Abstract
Investigation of the neurobiology of depression in humans depends on animal models that attempt to mimic specific features of the human disorder. However, frequently-used paradigms based on social stress cannot be easily applied to female mice which has led to a large sex bias in preclinical studies of depression. Furthermore, most studies focus on one or only a few behavioral assessments, with time and practical considerations prohibiting a comprehensive evaluation. In this study, we demonstrate that predator stress effectively induced depression-like behaviors in both male and female mice. By comparing predator stress and social defeat models, we observed that the former elicited a higher level of behavioral despair and the latter elicited more robust social avoidance. Furthermore, the use of machine learning (ML)-based spontaneous behavioral classification can distinguish mice subjected to one type of stress from another, and from non-stressed mice. We show that related patterns of spontaneous behaviors correspond to depression status as measured by canonical depression-like behaviors, which illustrates that depression-like symptoms can be predicted by ML-classified behavior patterns. Overall, our study confirms that the predator stress induced phenotype in mice is a good reflection of several important aspects of depression in humans and illustrates that ML-supported analysis can simultaneously evaluate multiple behavioral alterations in different animal models of depression, providing a more unbiased and holistic approach for the study of neuropsychiatric disorders.
Similar content being viewed by others
Introduction
Central to most contemporary theories of depression is the notion that chronic stress is a major predisposing factor [1,2,3]. Social conflicts are considered a major source of stress for humans that contribute to the pathogenesis of depression [4, 5]. However, in most mammalian species, a number of different laboratory-based social stress paradigms involve hierarchies that are clearly defined with more pronounced and dominant in males than in females [6]. For example, social stress in rodents is mimicked using a chronic social defeat model (CSDS), which is based on the resident-intruder paradigm where the intruder eventually becomes subordinate to the unfamiliar territorial resident conspecific that defends its home cage [7]. A major drawback of this model is the difficulty of initiating a natural attack by the male territorial resident on female intruder mice. It is also for this reason, the vast majority of studies on depression involving social stress have been carried out using only male laboratory mice [7, 8]. Therefore, a better animal model of stress is needed so that mechanistic details of depression in females can be studied to the same degree as in males.
Predation is a major force behind natural selection that endowed animals with various innate and automatically activated defense behaviors and helped shape the evolution of nervous systems [9]. Although modern humans rarely become prey to other species, our defense system against predators may have been co-opted during the course of evolutionary history to also cope with social conflicts [10, 11]. The response to psychosocial threats in humans shares underlying neurocircuitry and physiology with the response to predators in rodents and primates [12,13,14,15,16,17]. The pathology of depression may involve common defense systems that evolved to deal with predators and social threats. Indeed, predator stress in rodents has been used as a potent stress stimulus to induce physiological and behavioral changes in male mice similar to those elicited by social threats, such as increased anxiety level [9]. However, it remains unclear whether chronic exposure to predator stress has the potential to induce depression-like behaviors in both male and female mice and replace sex-biased paradigms for future mechanistic studies and drug screening.
Canonical screening tests for evaluating depression-like behaviors in rodents, such as the tail suspension test and sucrose preference test are used to reflect the behavioral despair and anhedonia observed in human depression. However, the major drawbacks are that these tests provide a reductionist perspective of a complex disorder and that these behavioral changes lie on a continuum that cannot be easily sectionalized [18, 19]. On the other hand, automated behavioral analysis using ML has been applied to extract more complex behavioral patterns that can reflect an animal’s internal state. These behavioral patterns become altered after experimental manipulation or in disease models [20, 21]. Therefore, we aim to better annotate the overall ‘psychological state’ of the rodent using the Behavior Atlas (BeA) [22], a high-dimensional behavior mapping tool based on ML and automated 3D video capture.
In this study, we demonstrate that predator stress can induce depression-like behaviors in both male and female mice. Both predator stress and social stress models induced anhedonia in male mice, while the former elicited a higher level of behavioral despair and the latter elicited more robust social avoidance. Our results suggest that the CRS and CSDS models, while both sharing core characteristics of the human disorder, also have the potential to be applied during investigation of the divergent mechanisms underlying different depression subtypes. Furthermore, we used ML-assisted 3D video analysis of spontaneous behavioral patterns and compared these between the two types of stress. We also reveal the related patterns of spontaneous behaviors corresponding to the status measured by canonical depression-like behaviors. In addition, CRS-induced alterations in ML-observed spontaneous behaviors can be attenuated by the antidepressant fluoxetine. Our study illustrates that ML-supported analysis of behavioral fingerprints may enable us to gain a better understanding of an individual’s underlying mental state and can be particularly useful in the diagnosis and assessment of mental disorders.
Materials and methods
Animals
All experimental procedures were approved by the Animal Care and Use Committees at the Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences. 8-week-old male and female C57BL6/J mice, 8–10-week-old male Sprague-Dawley rats (Beijing Vital River), and 4–6-month-old CD-1 male retired breeder mice (SiPeiFu Beijing) were used, all under a 12-h light/dark cycle with ad libitum access to food and water.
Behavioral assays
Chronic predator stress (CRS) [11] and chronic social defeat stress (CSDS) [23] were performed to induce depression-like behavior in mice. The social interaction test (SI), sucrose preference test (SPT) and tail suspension test (TST) were used to asses depression-like behaviors, while the looming test was employed to evaluate innate defensive behaviors. The SI ratio was calculated as (time spent in the SI zone with a social target) / (time spent in the SI zone without a social target) [24]. Sucrose preference (%) was calculated as [sucrose solution consumed] / [sucrose + water solutions consumed] × 100 [24]. The time spent immobile was recorded by an observer blinded to experimental conditions in last 5 min of 6 min recording [25]. More details are provided in the supplementary information.
3D motion-capture system and behavior decomposition framework
The setup was similar to our previous study [22]. Briefly, cameras on the four sides of the apparatus synchronously record spontaneous behaviors of mice, then an ML-based method was used to automatically identify the behavioral phenotypes of mice. Unsupervised behavioral movement clusters were further recognized by supervised classification. Behavior fractions were calculated as the total time performing one type of behavior movement divided by the total time of all behavior movements, and a behavioral transition represents one type of behavior movement translated to another. More details are provided in the supplementary information.
Statistical analysis
Sample sizes were determined according to previous studies [22, 24]. All relevant data were included in the analysis and mice were tested once, and no individual replicates were excluded. Before hypothesis testing, normality (Shapiro–Wilk normality test) and homoscedasticity (F-test) were verified. Statistics were performed in Prism 8.0 (GraphPad Software). Data are expressed as means ± SEM. Post hoc significance values were set as *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001. See also Table S1 for details.
Results
Chronic predator stress induces depression-like behaviors
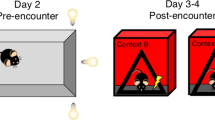
Our previous work showed that mice exposed to chronic predator stress induced by a rat (CRS) displayed sleep pattern changes similar to those seen in human depression [11]. We subjected male and female mice to 12 consecutive days of predator stress and subsequently evaluated depression-like behaviors that are considered to reflect the core symptoms of human depression. Futhermore, CSDS generates a variety of behavioral changes (anhedonia, social withdrawal, despair) which are thought to reflect many of the core symptoms observed in depressed humans [5, 26,27,28]. This CSDS paradigm has been used extensively in basic and preclinical research to study social defeat stress and depression. Therefore, we also subjected another group of male mice to CSDS and compared depression-like behaviors between the CRS and CSDS groups (Fig. 1A) [23, 29]. Following CRS or CSDS in C57BL/6 J male mice, we performed a sucrose preference test to evaluate interest in rewarding stimuli [30]. We found that the male CRS group (CRS-M) and male CSDS group (CSDS-M) exhibited a significantly lower sucrose preference than a male stress-naïve control group (Control-M in figures), suggesting that both CRS and CSDS can promote anhedonia-related behavior (Fig. 1B). Furthermore, female mice subjected to CRS (CRS-F) also exhibited a lower sucrose preference than female stress-naïve controls (Control-F) (Fig. 1B). All groups had similar total fluid intake (Fig. 1B). Next, we used a social interaction (SI) test to compare CRS- and CSDS-induced deficits in sociable behavior [31]. Compared to Control-M mice, CRS-M and CSDS-M mice had lower SI ratios (Fig. 1C). This result indicates that both CRS and CSDS lead to social avoidance in C57BL/6 J male mice. CRS induces similar social withdrawal behaviors in C57BL/6 J female mice (Fig. 1C). It is noteworthy that in C57BL/6 J male mice, CSDS produces stronger social avoidance than CRS. Lastly, behavioral despair was assayed using the tail suspension test (TST), in which animals were exposed to an inescapable aversive situation. In this situation, mice originally attempt vigorous escape but switch to a passive coping state after prolonged behavioral challenge [32]. Interestingly, we found that mice subjected to CRS displayed a significantly higher level of passive coping compared to both stress-naïve and CSDS groups (Fig. 1D). Together, these data demonstrate that both CRS and CSDS drive depression-like behaviors in male mice, and CRS also induces such behavior changes in female mice.
A Schematic of the CRS and CSDS behavioral paradigms. Mice created with BioRender.com. B–D Sucrose consumption (%) and total consumption (B), SI ratio and durations in the SI zone without/with target mouse present (C), and immobility time (D). See Table S1 for statistical details.
Anhedonia phenotypes induced by chronic predator stress correlate with an increased defensive state
High perceived stress level, an individual’s appraisal of their inability to cope with recent threats from stressors, is shown to be a critical etiological factor in the pathogenesis of depression [33, 34]. Given this, we investigated the premise that defensive behavior in response to perceived stress may be associated with depression-like behaviors. We used the looming assay (rapidly expanding dark overhead spots mimicking an approaching predator) to evaluate levels of defensive behavior [35, 36]. Both male and female mice subjected to CRS displayed enhanced defensive behavior (Fig.S1A-S1B). Interestingly, correlation analysis showed sucrose preference was negatively correlated with the time spent hiding in the refuge after mice experienced CRS (Fig.S1C-S1H).
Systemic evaluation of behavior profiles following CRS and CSDS using a 3D motion-capture system
To obtain detailed annotation of naturalistic behaviors in mice following CRS and CDS, we took advantage of a hierarchical 3D-motion learning framework and obtained behavioral phenotypes from both groups [22, 37]. Since CSDS was mainly used to drives depression-like behaviors in male mice, we first measured male mice so that behaviors induced by CRS and CSDS could be systematically compared. Male mice were divided into three groups: no treatment, CRS, or CSDS (Control-M, CRS-M, and CSDS-M, respectively). Spontaneous behaviors of these groups were recorded for 15 min (Fig. 2A). The data were automatically analyzed using the ML-based behavior analysis framework (BeA) [22], which extracted 40 behavioral motifs with unsupervised clustering (Fig. S3). We obtained 11 major behavior types from 74 animals by manually grouping these 40 behavioral motifs (Fig. 2A and Table 1). These behaviors included grooming, two types of rising (rising high and rising low), rearing, two types of stepping (stepping, head down and stepping, head up), rotating, three types of walking (walking, turn, walking, head down, and walking, head up), and trotting. We found several aligned and non-aligned differences in the 2 stress groups (CRS-M and CSDS-M) compared to controls in all 11 behavioral modules (Fig. 2B). In the CRS-M group, there were significant fewer instances in grooming, rearing, trotting, but more stepping, head down, and these are thereby defined as CRS-specific behaviors. Only the walking, head down behavior was significantly lower in the CSDS-M group and is thus defined as CSDS-specific. In addition, two behavioral features, rising high and rotating, showed similar changes in the CRS-M and CSDS-M groups and are thereby defined as both CRS and CSDS (Fig. 2D–F).
A Left: Pipeline of mouse behavior recording and analysis via 3D-motion learning framework. Right: 3D scatter plot illustrates the spatiotemporal feature space of the components of 11 behaviors of 1 mouse. B Up: averaged skeletons of 11 behavior movements. Down: comparison of the fractions of different movement types found in Control-M, CRS-M and CSDS-M mice. C Two-dimensional embedding of behavior fractions by t-SNE. The SVM decision boundary was identified by gaussian Kernel function. D Comparison of altered behavior movement numbers between CRS-M and CSDS groups. E Raster of the behavior movements. F Probability of behavior movements across time. See Table S1 for statistical details.
Next, we examined whether these behavioral features can effectively discriminate between the three groups. To do this, we embedded the behavioral fractions of 11 behavior movements from each mouse into a 2D t-SNE manifold. We found that individuals of the Control-M, CRS-M, and CSDS-M could be classified into three clusters by a Support Vector Machine (SVM) classifier (Fig. 2C). An organism’s behaviors can be decomposed into discrete, reproducible motifs and they can be described in terms of a small set of stereotyped motifs [38,39,40]. Therefore, we further quantified the transitional dynamics of these 11 behavioral features, whilst aiming to retain each animal’s full behavioral complexity. We found that the CRS-M group had significantly fewer behavioral transitions compared to the Control-M and CSDS-M groups, implying an impairment in behavioral flexibility (Fig. S3A and S3E). Both CRS-M and CSDS-M groups displayed various transitions between bouts of behavioral features which differed from the Control-M group, sometimes in a divergent manner (Fig. S3B, S3D and Fig. S4). Furthermore, we found that embedding the total 106 behavioral transitions into a 2D t-SNE manifold allowed for a more effective discrimination between the Control-M, CRS-M, and CSDS-M groups (Fig. S3C). These experiments demonstrate that, although sharing common features, each stress type elicits a specific pattern of behavior in mice.
Spontaneous behavior profiles correlate with depression-like behaviors
Although chronic stress caused both depression-like behaviors and changes in spontaneous behavior features, it was still unclear whether there is a correlation between them. Therefore, we evaluated the correlation between depression-like behaviors and behavior profiles using Pearson correlation analysis. Following either CRS or CSDS, mouse behavioral features were measured using the 3D motion-capture system and depression-like behaviors were also tested using the SI, SPT, and TST paradigms (Fig. 3A). Interestingly, we found that the fraction of grooming in mice positively correlated with social avoidance in the SI test and negatively correlated to behavioral despair in the TST test (Fig. 3B–D and Fig. S5). Furthermore, stepping, head down and walking, head down were positively correlated with behavioral despair and walking, head down was negatively correlated to social avoidance. Rearing and trotting were negatively correlated with behavioral despair (Fig. 3C, D). To further explore the correlation between behavioral transitions and depression-like behaviors, we matched the 106 types of transitions with depression-like behaviors (Fig. 3E and Fig. S6). We found that SI ratio and immobility time were correlated with 10 behavioral transition types, whereas no transitions correlated with sucrose preference. Given that spontaneous behavior fractions and transitions were correlated with depression-like behaviors, we examined whether we could use spontaneous behavior to predict depression-like behaviors. Fifty samples were partitioned into separate training and testing datasets. A linear model was trained using spontaneous behavior fractions and transitions that were significantly correlated with depression-like behavior to fit immobility time, sucrose preference and social ratio. After training, the model was applied to predict depression-like behaviors using spontaneous behavior fractions and transitions as predictors in the testing dataset (Fig. 3F). We found that prediction values fit with immobility time and social ratio, but poorly fit with sucrose preference (Fig. 3G). Additionally, the three-dimensional space comprised of depression-like behaviors revealed that, relative to the sucrose preference, the prediction and measurement data exhibited a more comparable distribution of immobility time and social ratio dimensions data points (Fig. 3H). To further evaluate this model, we performed K-means clustering to identify featured clusters (Fig. 3I). Based on the variations in the performance of behavior despair and social withdrawal across these clusters, we identified the clusters as ‘Relatively no change’, ‘Social withdrawal’, ‘Despair’ and ‘Despair + Social withdrawal’. The classification accuracy of the predicted data in these four groups was found to be 80 %, indicating that spontaneous behaviors can predict depression-like behaviors. Together, spontaneous behavioral profiles as well as the defensive level of an animal has the potential to identify depression-like symptoms.
A Schematic diagram of the behavioral tests. B Summary of the correlation analysis between depression-like behaviors and the fraction of each spontaneous behavior. The numbers above the dots indicate the correlation coefficient. C, D Representative correlation analysis between depression-like behaviors and the fraction of each spontaneous behavior. E Summary of the correlation analysis between behavioral transitions and two depression-like behaviors. The arrows between two behavioral states indicate the direction of transition. The numbers above the arrow indicate the correlation coefficient. See also Fig. S6. F Pipeline for ElasticNet linear regression model construction and prediction. G Model prediction of depression-like behaviors (left) and residual histograms (right). Coefficient of determination (R²) were labeled. H Measurement (up) and Prediction (down) values of depression-like behaviors were shown in the ‘Immobility time × sucrose preference × social ratio’ space. I K-means clustering (k = 4) of measurement (up) and prediction (down) values of immobility time and social ratio. Samples belonging to the same cluster in measurement and prediction clusters are labeled with yellow circles in the prediction data. Pearson correlation analysis.
Female mice display spontaneous behavioral changes following CRS similar to male mice
Although males and females do not differ in overall depression-like behaviors following CRS, it is not clear whether they differ in the finer behaviors tracked by the 3D motion‐capture system. Therefore, we compared spontaneous behaviors in the Control-F and CRS-F groups to examine the impact of CRS on female mice. Female mice following CRS showed similar significant differences in trotting, rising high and rotating fractions as male mice following CRS compared to their respective control groups (Fig. 4A). To compare behavioral patterns of female and male mice, we embedded the behavioral fractions from each sex into a 2D t-SNE manifold, and found that male and female mice before CRS showed similar distributions and that the distribution patterns of both sexes tended to move in the same direction in t-SNE feature space following CRS. Furthermore, a linear classifier effectively classified pre-CRS mice with post-CRS mice but was not able to distinguish sex, indicating that when it comes to overall behavioral changes, both male and female mice exhibit similar trends (Fig. 4B). In addition, CRS-F mice displayed significantly fewer behavioral transitions compared to Control-F, revealing a common feature where both female and male mice have an impairment in behavioral flexibility after CRS (Fig. S7C). The majority of the behavioral transition types showed similar trends in males and females (Fig. S7A–S7D). In addition, we embedded all behavioral transitions into a 2D t-SNE manifold and found that the linear classifier could effectively classify whether a mouse had experienced CRS, although it could not differentiate between male and female mice (Fig. 4C). Together, these data suggest that CRS-induced behavioral changes using an automated 3D-motion capture system are similar between sexes.
A Comparison of the fraction of spontaneous behavioral types of Control-F and CRS-F mice. B, C Two-dimensional embedding of behavioral fractions (B) and transitions (C) by t-SNE, Solid lines are the decision boundary identified by SVM. See Table S1 for statistical details.
Fluoxetine treatment eliminates spontaneous behavior changes following CRS
Behavioral measures intended for use as a new animal model of depression should initially satisfy the criteria of having strong predictive validity [41, 42]. Therefore, we further examined whether the behavioral changes induced by CRS can be attenuated by fluoxetine, a selective serotonin reuptake inhibitor (SSRI) for depression treatment [43, 44]. Fluoxetine treatment (20 mg/kg/day in 0.2 ml saline) was applied every day during the period when mice were subjected to CRS (Fig. 5A). The effects of fluoxetine treatment on the spontaneous behavior induced by CRS were inferred by the differences in behavioral fractions and transitions between treatment and control groups (Fig. 5B–E). In both male and female mice, chronic fluoxetine treatment (CRS+Fluoxetine) significantly eliminated the behavioral changes following CRS (CRS+Vehicle) (Fig. 5B and D). In addition, we embedded the fractions of 11 behaviors and 106 types of transitions of each mouse into a 2D space by t-SNE (Fig. 5C and E). We found that mice with or without fluoxetine treatment could be classified using a linear classifier. There was a similar distribution of data points between the CRS+Fluoxetine and stress-naïve control groups (Control+Vehicle). Both Control+Vehicle and CRS+Fluoxetine groups exhibited distinctly different distributions with CRS+Vehicle. In summary, the predictive validity criteria suggest that the spontaneous behaviors in CRS mice may be used as a reliable measure of depression.
A Schematic diagram of the behavioral tests. B Comparison of the fractions of each spontaneous behavioral type in Control+Vehicle, CRS+Vehicle and CRS+Fluoxetine mice. C Two-dimensional embedding of all behavioral fractions by t-SNE. D Representative behavioral transitions observed in the Control+vehicle, CRS+vehicle and CRS+Fluoxetine groups. E Two-dimensional embedding of all behavior transitions by t-SNE. See Table S1 for statistical details.
Discussion
Modeling human psychopathologies by means of laboratory animals requires several criteria, and among these, construct, face, and predictive validity have been given priority [45,46,47]. CSDS is considered a valid animal model of depression as it fulfills these validity criteria [48, 49]. For example, most common stressors in humans are psychosocial in nature and the neural correlates of processing social stress have been verified in rodents, establishing construct validity [50]. CSDS is also a model with high face validity, because it produces specific behaviors resembling the signs and symptoms of humans with depression, such as anhedonia, social avoidance, and despair [51,52,53]. However, not only is the CDS paradigm challenging to model in female laboratory mice, but it also has the potential for skin wounds on the test mouse as a result of attacks, which can impair health and preclude continued inclusion in the experiment [5]. The major advantages of our CRS model are that it does not involve physical stress, and that predator stress can be conducted on both sexes. The CRS model does not only have good face validity, but its predictive validity is also demonstrated by a lack of behavioral changes after chronic treatment with fluoxetine. The core components of defensive responses to predator stress at the circuit level are highly conserved across mammalian species [54], suggesting good construct validity of the CRS model. Interestingly, despite the commonalities between CRS and CSDS, there are some notable differences. There tend to be higher levels of behavioral despair following CRS, while CSDS elicits higher degrees of social avoidance. This suggests that these two models can be used for different representations of depression symptomatology. Clinical studies have identified two subtypes of major depressive disorder, atypical depression and melancholic depression [55,56,57]. Atypical depression is characterized by symptoms like interpersonal rejection sensitivity, which may lead to social withdrawal and avoidance, whereas melancholic depression typically includes symptoms like psychomotor retardation which is shown as loss of responsiveness to the individual’s environment. Patients with different subtypes of depression may exhibit differences in endocrinology, neurocircuitry changes, as well as differences in response to treatment, which requires suitable animal models to explore the mechanisms underlying different depression subtypes [58]. Gratifyingly, the CRS model closely resembles symptoms of melancholic depression, whereas the CSDS model captures some of the symptoms more frequently found in atypical depression, suggesting that these models have potential to be applied in studying the mechanisms of different depression subtypes. In summary, our study illustrates that the CRS-induced phenotype in mice is a good reflection of behavioral aspects of depression in humans, as currently understood.
This is the first study to dissect the correlation of animal defensive behavior with the depressive state. We found that time spent hiding in the nest in response to predator stimuli was correlated with the level of anhedonia in the sucrose preference test. This discovery indicates that hiding behavior, but not escape itself, may reflect the depression-like state in mice and suggests an adaptive function for this state. Given that animals being preyed upon often decrease feeding activity to take cover and avoid detection by predators [59], prolonged hiding and reduced reward gained from palatable food may constitute a coordinated response to predator stress, effectively reducing the time and energy allocated to foraging in a dangerous environment. Whether this lowered reward responsivity may have become maladaptive in humans who suffer from sustained stress or whether it constitutes an adaptive change in motivation [60] remains unkown. In either case, our results clearly suggest that anhedonia and increased defensive level expressed as hiding in a shelter following CRS may emerge from shared neural mechanisms. Furthermore, we also found that the group of mice subjected to CRS showed decreased struggling in the tail suspension test, but enhanced escape in the looming test. These seemingly contradictory results support the hypothesis that prior experience of CRS leads to optimized decision-making processes rather than a state of generalized “learned helplessness” [61]. Mice may learn to decide when to escape and when to give up, depending on a more realistic perception of control during stressful events. This is consistent with the still controversial view that, in humans, depressed individuals frequently show more accurate judgment of their ability to influence an outcome than healthy individuals [62], termed “depressive realism”.
Animals have complex behavioral patterns, the contents of which manifest brain function as the result of the manifold interactions of gene regulatory networks and neural circuits. Overly simplistic behavioral assays with low dimensionality can lead to significant difficulty to link drug effects to molecular mechanisms in complex diseases such as depression [18, 21]. ML tools have enabled the extraction and classification of more complex behavioral patterns, and the identification of pattern perturbation by drugs and disease models [20]. Furthermore, bodily expression and emotional processing are closely connected [63], and clinical studies have revealed that depressed individuals exhibit a change in body movements and posture [57, 64, 65]. For example, depressed patients often display a decrease in spontaneous movements and body postures[66], and measuring these behaviors can supply a wealth of information in clinical diagnosis [67,68,69]. In this study, we showed that mice following predator or social stress displayed increased rising and decreased rotating behaviors. Rising is usually associated with exploration and foraging, but may also be a sign of anxiety or fear, as rodents may stand upright in response to a perceived threat [70]. Rotating is a spontaneous cycling action that requires animals to be able to flexibly control their bodies. Interestingly, studies have shown that patients who are depressed often showed rigid postures such as reduced arm swing and head movements [64, 65], indicating that decreased rotating behavior may also be an expression of behavior rigidity in depression that manifests as a reduced range of motion. It may be the case that a mechanism common to both CRS and CSDS modulate these two behavioral changes, rising and rotating. Furthremore, we successfully captured the spontaneous behavioral differences between CRS and CSDS models, illustrating that ML-supported analysis can simultaneously evaluate multiple behavioral alterations in different animal models of depression.
With regards to testing for depression-like behaviors in rodents, canonical screening tests (such as the TST, SI and SPT) are used mainly to reflect specific clinical symptoms [62]. Our results indicate that CRS induces more behavior despair whereas CSDS triggers more social avoidance. Correlation analysis between ML-observed spontaneous behaviors and depression behavior indicators revealed that head-down movements showed a positive correlation with behavioral despair, whereas rearing and trotting showed a negative correlation. Interestingly, depressed patients show increased head-down postures and reduced behavior movements and speeds [64, 65], which is consistent with our observations. These results all suggest that changes in the motor network coincide with the development of behavioral despair. Moreover, we found that grooming was most strongly correlated with social avoidance. In line with our findings, it has been observed that mice lacking ephrin receptors display both increased self-grooming and impaired social interaction [71]. Furthermore, a shared mechanism has been shown to control these two behavioral phenomena, as stimulation of neurons in the posterior dorsal part of the medial amygdala can induce repetitive self-grooming in mice and suppress social interaction [72]. Furthermore, we showed that depression-like behaviors can be predicted by patterns of spontaneous behaviors. This evidence suggests that studying spontaneous behavior will help to explain the implications of behaviors in more constrained tasks. Together, our ML-based approach provides predictive classifiers for social and despair behavior in rodents, and overcomes several limitations in the classical behavioral paradigms including dependence on human observers and a heavy susceptibility to confounders [73].
We acknowledge several research limitations in our study. First, our ML-observed approach is performed in single mice and outside their normal living environment [74]. As Shemesh et al. proposed, it would be interesting to assess potential changes in complex behavioral patterns following stress in an enriched environment that include essential resources, potential mating partners, and social competitors [74]. This would require an automatic setup that enables the tracking of group-housed mice individually in complex 3D environments [74, 75]. Second, CRS-induced changes in behavioral fractions using ML-observed behavior were similar between sexes despite the fact that depression affects females around twice as often as it does males [76]. In response to environmental stimuli, such as predator odor, mice can display fear-related behaviors that are not composed of new syllables but rather of new sequences of the same syllables [77]. Therefore, future study should be directed towards investigations comparing the behavioral sequences between sexes and longitudinally characterizing the behavioral patterns of both female and male mice several weeks after the end of the stress paradigm.
Code availability
Behavior atlas (https://behavioratlas.tech//) was used for spontaneous behavior analysis, all other scripts will provide upon request.
References
Hill MN, Hellemans KG, Verma P, Gorzalka BB, Weinberg J. Neurobiology of chronic mild stress: parallels to major depression. Neurosci Biobehav Rev. 2012;36:2085–117.
Mazure CM, Maciejewski PK. A model of risk for major depression: effects of life stress and cognitive style vary by age. Depress Anxiety. 2003;17:26–33.
Willner P, Scheel-Krüger J, Belzung C. The neurobiology of depression and antidepressant action. Neurosci Biobehav Rev. 2013;37:2331–71.
Charney DS, Manji HK. Life stress, genes, and depression: multiple pathways lead to increased risk and new opportunities for intervention. Sci STKE. 2004;2004:re5.
Huhman KL. Social conflict models: can they inform us about human psychopathology? Horm Behav. 2006;50:640–6.
Hannibal DL, Bliss-Moreau E, Vandeleest J, McCowan B, Capitanio J. Laboratory rhesus macaque social housing and social changes: Implications for research. Am J Primatol. 2017;79:1–14.
Toyoda A. Social defeat models in animal science: What we have learned from rodent models. Anim Sci J. 2017;88:944–52.
Blanchard RJ, McKittrick CR, Blanchard DC. Animal models of social stress: effects on behavior and brain neurochemical systems. Physiol Behav. 2001;73:261–71.
Kotrschal A, Deacon AE, Magurran AE, Kolm N. Predation pressure shapes brain anatomy in the wild. Evol Ecol. 2017;31:619–33.
LeDoux J, Daw ND. Surviving threats: neural circuit and computational implications of a new taxonomy of defensive behaviour. Nat Rev Neurosci. 2018;19:269–82.
Tseng YT, Zhao B, Chen S, Ye J, Liu J, Liang L, et al. The subthalamic corticotropin-releasing hormone neurons mediate adaptive REM-sleep responses to threat. Neuron. 2022;110:1223–39.e8.
Kozlowska K, Walker P, McLean L, Carrive P. Fear and the defense Cascade: clinical implications and management. Harv Rev Psychiatry. 2015;23:263–87.
Bolles RC. Species-specific defense reactions and avoidance learning. Psychol Rev. 1970;77:32–48.
Bolles RC, Fanselow MS. A perceptual-defensive-recuperative model of fear and pain. Behav Brain Sci. 1980;3:291–301.
Fanselow MS, Lester LS. Evolution and learning. NJ: Lawrence Erlbaum Associates, Inc; 1988. A functional behavioristic approach to aversively motivated behavior: predatory imminence as a determinant of the topography of defensive behavior; p. 185–212.
Qi S, Hassabis D, Sun J, Guo F, Daw N, Mobbs D. How cognitive and reactive fear circuits optimize escape decisions in humans. Proc Natl Acad Sci USA 2018;115:3186–91.
Vieira JB, Schellhaas S, Enström E, Olsson A. Help or flight? Increased threat imminence promotes defensive helping in humans. Proc Biol Sci. 2020;287:20201473.
Gururajan A, Reif A, Cryan JF, Slattery DA. The future of rodent models in depression research. Nat Rev Neurosci. 2019;20:686–701.
Leite-Almeida H, Castelhano-Carlos MJ, Sousa N. New horizons for phenotyping behavior in rodents: the example of depressive-like behavior. Front Behav Neurosci. 2021;15:811987.
Flavell SW, Gogolla N, Lovett-Barron M, Zelikowsky M. The emergence and influence of internal states. Neuron 2022;110:2545–70.
Wiltschko AB, Tsukahara T, Zeine A, Anyoha R, Gillis WF, Markowitz JE, et al. Revealing the structure of pharmacobehavioral space through motion sequencing. Nat Neurosci. 2020;23:1433–43.
Huang K, Han Y, Chen K, Pan H, Zhao G, Yi W, et al. A hierarchical 3D-motion learning framework for animal spontaneous behavior mapping. Nat Commun. 2021;12:2784.
Golden SA, Covington HE 3rd, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 2011;6:1183–91.
Morel C, Montgomery SE, Li L, Durand-de Cuttoli R, Teichman EM, Juarez B, et al. Midbrain projection to the basolateral amygdala encodes anxiety-like but not depression-like behaviors. Nat Commun. 2022;13:1532.
Lee EH, Park JY, Kwon HJ, Han PL. Repeated exposure with short-term behavioral stress resolves pre-existing stress-induced depressive-like behavior in mice. Nat Commun. 2021;12:6682.
Von Frijtag JC, Reijmers LG, Van der Harst JE, Leus IE, Van den Bos R, Spruijt BM. Defeat followed by individual housing results in long-term impaired reward- and cognition-related behaviours in rats. Behav Brain Res. 2000;117:137–46.
van Kampen M, Kramer M, Hiemke C, Flügge G, Fuchs E. The chronic psychosocial stress paradigm in male tree shrews: evaluation of a novel animal model for depressive disorders. Stress. 2002;5:37–46.
Rygula R, Abumaria N, Flügge G, Fuchs E, Rüther E, Havemann-Reinecke U. Anhedonia and motivational deficits in rats: impact of chronic social stress. Behav Brain Res. 2005;162:127–34.
Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404.
Forbes NF, Stewart CA, Matthews K, Reid IC. Chronic mild stress and sucrose consumption: validity as a model of depression. Physiol Behav. 1996;60:1481–4.
Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–9.
Koolhaas JM, Korte SM, De Boer SF, Van Der Vegt BJ, Van Reenen CG, Hopster H, et al. Coping styles in animals: current status in behavior and stress-physiology. Neurosci Biobehav Rev. 1999;23:925–35.
Ding Y, Dai J. Advance in stress for depressive disorder. Adv Exp Med Biol. 2019;1180:147–78.
Becker M, Pinhasov A, Ornoy A. Animal models of depression: what can they teach us about the human disease? Diagnostics (Basel). 2021;11:123.
Zhou Z, Liu X, Chen S, Zhang Z, Liu Y, Montardy Q, et al. A VTA GABAergic neural circuit mediates visually evoked innate defensive responses. Neuron 2019;103:473–88.e6.
Wei P, Liu N, Zhang Z, Liu X, Tang Y, He X, et al. Processing of visually evoked innate fear by a non-canonical thalamic pathway. Nat Commun. 2015;6:6756.
Liu X, Feng X, Huang H, Huang K, Xu Y, Ye S, et al. Male and female mice display consistent lifelong ability to address potential life-threatening cues using different post-threat coping strategies. BMC Biol. 2022;20:281.
Altmann J. Observational study of behavior: sampling methods. Behaviour 1974;49:227–67.
Lehner, P. N. Handbook of ethological methods. UK: Cambridge University Press; 1998.
Berman GJ, Choi DM, Bialek W, Shaevitz JW. Mapping the stereotyped behaviour of freely moving fruit flies. J R Soc Interface. 2014;11:20140672.
Alcaro A, Cabib S, Ventura R, Puglisi-Allegra S. Genotype- and experience-dependent susceptibility to depressive-like responses in the forced-swimming test. Psychopharmacol (Berl). 2002;164:138–43.
Willner P. The validity of animal models of depression. Psychopharmacol (Berl). 1984;83:1–16.
Taylor MJ, Freemantle N, Geddes JR, Bhagwagar Z. Early onset of selective serotonin reuptake inhibitor antidepressant action: systematic review and meta-analysis. Arch Gen Psychiatry. 2006;63:1217–23.
Wong DT, Perry KW, Bymaster FP. Case history: the discovery of fluoxetine hydrochloride (Prozac). Nat Rev Drug Disco. 2005;4:764–74.
Gaszner T, Farkas J, Kun D, Ujvári B, Berta G, Csernus V, et al. Fluoxetine treatment supports predictive validity of the three hit model of depression in male PACAP heterozygous mice and underpins the impact of early life adversity on therapeutic efficacy. Front Endocrinol (Lausanne). 2022;13:995900.
Willner P. Animal models of depression: validity and applications. Adv Biochem Psychopharmacol. 1995;49:19–41.
Farkas J, Kovács L, Gáspár L, Nafz A, Gaszner T, Ujvári B, et al. Construct and face validity of a new model for the three-hit theory of depression using PACAP mutant mice on CD1 background. Neuroscience. 2017;354:11–29.
Czéh B, Simon M. Benefits of animal models to understand the pathophysiology of depressive disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2021;106:110049.
Wang W, Liu W, Duan D, Bai H, Wang Z, Xing Y. Chronic social defeat stress mouse model: current view on its behavioral deficits and modifications. Behav Neurosci. 2021;135:326–35.
Patel D, Kas MJ, Chattarji S, Buwalda B. Rodent models of social stress and neuronal plasticity: Relevance to depressive-like disorders. Behav Brain Res. 2019;369:111900.
Chaouloff F. Social stress models in depression research: what do they tell us? Cell Tissue Res. 2013;354:179–90.
Fuchs E, Flügge G. Stress, glucocorticoids and structural plasticity of the hippocampus. Neurosci Biobehav Rev. 1998;23:295–300.
Hammels C, Pishva E, De Vry J, van den Hove DL, Prickaerts J, van Winkel R, et al. Defeat stress in rodents: from behavior to molecules. Neurosci Biobehav Rev. 2015;59:111–40.
McFadyen J, Dolan RJ, Garrido MI. The influence of subcortical shortcuts on disordered sensory and cognitive processing. Nat Rev Neurosci. 2020;21:264–76.
Gold PW, Chrousos GP. The endocrinology of melancholic and atypical depression: relation to neurocircuitry and somatic consequences. Proc Assoc Am Physicians. 1999;111:22–34.
Hwa-Young L, Yong-Ku K. Different mechanisms between melancholic and atypical depression. In: Yong-Ku K, editor Major Depressive Disorder. Rijeka: IntechOpen;2015.
Diagnostic and statistical manual of mental disorders: DSM-5™, 5th ed. VA: American Psychiatric Publishing, Inc.;2013.
Planchez B, Surget A, Belzung C. Animal models of major depression: drawbacks and challenges. J Neural Transm (Vienna). 2019;126:1383–408.
Tigreros N, Wang EH, Thaler JS. Prey nutritional state drives divergent behavioural and physiological responses to predation risk. Funct Ecol. 2018;32:982–89.
Andrews PW, Maslej MM, Thomson JA Jr, Hollon SD. Disordered doctors or rational rats? Testing adaptationist and disorder hypotheses for melancholic depression and their relevance for clinical psychology. Clin Psychol Rev. 2020;82:101927.
Daviu N, Füzesi T, Rosenegger DG, Rasiah NP, Sterley TL, Peringod G, et al. Paraventricular nucleus CRH neurons encode stress controllability and regulate defensive behavior selection. Nat Neurosci. 2020;23:398–410.
Alloy LB, Abramson LY. Judgment of contingency in depressed and nondepressed students: sadder but wiser? J Exp Psychol Gen. 1979;108:441–85.
Niedenthal PM. Embodying emotion. Science. 2007;316:1002–5.
Michalak J, Troje NF, Fischer J, Vollmar P, Heidenreich T, Schulte D. Embodiment of sadness and depression—gait patterns associated with dysphoric mood. Psychosom Med. 2009;71:580–7.
Wang Y, Wang J, Liu X, Zhu T. Detecting depression through gait data: examining the contribution of gait features in recognizing depression. Front Psychiatry. 2021;12:661213.
Canales JZ, Cordás TA, Fiquer JT, Cavalcante AF, Moreno RA. Posture and body image in individuals with major depressive disorder: a controlled study. Braz J Psychiatry. 2010;32:375–80.
Fu G, Yu Y, Ye J, Zheng Y, Li W, Cui N, et al. A method for diagnosing depression: facial expression mimicry is evaluated by facial expression recognition. J Affect Disord. 2023;323:809–18.
Yu Y, Li W, Zhao Y, Ye J, Zheng Y, Liu X, et al. Depression and severity detection based on body kinematic features: using kinect recorded skeleton data of simple action. Front Neurol. 2022;13:905917.
Willmore L, Cameron C, Yang J, Witten IB, Falkner AL. Behavioural and dopaminergic signatures of resilience. Nature. 2022;611:124–32.
Sturman O, Germain PL, Bohacek J. Exploratory rearing: a context- and stress-sensitive behavior recorded in the open-field test. Stress. 2018;21:443–52.
Wurzman R, Forcelli PA, Griffey CJ, Kromer LF. Repetitive grooming and sensorimotor abnormalities in an ephrin-A knockout model for Autism Spectrum Disorders. Behav Brain Res. 2015;278:115–28.
Hong W, Kim DW, Anderson DJ. Antagonistic control of social versus repetitive self-grooming behaviors by separable amygdala neuronal subsets. Cell. 2014;158:1348–61.
von Mücke-Heim IA, Urbina-Treviño L, Bordes J, Ries C, Schmidt MV, Deussing JM. Introducing a depression-like syndrome for translational neuropsychiatry: a plea for taxonomical validity and improved comparability between humans and mice. Mol Psychiatry. 2023;28:329–340.
Shemesh Y, Chen A. A paradigm shift in translational psychiatry through rodent neuroethology. Mol Psychiatry. 2023;28:993–1003.
Han Y, Chen K, Wang Y, Liu W, Wang X, Liao J, et al. Social Behavior Atlas: A computational framework for tracking and mapping 3D close interactions of free-moving animals. bioRxiv. 2023. https://doi.org/10.1101/2023.03.05.531235.
Van de Velde S, Bracke P, Levecque K. Gender differences in depression in 23 European countries. Cross-national variation in the gender gap in depression. Soc Sci Med. 2010;71:305–13.
Wiltschko AB, Johnson MJ, Iurilli G, Peterson RE, Katon JM, Pashkovski SL, et al. Mapping sub-second structure in mouse behavior. Neuron. 2015;88:1121–35.
Acknowledgements
We thank Kang Huang for helpful comments and Bayone BioTech Co., LTD for technical support.
Funding
This work was supported by grants from the National Natural Science Foundation of China (32230042 and 31930047 to LW; 32200826 to Y-TT), the Financial Support for Outstanding Talents Training Fund in Shenzhen (to LW), the Shenzhen Science and Technology Program (JCYJ20220530154412028 to Y-TT; KCXFZ20211020163549011 to BS), the Guangdong Basic and Applied Basic Research Foundation (2021A1515111030 to Y-TT).
Author information
Authors and Affiliations
Contributions
Y-TT and LW designed the study; Y-TT, BZ, HD, and LL collected the data and performed statistical analysis. Y-TT, BZ, and BS wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tseng, YT., Zhao, B., Ding, H. et al. Systematic evaluation of a predator stress model of depression in mice using a hierarchical 3D-motion learning framework. Transl Psychiatry 13, 178 (2023). https://doi.org/10.1038/s41398-023-02481-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-023-02481-8
- Springer Nature Limited
This article is cited by
-
Defensive responses: behaviour, the brain and the body
Nature Reviews Neuroscience (2023)