study is funded by the Centre for Addiction Studies, Department of Psychiatry, Faculty of Medicine, Chulalongkorn University, and the Thai Health Promotion Foundation to RK. The study is funded by the Thailand Science Research and Innovation Fund Chulalongkorn University (HEA663000016), and the Ratchadapiseksompotch Fund, Faculty of Medicine, Chulalongkorn University, grant number. RA66/016.
Author information
Authors and Affiliations
Contributions
RK carried out the current study’s design. The data was collected by ST, KD, and RK. The statistical analyses were conducted by MM. The first draft was written by MM and RK. Immune analyses were performed by SS. All authors gave their consent for submission of the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The project was approved by the Institutional Review Board of the Faculty of Medicine of Chulalongkorn University and PMNIDAT (No. 28/2565). All participants or their guardians gave written informed consent prior to participation.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kalayasiri, R., Dadwat, K., Thika, S. et al. Methamphetamine (MA) use and MA-induced psychosis are associated with increasing aberrations in the compensatory immunoregulatory system, interleukin-1α, and CCL5 levels. Transl Psychiatry 13, 361 (2023). https://doi.org/10.1038/s41398-023-02645-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-023-02645-6
- Springer Nature Limited