Abstract
Selective serotonin reuptake inhibitors (SSRIs) and internet-based cognitive behavioral therapy (ICBT) are recommended treatments of social anxiety disorder (SAD), and often combined, but their effects on monoaminergic signaling are not well understood. In this multi-tracer positron emission tomography (PET) study, 24 patients with SAD were randomized to treatment with escitalopram+ICBT or placebo+ICBT under double-blind conditions. Before and after 9 weeks of treatment, patients were examined with positron emission tomography and the radioligands [11C]DASB and [11C]PE2I, probing the serotonin (SERT) and dopamine (DAT) transporter proteins respectively. Both treatment combinations resulted in significant improvement as measured by the Liebowitz Social Anxiety Scale (LSAS). At baseline, SERT-DAT co-expression was high and, in the putamen and thalamus, co-expression showed positive associations with symptom severity. SERT-DAT co-expression was also predictive of treatment success, but predictor-outcome associations differed in direction between the treatments. After treatment, average SERT occupancy in the SSRI + ICBT group was >80%, with positive associations between symptom improvement and occupancy in the nucleus accumbens, putamen and anterior cingulate cortex. Following placebo+ICBT, SERT binding increased in the raphe nuclei. DAT binding increased in both groups in limbic and striatal areas, but relations with symptom improvement differed, being negative for SSRI + ICBT and positive for placebo + ICBT. Thus, serotonin-dopamine transporter co-expression exerts influence on symptom severity and remission rate in the treatment of social anxiety disorder. However, the monoamine transporters are modulated in dissimilar ways when cognitive-behavioral treatment is given concomitantly with either SSRI-medication or pill placebo.
Similar content being viewed by others
Introduction
Social anxiety disorder (SAD) is a debilitating and chronic psychiatric disorder associated with severe suffering for the individual, negative impact on working-life and relationships [1] and high barriers to seek treatment [2]. Existing treatments, predominantly cognitive behavioral therapy (CBT) and selective serotonin reuptake inhibitors (SSRIs), are often successful [3] and combining the two may further enhance clinical efficacy [4]. Neuroimaging studies support that CBT, SSRIs, as well as combined treatment attenuate amygdala activity and connectivity during emotional conditions in SAD [4,5,6,7,8,9,10]. Despite these advances, response rates for first line treatments are only 50–65%, indicating that many patients do not achieve remission [11]. A better understanding of the biological mechanisms underlying treatment efficacy in SAD is therefore needed.
Serotonin has been implicated as a key neurotransmitter in the neurobiology of anxiety [12]. Positron emission tomography (PET) studies of serotonin synthesis capacity, serotonin 1A-receptor and transporter availability have suggested increased pre-synaptic serotonin activity in SAD patients [13,14,15] and the beneficial effects of SSRIs [3] further point to serotonergic involvement. The primary action of SSRIs is to block the serotonin transporter (SERT) protein that facilitates transmembrane reuptake of serotonin into the pre-synaptic cell [16] and 76–85% occupancy has been suggested to exert efficient symptom relief [17, 18]. Adequate SSRI occupancy of the SERT has been verified in SAD [19, 20]. However, it is not clear if occupancy rate is linearly related to clinical SSRI responses. In a previous PET study, we failed to demonstrate such a relationship in SAD [20], consistent with several studies of major depression [18, 21,22,23], although it has been observed for certain subpopulations of depression [24, 25]. Despite the well-characterized blockade of SERT by SSRIs, the anxiolytic mechanism of action is still debated [26,27,28]. A number of downstream effects have been proposed to mediate the clinical effect, for example interactions with dopamine signaling [29,30,31].
An emerging body of evidence points to the importance of dopamine in SAD [32,33,34,35,36,37]. While single photon emission computed tomography (SPECT) studies of the dopamine transporter (DAT) have yielded mixed findings [35, 38,39,40], we recently demonstrated, by use of PET, that SAD symptom severity was associated with increased striatal DAT binding and that DAT-SERT co-expression was higher in SAD patients relative to healthy controls [15]. There is considerable interaction between the serotonin and dopamine systems [41,42,43], for example, DATs may contribute to serotonin reuptake [44,45,46,47]. Molecular imaging studies have demonstrated changes in DAT availability with SSRI treatment [48,49,50,51,52] suggesting that SSRIs could exert secondary effects on the DAT. We recently showed that striatal DAT binding was associated with SSRI anti-anxiety effects and can be shaped by psychological expectancies[20]. CBT may affect dopamine D2 receptors [53] but, to our knowledge, no earlier PET-study has investigated concurrent changes in serotonin and dopamine transporters resulting from CBT or combined SSRI + CBT treatment.
The aim of this study was to evaluate changes in serotonin and dopamine transporter availability in SAD patients after 9 weeks of treatment with Internet-delivered CBT (ICBT) [54, 55] combined with an SSRI (escitalopram) or pill-placebo under double-blind conditions, and if such changes are associated with symptom improvement. In a PET subsample of a previously reported RCT [4], we measured SERT and DAT binding with the two highly selective radioligands [11C]DASB and [11C]PE2I. We expected marked SERT occupancy (lowered binding potential) specifically with SSRI-treatment whereas we did not have directed hypotheses regarding DAT changes. We also examined the relationships between transporter co-expression at baseline and symptom severity as well as symptom remission with treatment.
Patients and methods
Participants and design
The study was a double-blind clinical trial with a treatment duration of 9 weeks (trial registration: ISRCTN24929928), conducted between September 2011 and September 2013, and details have been described previously [4]. Briefly, participants were recruited through media advertisements and screened for SAD using online versions of the Social Phobia Screening Questionnaire (SPSQ) [56] and the Montgomery-Åsberg Depression Rating Scale–self rated version (MADRS-S) [57]. The Mini International Neuropsychiatric Interview (MINI) [58], and the SAD section of the Structured Clinical Interview for DSM-IV (SCID-I) [59] were thereafter administered via telephone and subjects deemed to fulfill the criteria for SAD went through a medical examination.
Exclusion criteria were previous PET-scan, treatment of any psychiatric condition during the last three months, ongoing serious somatic or psychiatric disorder, drug or substance abuse or dependency, menopause, pregnancy and MRI contraindications. All subjects provided written informed consent. The study was approved by the regional ethics committee, the Radiation Safety Committee and the Medical Products Agency in Sweden.
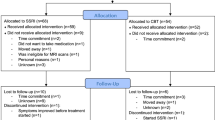
After screening, 48 subjects were enrolled and randomized to treatment with SSRI + ICBT or placebo+ICBT [4]. SAD was the primary diagnosis for all participants. Randomization, matched for age and sex was performed by Apoteket Production and Laboratories (APL), Stockholm, Sweden. All subjects underwent functional and structural MRI and 26 of these participants were again matched on age and sex and underwent additional PET assessments. The PET pairs were drawn from the whole sample, although no random sampling was applied. The Uppsala University Hospital Pharmacy kept all randomization codes secret until unblinding was due. One participant opted out of the post-treatment PET assessment, and one participant dropped out of the study before scanning procedures, leaving 24 participants (12 women) with complete PET data. There were 6 women and 6 men in each group and mean age±SD was 37.2 ± 11.32 and 31.25 ± 8.11 years for the SSRI + ICBT and the placebo + ICBT groups respectively. Sex and age distributions were not significantly different between the groups (Ps > 0.156).
Treatment
During the 9-week treatment period, the SSRI group was prescribed with a 20 mg daily dose of escitalopram, (H.Lundbeck AB, Helsingborg, Sweden) starting with 10 mg daily dose during the first week. SSRI and placebo oral suspension capsules were identical, and prepared by APL, Stockholm, Sweden. Compliance to escitalopram treatment was assessed by analyzing blood metabolites of escitalopram at the time for the last PET-scan. SSRI and placebo treatments were supervised by an experienced psychiatrist (K.W).
In tandem with the first SSRI or placebo therapy, ICBT was started as well. The ICBT program has been found to be effective in several RCTs [55, 60, 61], and found to be as effective as face-to-face CBT [54, 62]. The program is partly based on the Clark and Wells model of SAD [63] and includes 9 weekly modules: Introductory reading about SAD and CBT (module 1), the cognitive model for SAD and cognitive restructuring (modules 2–4), exercises for exposure (modules 5–7), social skills and relapse prevention (modules 8–9). The treatment was guided by trained therapists who gave the participants weekly homework, feedback on assignments, and introduced the next week’s module. All homework and completion of modules were registered to assess participants’ compliance to the treatment [4].
Behavioral measures
Severity of social anxiety symptoms was measured using the clinician-administered Liebowitz Social Anxiety Scale [64] before and after treatment. Treatment response was assessed using the Clinical Global Impression Improvement (CGI-I), scores of 1 or 2 denoting responders and ≥3 non-responders. Both instruments were administered by the same experienced psychiatrist.
Imaging procedure
PET-assessments
Participants were instructed to fast for 3 h before the scanning session and to abstain from nicotine, alcohol and caffeine 12 h before. Image acquisition was performed using a Siemens ECAT EXACT HR + 32-ring high-resolution scanner with 63 contiguous 2.46 mm slices. Participants were placed in the PET scanner with their heads lightly fixated and a transmission scan was performed using 3 retractable Germanium 68 rotating line sources for 10 min before the tracer was delivered with a rapid bolus injection through a venous catheter inserted in the arm. Dynamic PET image acquisition was initiated at the time of bolus injection. For both [11C]DASB and [11C]PE2I, the same procedure was used during pre- and posttreatment.
[11C]PE2I image acquisition: Twenty-two [11C]PE2I images, probing DAT availability, were collected during 80 min (4 × 60s, 2 × 120s, 4 × 180s, 12 × 300s). Mean ± SD injected activity was 332.38 ± 16.93 MBq at pretreatment and 319.33 ± 29.01 MBq at posttreatment.
[11C]DASB image acquisition: After a waiting period of 45–60 min to allow sufficient radioligand decay (less than 1% [11C]PE2I activity left at [11C]DASB injection), 22 [11C]DASB images, probing SERT availability, were collected during 60 min (1 × 60s, 4 × 30s, 3 × 60s, 4 × 120s, 2 × 180s, 8 × 300s). Mean±SD injected activity at pre-treatment was 329.50 ± 26.90 and at post- treatment, 318.75 ± 37.62 MBq.
MRI-assessments
Anatomical MRI was performed to allow co-registration of PET images to anatomical T1-weighted images. The T1 images (echo time (TE) = 15 ms; repetition time (TR) = 5700 ms; inversion time = 400 ms; field of view = 230 × 230 mm2; voxel size = 0.8 × 1.0 × 2.0 mm3; 60 contiguous axial slices) were acquired with a Philips Achieva 3.0 T whole body MR scanner (Philips Medical Systems, Best, The Netherlands) using an 8-channel head coil.
Preprocessing
Parametric [11C]PE2I binding potential (BPND) images were generated using receptor parametric mapping [65] which is based on a simplified reference region compartmental model and [11C]DASB BPND images were calculated using the reference Logan [66] method. When using reference Logan, BPND is derived by subtracting 1 from the distribution volume ratio. Cerebellum gray matter was used as a reference region for both [11C]DASB and [11C]PE2I due to its negligible levels of SERT and DAT. The cerebellum was defined in a user-independent fashion using the PVElab [67] software on each participant’s T1-weighted image, which was co-registered to the PET images.
Further preprocessing steps were performed in Statistical Parametric Mapping 8 (SPM8; (Wellcome Department of Cognitive Neurology, University College London, www.fil.ion.ucl.ac.uk) implemented in MATLAB 2018a (Mathworks Inc., Natick, MA, USA). Each participant’s BPND images were co-registered to the anatomical T1-weighted image, which was then segmented and normalized to MNI standard space. Transformation parameters from segmentation were then applied to the BPND images yielding parametric images with 2 mm isotropic voxels in MNI space. Lastly, images were smoothed with a 12 mm Gaussian kernel.
Statistical analysis
To examine treatment effects on SERTs and DATs, pre to post treatment diff-images were prepared. SSRI occupancy images (100 × ([PRE − POST] / PRE)) of [11C]DASB BPND and percent change images (100 × ([POST − PRE]/PRE)) of [11C]PE2I BPND were calculated for the SSRI + ICBT treatment arm. Occupancy is a measure of the proportion of the transporters available pre-treatment that are occupied by SSRI at post-treatment. Hence, occupancy > 0% is reflected by a decrease in binding potential after treatment, whereas percent change > 0% signifies an increase in binding potential. Since there is no SERT occupancy of SSRIs in the placebo+ICBT treatment arm, percent change images were calculated for both [11C]DASB BPND and [11C]PE2I BPND. The LSAS post-scores were subtracted from the pre measurement scores with higher positive scores reflecting a larger symptom improvement.
A priori regions of interest (ROIs) were selected based on earlier neuroimaging research in SAD and tracer binding [13, 68, 69]. [11C]PE2I binding is specific to regions rich in dopamine transporters, such as the dorsal (putamen, caudate nucleus) and ventral striatum (nucleus accumbens (NAcc), but adequate levels can also be found in the amygdala, hippocampus, thalamus and pallidum. BPND distributions were tested for heterogeneity and normality and were deemed adequate for parametric analyses. [11C]DASB BPND analyses were performed in the same ROIs but also extended to the insular cortex, anterior cingulate cortex (ACC) and the raphe nuclei. Anatomical regions were defined by masks available in the Automated Anatomical Labeling (AAL) library found in the Wake Forest University Pickatlas [70]. The raphe nuclei were defined using PVElab, and NAcc with the Hammersmith atlas [71].
Within group effects
Effects of treatment on SERT and DAT BPND were evaluated with one-sample t-tests using the occupancy and percent change diff-images for the two tracers separately. Associations between changes in transporter binding and symptom reduction were evaluated in SPM 8 using multiple regressions with age and sex as covariates. Family wise error (FWE) correction was used within each ROI and the statistical threshold was set at PFWE < 0.05. Co-expression of SERT and DAT was analyzed in Matlab 2018a, (Mathworks Inc, Natick, MA, USA) using voxel-wise partial Pearson’s correlations (age and sex as controlling variables) with the statistical threshold set at P < 0.05. To examine if changes (Δ) in SERT and DAT availability were associated with altered symptom severity, multiple regressions were performed with ΔSERT, ΔDAT, their interaction term, age and sex, as regressors. A similar regression using initial values of SERT and DAT BPND and their interaction term as predictors, and the change in LSAS as outcome, examined if initial SERT-DAT balance predicted treatment outcome.
Between group effects
To examine treatment group differences in changes in DAT binding, a two-sample t-test was performed in SPM8 with age and sex as covariates and the statistical threshold set to PFWE < 0.05. Additionally, a multiple regression was used to compare the relation between the percentage change in DAT BPND and symptom reduction between groups. Note that the high affinity of escitalopram to the SERT precluded the possibility of group comparisons of changes in SERT and SERT × DAT interactions.
Results
Treatment outcome
Initial LSAS scores and depression comorbidity data are found in Table 1. No initial difference in social anxiety symptoms was found between the two groups (t(22) = 0.55, P = 0.59). Repeated measures ANOVA revealed statistically significant symptom improvement (LSAS scores) in both groups from pre to post-treatment (F(1, 22) = 43.12, P < 0.001, Cohen’s d = 1.32). Follow up t-tests verified significant symptom improvement in the SSRI + ICBT (t(11) = 5.16, P < 0.001) as well as in the placebo+ICBT group (t(11) = 4.07, P = .002). No effect of group on symptom improvement (F(1,22) = 1.51, P = 0.232) or group × time effect (F(1,1) = 1.83, P = 0.186) was detected. According to CGI-I assessments, there were 10 responders (83%) in the SSRI + ICBT group and 5 (42%) in the placebo+ICBT group (Fisher’s exact test: P = 0.089), congruent with the generally better outcome for SSRI + ICBT reported in the full treatment sample [4].
Serotonin transporter binding
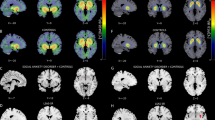
Groups did not differ in initial SERT binding (see Fig. 1 for whole sample SERT and DAT BPND). Symptom severity, measured with LSAS, showed negative associations with whole sample SERT BPND in the left dorsal ACC as previously reported [13]. Injected activity for both tracers can be found in Supplementary Table 1.
Regarding SSRI + ICBT treatment effects, the mean occupancy of the SERT across the a priori selected ROIs was >80% (Table 2, Supplementary Table 2) and there were significant positive relations between SERT occupancy and symptom improvement in the right NAcc, left putamen and left ACC in this group (Table 3, Fig. 2). The mean (±SD) concentrations (nMol/l) of blood serum escitalopram and desmethylescitalopram after treatment were 78.3 (±42.7) and 35.0 (±14.4) respectively in the SSRI + ICBT group. Concentrations were 0 for the same measures after placebo+ICBT. Neither of these measures were significantly correlated with symptom improvement (P > 0.27) or SERT occupancy (P > 0.11).
In the placebo+ICBT arm, there was a significant increase in SERT binding potential from pre- to posttreatment in the raphe nuclei (Table 2) and, when applying a more lenient statistical threshold (PFWE < 0.10), also in the right amygdala (PFWE = 0.068, MNI: 20 4 –16, Z = 2.63), right putamen (PFWE = 0.056, MNI: 20 18 –10, Z = 3.16) and right NAcc (PFWE = 0.061, MNI: 12 12 –12, Z = 2.18). No significant associations between change in SERT binding and symptom improvement were detected in the placebo+ICBT group.
Dopamine transporter binding
Baseline DAT BPND did not differ between groups and was not significantly related to symptom severity. Both groups showed increases in DAT availability after treatment in the bilateral amygdala, hippocampus, NAcc, and putamen (Table 2). However, groups differed significantly in their association between the pre-post change in DAT BPND and symptom improvement (LSAS) (Table 3, Fig. 3). In the SSRI + ICBT group, increased DAT BPND in the left amygdala (Table 3) and less robustly also in the left NAcc (PFWE = 0.087, MNI: −6 12 −8, Z = 2.12), was related to lesser symptom improvement. Conversely, in the placebo+ICBT group, increased DAT binding in the left NAcc was associated with larger improvement (Table 3).
Serotonin-dopamine transporter co-expression
At baseline, voxel-wise regressions of binding potentials for the whole sample revealed significant SERT-DAT co-expression (positive beta values) in all ROIs, which remained for most regions at post treatment (Supplementary Table 3). Higher baseline co-expression in the left putamen and left thalamus was associated with more severe social anxiety (Supplementary Table 4). In SSRI + ICBT subjects, higher pre-treatment SERT-DAT co-expression in the right NAcc, left putamen, right pallidum and right thalamus, significantly predicted symptom reduction with treatment (Supplementary Table 5). In placebo + ICBT subjects, the same pattern was found in the thalamus, whereas in the right amygdala, bilateral hippocampus, left putamen and right pallidum initially lower SERT-DAT co-expression was significantly associated with larger symptom reduction following treatment. Neither SERT nor DAT binding at baseline was predictive of treatment outcome by themselves. See also supplementary material.
Discussion
By use of PET and two selective radioligands, we examined parallel changes in serotonin and dopamine transporters resulting from 9 weeks of combined pharmacologic (SSRI) and psychological (ICBT) treatment for social anxiety under double-blind randomized conditions. Both SSRI + ICBT and placebo + ICBT resulted in significant improvement on the main social anxiety outcome (LSAS), with a trend towards higher number of responders in the SSRI arm. Since the clinical measures of the full cohort has already been evaluated [4], the aim of the current study was not to verify differential treatment efficacy, but to evaluate if monoaminergic transporter mechanisms underlying symptom improvement differ between the two treatment modalities. Both treatment combinations yielded similar pre-to-post increases in DAT availability in limbic and striatal regions but associations with symptom reduction differed in direction across treatment groups. Baseline SERT-DAT co-expression was high, and showed positive relations with initial symptom severity. Co-expression also predicted treatment outcome, albeit again in different directions in the two groups.
As expected, only the SSRI + ICBT combination yielded a SERT occupancy rate consistent with SSRI efficacy. The mean level of all investigated ROIs was >80%, indicating good compliance with SSRI medication, as also verified by analyses of serum metabolites. It has been suggested that an occupancy rate of 76–85% is sufficient to yield a therapeutic effect [17, 18]. Moreover, in the NAcc, putamen and ACC, SERT occupancy was significantly associated with symptom improvement which has not been reported before in SAD [19, 20], but has been infrequently observed in other disorders like geriatric depression [25]. With the current design, it cannot be excluded that ICBT moderated this effect.
Studies of depression have suggested that SERT binding increases after CBT [72, 73]. Consistently, in the placebo+ICBT arm, we observed increased (pre-post) SERT binding in the raphe nuclei, although this effect was not related to clinical improvement. The raphe is regarded as an important target for SSRIs due to its high concentration of serotonergic neurons, and PET studies of SAD have demonstrated lower serotonin 1 A binding [14] and increased serotonin synthesis [13] in this region before treatment. The current results indicate that raphe serotonergic activity could also be modulated by ICBT, in line with findings of reduced serotonin 1B receptor binding in raphe after ICBT for major depressive disorder [74]. Increased SERT binding, suggesting faster serotonin clearance, is interesting in the context of reduced serotonin synthesis reported after anxiolytic treatment [68]. However, ICBT effects on raphe SERTs did not occur concomitantly with altered serotonin transport in other regions.
Regarding dopamine transport, both groups exhibited a general increase in DAT binding with treatment. In SAD, similar effects of escitalopram have previously been reported in a SPECT study, where DAT increases were limited to the left dorsal striatum and did not correlate with symptom improvement [48]. Additionally, in a recent PET study from our group on SSRI response expectancies [20], DAT BPND increases were found in the hippocampus and pallidum, but only in the treatment group with lowered expectancies of improvement induced by verbal instructions. Since similar DAT increases were observed in both groups in the current study, it is possible that these changes were mainly driven by ICBT, i.e., dopaminergic changes might be more pronounced with ICBT than SSRI-treatment.
Despite common DAT increases, the two treatment groups showed inverse associations between DAT BPND change and treatment outcome. With SSRI + ICBT, symptom improvement was negatively associated with DAT change in the amygdala. Further, in the ventral striatum and thalamus, symptom improvement was associated with smaller DAT increases in the SSRI + ICBT arm relative to placebo+ICBT where positive associations were noted. Similarly, we previously observed that pre-post reductions in DAT improved symptoms in SAD patients treated openly with escitalopram [20]. The current data, however, suggests a different role of dopamine in ICBT. Stronger association between increased DAT BPND in NAcc and symptom reduction with placebo+ICBT could possibly reflect that ICBT has a greater influence on the ventral striatum, known to be important for approach-avoidance conflict resolution, reward processing and plays a major role in placebo responses [75,76,77]. The noted association between ICBT outcome and the overall DAT increases suggest that further study of appetitive/approach elements of CBT and their associations with dopamine function is warranted. Speculatively, SSRIs may act more by modulating amygdala threat signaling. For example, SSRI + ICBT yielded stronger attenuation of amygdala BOLD reactivity to emotional faces than placebo+ICBT in the larger cohort [4]. Very few studies have examined dopaminergic changes in SAD, non-confounded by pharmacological treatment, but Cervenka and coworkers [53] found increased D2 receptor binding potential in limbic and pre-frontal areas with ICBT. Since changes both in D2 and DAT parameters can be linked to treatment outcome in SAD, and since D2 autoreceptors regulate dopamine synthesis and DAT expression [78], further research on both dopamine sub-systems is warranted. As we mentioned previously in a study of a different cohort [20], our PET data may indicate dopaminergic dysfunction in SAD similar to at least some subgroups of treatment resistant depression for which dopamine agonists could be effective [79]. However, dopaminergic medications have not stood out as particularly effective on their own, although this topic is understudied [80, 81].
In all evaluated brain regions, there was significant positive co-expression of SERT and DATs at baseline which exhibited a positive relation with symptom severity. We have reported a similar relationship in a different cohort, i.e., a significantly higher correlation coefficient between SERT and DAT BPND in SAD patients relative to healthy controls [15]. Thus, upregulated monoamine co-expression could be involved in the pathophysiology of SAD. Differences were also noted regarding prediction of treatment outcome. In the SSRI + ICBT group, high initial SERT-DAT co-expression in striatal-thalamic areas predicted better treatment outcome, which was also found for the thalamus in the placebo+ICBT group, whereas for other brain regions, high initial SERT-DAT co-expression was generally disadvantageous for treatment success with placebo+ICBT. Similarly, in the larger cohort, we previously demonstrated that initial neural activations of the dACC in response to emotional faces, predicted outcome in different directions in the two treatment modalities [82].
The multi-tracer PET methodology enabling analysis of transporter co-expression, the double-blind RCT design, and inclusion of a non-pharmacologic treatment group are major strengths of our study, but there are also limitations to consider. First, an additional control group to the ICBT condition, e.g., a waiting-list, no-treatment, or placebo-only control, would have been helpful to capture the complete contribution of ICBT. Second, voxel-based analyses are likely more spatially sensitive than regional mean approaches but might also be more susceptible to noise due to the smaller number of activity counts detected within the limited volume and due to smoothing of parametric images especially in smaller ROIs. Also, the complex dynamics between serotonin and dopamine signaling cannot be uncovered by PET data on transporters only and the longevity of the transporter changes needs further evaluation. Another limitation is that analyses were not adjusted for menstrual cycle phase. Moreover, although PET is a more sensitive and precise imaging technique than SPECT, the restricted sample size warrants some caution, especially regarding the SERT×DAT interactions linked to symptom reduction (see supplementary material), because the number of regressors were large in relation to sample size.
In conclusion, the current study replicates and extends several of our previous PET findings in SAD [15, 20], mainly that SAD patients before treatment exhibit strong positive SERT-DAT associations related to symptom severity, that clinical doses of escitalopram result in high (>80%) SERT occupancy, here associated with clinical improvement, and that reductions or lesser increases of DAT availability are associated with better outcome in SSRI-treated patients. Results further suggest that monoamine transporter co-expression has an impact on symptom remission with treatment and that pharmacologic and psychosocial treatments modulate the transporter proteins in disparate ways.
References
Stein MB, Stein DJ. Social anxiety disorder. Lancet. 2008;371:1115–25.
Wang PS, Lane M, Olfson M, Pincus HA, Wells KB, Kessler RC. Twelve-month use of mental health services in the United States. Arch Gen Psychiatry. 2005;62:629.
Mayo-Wilson E, Dias S, Mavranezouli I, Kew K, Clark DM, Ades AE, et al. Psychological and pharmacological interventions for social anxiety disorder in adults: a systematic review and network meta-analysis. lancet Psychiatry. 2014;1:368–76.
Gingnell M, Frick A, Engman J, Alaie I, Björkstrand J, Faria V, et al. Combining escitalopram and cognitive–behavioural therapy for social anxiety disorder: Randomised controlled fMRI trial. Br J Psychiatry. 2016;209:229–35.
Doehrmann O, Ghosh SS, Polli FE, Reynolds GO, Horn F, Keshavan A et al. Predicting treatment response in social anxiety disorder from functional magnetic resonance imaging. JAMA Psychiatry. 2013;70:87–97.
Goldin PR, Ziv M, Jazaieri H, Hahn K, Heimberg R, Gross JJ. Impact of cognitive behavioral therapy for social anxiety disorder on the neural dynamics of cognitive reappraisal of negative self-beliefs: randomized clinical trial. JAMA Psychiatry. 2013;70:1048–56.
Klumpp H, Fitzgerald DA, Phan KL. Neural predictors and mechanisms of cognitive behavioral therapy on threat processing in social anxiety disorder. Prog Neuro Psychopharmacol Biol Psychiatry. 2013;45:83–91.
Furmark T, Tillfors M, Marteinsdottir I, Fischer H, Pissiota A, Långström B, et al. Common changes in cerebral blood flow in patients with social phobia preated with citalopram or cognitive-behavioral therapy. Arch Gen Psychiatry. 2002;59:425–33.
Månsson KNTT, Carlbring P, Frick A, Engman J, Olsson C-JJ, Bodlund O, et al. Altered neural correlates of affective processing after internet-delivered cognitive behavior therapy for social anxiety disorder. Psychiatry Res Neuroimaging. 2013;214:229–37.
Furmark T, Appel L, Michelgård Å, Wahlstedt K, Åhs F, Zancan S, et al. Cerebral blood flow changes after treatment of social phobia with the neurokinin-1 antagonist GR205171, citalopram, or placebo. Biol Psychiatry. 2005;58:132–42.
Leichsenring F, Leweke F. Social anxiety disorder. N Engl J Med. 2017;376:2255–64.
Griebel G. 5-Hydroxytryptamine-interacting drugs in animal models of anxiety disorders: More than 30 years of research. Pharm Ther. 1995;65:319–95.
Frick A, Åhs F, Engman J, Jonasson M, Alaie I, Björkstrand J, et al. Serotonin synthesis and reuptake in social anxiety disorder a positron emission tomography study. JAMA Psychiatry. 2015;72:794–802.
Lanzenberger RR, Mitterhauser M, Spindelegger C, Wadsak W, Klein N, Mien L, et al. Reduced serotonin-1A receptor binding in social anxiety disorder. Biol Psychiatry. 2006;61:1081–9.
Hjorth OR, Frick A, Gingnell M, Hoppe JM, Faria V, Hultberg S et al. Expression and co-expression of serotonin and dopamine transporters in social anxiety disorder: a multitracer positron emission tomography study. Mol Psychiatry. 2021;29:3970–79.
Hiemke C, Härtter S. Pharmacokinetics of selective serotonin reuptake inhibitors. Pharm Ther. 2000;85:11–28.
Voineskos AN, Wilson AA, Boovariwala A, Sagrati S, Houle S, Rusjan P, et al. Serotonin transporter occupancy of high-dose selective serotonin reuptake inhibitors during major depressive disorder measured with [11C]DASB positron emission tomography. Psychopharmacology. 2007;193:539–45.
Meyer JH, Wilson AA, Sagrati S, Hussey D, Carella A, Potter WZ, et al. Serotonin transporter occupancy of five selective serotonin reuptake inhibitors at different doses: an [11C]DASB positron emission tomography study. Am J Psychiatry. 2004;161:826–35.
Kent J, Coplan J, Lombardo I, Hwang D-R, Huang Y, Mawlawi O, et al. Occupancy of brain serotonin transporters during treatment with paroxetine in patients with social phobia: a positron emission tomography study with [11 C]McN 5652. Psychopharmacology. 2002;164:341–8.
Hjorth OR, Frick A, Gingnell M, Hoppe JM, Faria V, Hultberg S, et al. Expectancy effects on serotonin and dopamine transporters during SSRI treatment of social anxiety disorder: a randomized clinical trial. Transl Psychiatry. 2021;11:559.
Catafau AM, Perez V, Plaza P, Pascual J-C, Bullich S, Suarez M, et al. Serotonin transporter occupancy induced by paroxetine in patients with major depression disorder: a 123I-ADAM SPECT study. Psychopharmacology. 2006;189:145–53.
Cavanagh J, Patterson J, Pimlott S, Dewar D, Eersels J, Dempsey MF, et al. Serotonin transporter residual availability during long-term antidepressant therapy does not differentiate responder and nonresponder unipolar patients. Biol Psychiatry. 2006;59:301–8.
Herold N, Uebelhack K, Franke L, Amthauer H, Luedemann L, Bruhn H, et al. Imaging of serotonin transporters and its blockade by citalopram in patients with major depression using a novel SPECT ligand [123I]-ADAM. J Neural Transm. 2006;113:659–70.
Ruhé HG, Ooteman W, Booij J, Michel MC, Moeton M, Baas F, et al. Serotonin transporter gene promoter polymorphisms modify the association between paroxetine serotonin transporter occupancy and clinical response in major depressive disorder. Pharmacogenet Genom. 2009;19:67–76.
Smith GS, Kahn A, Sacher J, Rusjan P, van Eimeren T, Flint A, et al. Serotonin transporter occupancy and the functional neuroanatomic effects of citalopram in geriatric depression. Am J Geriatr Psychiatry. 2011;19:1016–25.
Harmer CJ. Serotonin and emotional processing: does it help explain antidepressant drug action? Neuropharmacology. 2008;55:1023–8.
Harmer CJ. Have no fear: the neural basis of anxiolytic drug action in generalized social phobia. Biol Psychiatry. 2013;73:300–1.
Gałecki P, Mossakowska-Wójcik J, Talarowska M. The anti-inflammatory mechanism of antidepressants – SSRIs, SNRIs. Prog Neuro Psychopharmacol Biol Psychiatry. 2018;80:291–4.
Kitaichi Y, Inoue T, Nakagawa S, Boku S, Kakuta A, Izumi T, et al. Sertraline increases extracellular levels not only of serotonin, but also of dopamine in the nucleus accumbens and striatum of rats. Eur J Pharm. 2010;647:90–96.
Tritschler L, Gaillard R, Gardier AM, David DJ, Guilloux J-P. Consequences of the monoaminergic systems cross-talk in the antidepressant activity. Encephale. 2018;44:264–73.
Macgillivray LES. The regulation of brain serotonergic and dopaminergic neurons: the modulatory effects of selective serotonin reuptake inhibitors, atypical neuroleptics and environmental enrichment. McMaster University; Hamilton, ON, 2012.
Hood SD, Potokar JP, Davies SJC, Hince DA, Morris K, Sedon KM, et al. Dopaminergic challenges in social anxiety disorder: evidence for dopamine D 3 desensitisation following successful treatment with serotonergic antidepressants. J Psychopharmacol. 2010;24:709–16.
Schneier FR, Liebowitz MR, Abi-dargham A, Zea-ponce Y, Lin S-H, Laruelle M. Low dopamine D 2 receptor binding potential in social phobia. Am J Psychiatry. 2000;157:457–9.
Bergman O, Åhs F, Furmark T, Appel L, Linnman C, Faria V, et al. Association between amygdala reactivity and a dopamine transporter gene polymorphism. Transl Psychiatry. 2014;4:e420.
Schneier FR, Abi-Dargham A, Martinez D, Slifstein M, Hwang D-R, Liebowitz MR, et al. Dopamine transporters, D2 receptors, and dopamine release in generalized social anxiety disorder. Depress Anxiety. 2009;8:1–8.
Prediger RDS, Matheus FC, Schwarzbold ML, Lima MMS, Vital MABF. Anxiety in Parkinson’s disease: a critical review of experimental and clinical studies. Neuropharmacology. 2012;62:115–24.
Plavén Sigray P, Hedman E, Victorsson P, Matheson GJ, Forsberg A, Radu Djurfeldt D et al. Extrastriatal dopamine D2-receptor availability in social anxiety disorder. Eur Neuropsychopharmacol. 2017. https://doi.org/10.1016/j.euroneuro.2017.03.007.
Tiihonen J, Kuikka J, Bergström K, Lepola U, Koponen H, Leinonen E. Dopamine reuptake site densities in patients with social phobia. Am J Psychiatry. 1997;154:239–42.
van der Wee NJ, van Veen JF, Stevens H, van Vliet IM, van Rijk PP, Westenberg HG. Increased serotonin and dopamine transporter binding in psychotropic medication-naive patients with generalized social anxiety disorder shown by 123I-beta-(4-iodophenyl)-tropane SPECT. J Nucl Med. 2008;49:757–63.
Moriyama TS, Felicio AC, Chagas MHN, Tardelli VS, Ferraz HB, Tumas V, et al. Increased dopamine transporter density in Parkinson’s disease patients with Social Anxiety Disorder. J Neurol Sci. 2011;310:53–57.
Esposito E, Di Matteo V, Di Giovanni G. Serotonin-dopamine interaction: an overview. Prog Brain Res. 2008;172:3–6.
Di Matteo V, Di Giovanni G, Pierucci M, Esposito E. Serotonin control of central dopaminergic function: focus on in vivo microdialysis studies. Prog Brain Res. 2008;172:7–44.
Dremencov E, Mansari MEL, Blier P. Effects of sustained serotonin reuptake inhibition on the firing of dopamine neurons in the rat ventral tegmental area. J Psychiatry Neurosci. 2009;34:223–9.
Callaghan PD, Irvine RJ, Daws LC. Differences in the in vivo dynamics of neurotransmitter release and serotonin uptake after acute para-methoxyamphetamine and 3,4-methylenedioxymethamphetamine revealed by chronoamperometry. Neurochem Int. 2005;47:350–61.
Zhou F-M, Liang Y, Salas R, Zhang L, De Biasi M, Dani JA. Corelease of dopamine and serotonin from striatal dopamine terminals. Neuron. 2005;46:65–74.
Daws LC. Unfaithful neurotransmitter transporters: focus on serotonin uptake and implications for antidepressant efficacy. Pharm Ther. 2009;121:89–99.
Mössner R, Simantov R, Marx A, Lesch KP, Seif I. Aberrant accumulation of serotonin in dopaminergic neurons. Neurosci Lett. 2006;401:49–54.
Warwick JM, Carey PD, Cassimjee N, Lochner C, Hemmings S, Moolman-Smook H, et al. Dopamine transporter binding in social anxiety disorder: the effect of treatment with escitalopram. Metab Brain Dis. 2012;27:151–8.
Kugaya A, Seneca NM, Snyder PJ, Williams SA, Malison RT, Baldwin RM, et al. Changes in human in vivo serotonin and dopamine transporter availabilities during chronic antidepressant administration. Neuropsychopharmacology. 2003;28:413–20.
Pogarell O, Poepperl G, Mulert C, Hamann C, Sadowsky N, Riedel M, et al. SERT and DAT availabilities under citalopram treatment in obsessive-compulsive disorder (OCD). Eur Neuropsychopharmacol. 2005;15:521–4.
Rominger A, Cumming P, Brendel M, Xiong G, Zach C, Karch S, et al. Altered serotonin and dopamine transporter availabilities in brain of depressed patients upon treatment with escitalopram: a [123I]$β$-CIT SPECT study. Eur Neuropsychopharmacol. 2015;25:873–81.
Booij J, de Jong J, de Bruin K, Knol R, de Win MML, van Eck-Smit BLF. Quantification of striatal dopamine transporters with 123I-FP-CIT SPECT is influenced by the selective serotonin reuptake inhibitor paroxetine: a double-blind, placebo-controlled, crossover study in healthy control subjects. J Nucl Med. 2007;48:359–66.
Cervenka S, Hedman E, Ikoma Y, Djurfeldt DR, Rück C, Halldin C, et al. Changes in dopamine D2-receptor binding are associated to symptom reduction after psychotherapy in social anxiety disorder. Transl Psychiatry. 2012;2:e120.
Carlbring P, Andersson G, Cuijpers P, Riper H, Hedman-Lagerlöf E. Internet-based vs. face-to-face cognitive behavior therapy for psychiatric and somatic disorders: an updated systematic review and meta-analysis. Cogn Behav Ther. 2018;47:1–18.
Andersson G, Carlbring P, Holmström A, Sparthan E, Furmark T, Nilsson-Ihrfelt E, et al. Internet-based self-help with therapist feedback and in vivo group exposure for social phobia: a randomized controlled trial. J Consult Clin Psychol. 2006;74:677–86.
Furmark T, Tillfors M, Everz P-OO, Marteinsdottir I, Gefvert O, Fredrikson M. Social phobia in the general population: prevalence and sociodemographic profile. Soc Psychiatry Psychiatr Epidemiol. 1999;34:416–24.
Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9.
Sheehan D V, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59: 22–33;quiz 34-57.
First MB, Spitzer RL, Gibbon MB, Williams J. Structured clinical interview for DSM-IV axis i disorders – non-patient edition. New York: New York State Psychiatric Institute. 1997.
Carlbring P, Gunnarsdóttir M, Hedensjo L, Andersson G, Ekselius L, Furmark T. Treatment of social phobia: randomised trial of internet-delivered cognitive- behavioural therapy with telephone support. Br J Psychiatry. 2007;190:123–8.
Furmark T, Carlbring P, Hedman E, Sonnenstein A, Clevberger P, Bohman B, et al. Guided and unguided self-help for social anxiety disorder: randomised controlled trial. Br J Psychiatry. 2009;195:440–7.
Hedman E, Andersson G, Ljótsson B, Andersson E, Rück C, Mörtberg E, et al. Internet-based cognitive behavior therapy vs. cognitive behavioral group therapy for social anxiety disorder: a randomized controlled non-inferiority trial. PLoS One. 2011;6:e18001.
Clark DM, Wells A. A cognitive model of social anxiety. In: Heimberg RG, Liebowitz MR, Hope DA, Schneier FR, editors. Social phobia: diagnosis, assessment and treatment. Guilford Press: New York, NY, 1995, pp 69–93.
Fresco DM, Coles ME, Heimberg RG, Liebowitz MR, Hami S, Stein MB, et al. The Liebowitz Social Anxiety Scale: a comparison of the psychometric properties of self-report and clinician-administered formats. Psychol Med. 2001;31:1025–35.
Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ. Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. Neuroimage. 1997;6:279–87.
Logan J, Fowler JS, Volkow ND, Wang G-J, Ding Y-S, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16:834–40.
Svarer C, Madsen K, Hasselbalch SG, Pinborg LH, Haugbøl S, Frøkjær VG, et al. MR-based automatic delineation of volumes of interest in human brain PET images using probability maps. Neuroimage. 2005;24:969–79.
Frick A, Åhs F, Appel L, Jonasson M, Wahlstedt K, Bani M, et al. Reduced serotonin synthesis and regional cerebral blood flow after anxiolytic treatment of social anxiety disorder. Eur Neuropsychopharmacol. 2016;26:1775–83.
Hjorth OR, Frick A, Gingnell M, Motilla-Hoppe J, Faria V, Hultberg S et al. Regional co-expression of serotonin and dopamine transporters in social anxiety disorder: a multi-tracer positron emission tomography study. submitted. 246:1–24.
Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–9.
Hammers A, Allom R, Koepp MJ, Free SL, Myers R, Lemieux L, et al. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum Brain Mapp. 2003;19:224–47.
Amsterdam JD, Newberg AB, Newman CF, Shults J, Wintering N, Soeller I. Change over time in brain serotonin transporter binding in major depression: effects of therapy measured with [123 I]-ADAM SPECT. J Neuroimaging. 2013;23:469–76.
Svensson JE, Svanborg C, Plavén-Sigray P, Kaldo V, Halldin C, Schain M, et al. Serotonin transporter availability increases in patients recovering from a depressive episode. Transl Psychiatry. 2021;11:264.
Tiger M, Rück C, Forsberg A, Varrone A, Lindefors N, Halldin C, et al. Reduced 5-HT1B receptor binding in the dorsal brain stem after cognitive behavioural therapy of major depressive disorder. Psychiatry Res Neuroimaging. 2014;223:164–70.
Hamel L, Thangarasa T, Samadi O, Ito R. Caudal nucleus accumbens core is critical in the regulation of cue-elicited approach-avoidance decisions. eneuro. 2017;4:ENEURO.0330–16.2017.
Kohls G, Perino MT, Taylor JM, Madva EN, Cayless SJ, Troiani V, et al. The nucleus accumbens is involved in both the pursuit of social reward and the avoidance of social punishment. Neuropsychologia. 2013;51:2062–9.
Petrovic P, Dietrich T, Fransson P, Andersson J, Carlsson K, Ingvar M. Placebo in emotional processing–induced expectations of anxiety relief activate a generalized modulatory network. Neuron. 2005;46:957–69.
Ford CP. The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience. 2014;282:13–22.
Hori H, Kunugi H. Dopamine agonist-responsive depression. Psychogeriatrics. 2013;13:189–95.
Simpson HB, Schneier FR, Marshall RD, Campeas RB, Vermes D, Silvestre J, et al. Low dose selegiline (L-Deprenyl) in social phobia. Depress Anxiety. 1998;7:126–9.
Villarreal G, Johnson MR, Rubey R, Lydiard RB, Ballanger JC. Treatment of social phobia with the dopamine agonist pergolide. Depress Anxiety. 2000;11:45–47.
Frick A, Engman J, Wahlstedt K, Gingnell M, Fredrikson M, Furmark T. Anterior cingulate cortex activity as a candidate biomarker for treatment selection in social anxiety disorder. BJPsych Open. 2018;4:157–9.
Acknowledgements
The study was supported by the Swedish Research Council, the Swedish Brain Foundation and Riksbankens Jubileumsfond - the Swedish Foundation for Humanities and Social Sciences.
Funding
Open access funding provided by Uppsala University.
Author information
Authors and Affiliations
Contributions
Conceptualization: OH, AF, TF Data curation: OH, AF, Formal analysis: OH, Funding acquisition: TF Investigation: AF, VF, MG, GA, PC, JE, MR, JB, MJ, ML, IA, KW, MF, TF Supervision: MF, TF Writing – original draft: OH, AF, TF Writing – review & editing: OH, AF, VF, MG, GA, PC, JE, MR, JB, MJ, ML, IA, KW, MF, TF.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hjorth, O., Frick, A., Gingnell, M. et al. Serotonin and dopamine transporter availability in social anxiety disorder after combined treatment with escitalopram and cognitive-behavioral therapy. Transl Psychiatry 12, 436 (2022). https://doi.org/10.1038/s41398-022-02187-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-022-02187-3
- Springer Nature Limited