Abstract
Background
Several pioneering studies investigated deep brain stimulation (DBS) in treatment-refractory anorexia nervosa (AN) patients, but overall effects remain yet unclear. Aim of this study was to obtain estimates of efficacy of DBS in AN-patients using meta-analysis.
Methods
We searched three electronic databases until 1st of November 2021, using terms related to DBS and AN. We included trials that investigated the clinical effects of DBS in AN-patients. We obtained data including psychiatric comorbidities, medication use, DBS target, and study duration. Primary outcome was Body Mass Index (BMI), secondary outcome was quality of life, and the severity of psychiatric symptoms, including eating disorder, obsessive-compulsive, depressive, and anxiety symptoms. We assessed the risk of bias using the ROBINS-I tool.
Results
Four studies were included for meta-analysis, with a total of 56 patients with treatment-refractory AN. Follow-up ranged from 6–24 months. Random effects meta-analysis showed a significant increase in BMI following DBS, with a large effect size (Hedges’s g = 1 ∙ 13; 95% CI = 0 ∙ 80 to 1 ∙ 46; Z-value = 6 ∙ 75; P < 0 ∙ 001), without heterogeneity (I2 = 0 ∙ 00, P = 0 ∙ 901). Random effects meta-analysis also showed a significant increase in quality of life (Hedges’s g = 0 ∙ 86; 95% CI = 0 ∙ 44 to 1 ∙ 28; Z-value = 4 ∙ 01, P < 0 ∙ 001). Furthermore, DBS decreased the severity of psychiatric symptoms (Hedges’s g = 0 ∙ 89; 95% CI = 0 ∙ 57 to 1 ∙ 21; Z-value = 5 ∙ 47; P < 0 ∙ 001, I2 = 4 ∙ 29, P = 0 ∙ 371).
Discussion
In this first meta-analysis, DBS showed statistically large beneficial effects on weight restoration, quality of life, and reduction of psychiatric symptoms in patients with treatment-refractory AN. These outcomes call for more extensive naturalistic studies to determine the clinical relevance for functional recovery.
This study is preregistered in PROSPERO,CRD42022295712.
Similar content being viewed by others
Introduction
Anorexia nervosa (AN) has an alarming mortality rate [1,2,3]. About 20% of AN patients remain treatment-refractory to psychotherapy and pharmacological treatment aimed at weight restoration [4]. To ameliorate this grim perspective, there is an urgent need for novel treatment options.
One promising new treatment is deep brain stimulation (DBS). DBS is neuromodulation therapy involving implantation of electrodes at targeted brain areas, which conduct electrical impulses to the brain tissue. These electrical impulses are controlled by a neurostimulator, a device similar to a pacemaker [5]. DBS is already established as an effective treatment for Parkinson’s disease (PD), essential tremor, idiopathic dystonia, epilepsy, and obsessive-compulsive disorder (OCD) [6, 7].
The idea of treating AN with DBS came from serendipitous positive effects that were noted in earlier DBS studies for other indications. In these case reports, patients were being treated with DBS for major depressive disorder (MDD) [8, 9] or OCD [10, 11], while they simultaneously suffered from comorbid AN. Follow-up showed not only a decrease of the MDD or OCD symptoms, but also an improvement of the AN symptoms, including cognitive and emotional symptoms. Patients presented significant improvement in BMI and decreased anxiety and distress in relation to weight gain [8,9,10,11].
DBS also holds promise for understanding AN from a pathophysiological perspective. The reward system has been proposed as a key brain circuit in AN [12]. This system regulates motivation and hedonic experience of food intake and provides feedback on the value of a specific food. Several lines of evidence show disturbances in the reward system in AN-patients, including failure to connect appropriate responses to stimuli [13], and limited awareness of intero- and exteroceptive homoeostatic triggers [14, 15]. Moreover, these disturbances in the reward system are linked to formation of habits [16], reflected in repetitive anorectic behaviours and associated altered activation of striatal brain areas [13, 17]. DBS has been shown to directly influence the reward and habit brain circuits, normalizing the aberrant activity associated with psychiatric disorders [18].
Based on these serendipitous clinical observations and pathophysiological insights, pioneering studies tested the effects of DBS in patients with AN. In 2013, Lipsman et al. hypothesized the subcallosal cingulate cortex (SCC) and the nucleus accumbens (NAcc) to be effective DBS targets for AN because they met the following criteria; (1) prominent afferent and efferent connection with the anterior insula, (2) involved in reward-processing, (3) involved in AN-related provocation and imaging studies, and (4) involved in anxiety and dysphoric mood [14]. Indeed, studies observed positive effects of both NAcc and SCC DBS on AN symptoms [6, 9]. Moreover, based on effectiveness in psychiatric disorders phenomenologically related to AN, including treatment-refractory OCD and depression, other targets such as the ventral anterior limb of the internal capsule (vALIC) have been successfully tested in AN [15, 19, 20].
Despite promising trial results, to our knowledge, a meta-analysis on the effects of DBS in AN had yet to be performed. In order to provide an estimate on the overall effect of DBS in AN, we performed such a meta-analysis, combining all available evidence from treatment trials. Based on previous case reports, we hypothesized a beneficial effect of DBS on weight restoration, quality of life, and reduction of psychiatric symptoms in AN patients. Results of this meta-analysis may justify further clinical application in future and more extensive naturalistic research.
Methods and materials
Following PRISMA guidelines [21], the systematic review and meta-analysis protocol was registered in PROSPERO [22].
Search strategy
A literature search was performed on MEDLINE, Embase, and PsycInfo, in the period between the last week of August 2020 and up to the 1st of November 2021 (Appendix 1). Publication date was not a restriction.
Selection process
Two independent reviewers (DMK, PC) screened titles and abstracts of found studies. We included clinical studies investigating the effects of DBS in patients with AN. Duplicates, case reports, and reviews were excluded. Articles were excluded if they did not cover AN and/or DBS, were based on animals, or contained <four participants. We did not exclude studies based on the availability of a control group, studies comparing outcomes before and after DBS will also be included. Inconsistencies were solved by means of discussion, if necessary, with a third and fourth reviewer (MSO, RJTM).
Critical appraisal and quality assessment
Critical appraisal of all included studies was performed independently in duplicate (DK, PC) by using the ROBINS-I tool (Risk Of Bias In Non-randomised Studies—of Interventions) [23]. Furthermore, we used GRADE [24] for assessing the overall quality of evidence. Inconsistencies were solved by means of discussion.
Data extraction and outcome data
The extracted data consisted of the following study characteristics: number of participants, number of time points, participants’ characteristics, study duration, and study outcome data. BMI change after DBS was considered our primary outcome. One study used the mean BMI achieved either in the year or in the 3-month prior to surgery, chosen depending on the characteristics of every patient [25]. The combined effect on psychiatric symptoms at the last observation was considered our secondary outcome. In this meta-analysis no other eating disorder related symptoms than BMI could be used as primary outcome data, because of inconsistencies in outcome assessment selection between the included studies. The secondary outcome data was represented using several scales: Yale-Brown-Cornell Eating Disorder Scale [26], Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) [27], Hamilton Anxiety Rating Scale (HAM-A) [28], and Hamilton Depression Rating Scale (HAM-D) [29]. Quality of life was assessed using the Quality of Life Scale [30], SF36 [31], and Eating Disorder Quality of Life [32]. Next to effectiveness data, we also extracted safety data including adverse effects and complications.
Statistical analyses
Main analyses
We used random effect models with Comprehensive Meta-Analysis V3 for our quantitative data synthesis. We used Hedges’ g for continuous results, with a 95% confidence interval and two-tailed p-values. Sensitivity analyses were performed using pre-operative BMI measures of Villalba et al. instead of the calculated reference BMI value [25]. Forest plots with I2 statistics were used to examine any study heterogeneity.
Publication bias
A funnel plot was plotted to assess publication bias. The classic and Orwin’s fail-safe N, Begg and Mazumdar rank correlation, and Egger’s regression intercept were calculated. Should it be deemed necessary Duval and Tweedie’s trim-and-fill method was used to report adjusted values.
Results
Study selection
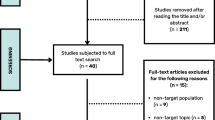
The literature search provided a set of 290 articles. 276 articles were excluded based on title and abstract [Fig. 1]. The full text of the fourteen remaining articles was reviewed. Duplicates and articles that turned out to be protocols were removed from analysis. Four of these fourteen articles were included for meta-analysis. Fig. 1 shows the process of study selection.
Study characteristics
Four included non-randomized non-controlled clinical trials contained a total of 56 participants. The follow-up period was either 6 [25], 12 [15, 33] or 24 months [4]. The average age of the participants over all studies was 29 ∙ 8 years, with a range of 18–57 years. The mean illness duration over the four included studies was 12 ∙ 8 years. All but one of the participants were female. All patients were diagnosed with AN, with either the restrictive (n = 28) or the (binge-)purging subtype (n = 28). All participants were defined as treatment-refractory, which for instance included numerous hospital admissions and extensive psychiatric treatment. Most patients suffered from psychiatric comorbidities (n = 54). These comorbidities were diagnosed as: MDD (n = 33), OCD (n = 19), anxiety/generalised anxiety disorder (GAD) (n = 11), post-traumatic stress disorder (PTSD) (n = 10), panic disorder (n = 3), borderline personality disorder (BPD) (n = 3), personality disorder not otherwise specified (PD-NOS) (n = 2), and substance use disorder (n = 2). Supplementary Table 1 shows all study and patients characteristics.
At time of surgery, eight participants were not taking medication, six used one drug and 42 used two drugs or more. The types of drugs consisted of SSRIs (selective and non-selective), benzodiazepine agonists, atypical antipsychotics, antiepileptics, and tetracyclic antidepressants. No major psychopharmacological adjustments were made during the follow-up.
32 out of 56 participants received DBS to the NAcc, 20 patients received DBS to the SCC, and 4 to the vALIC. Three of the studies [4, 15, 33] used either the SCC, NAcc, or the vALIC as the DBS target, while the fourth study [25] used either the SCC or NAcc for their patients, based on their primary comorbidity. 4 out of 8 participants received DBS to the SCC, and 4 to the NAcc in the last mentioned study. Overall, 3 out of 56 participants had their electrodes explanted before the end of follow-up [4, 33, 34].
Main analyses: effects on BMI
Random effects meta-analysis showed a significant increasing effect of DBS on primary outcome BMI change after DBS, with a large effect size (Hedges’s g = 1 ∙ 13; 95% CI = 0 ∙ 80 to 1 ∙ 46; Z-value = 6 ∙ 75; P < 0 ∙ 001, Fig. 2), without heterogeneity (I2 = 0 ∙ 00, P = 0 ∙ 901).
Secondary analysis: effects on psychiatric symptom domains
DBS also had a beneficial effect on secondary outcome combined psychiatric symptom severity at last observation (Hedges’s g = 0 ∙ 89; 95% CI = 0 ∙ 57 to 1 ∙ 21; Z-value = 5 ∙ 47; P < 0 ∙ 001, I2 = 4 ∙ 29, P = 0 ∙ 371, Supplementary Fig. 2).
Eating disorder symptoms
Three non-randomized non-controlled clinical trials [15, 25, 33] assessed eating disorder symptoms. Random effects analyses showed a beneficial main effect of 0 ∙ 98 (Hedges’s g; 95% CI = 0 ∙ 28 to 1 ∙ 68; Z-value = 2 ∙ 74; P = 0 ∙ 006; Supplementary Fig. 3).
Symptoms of depression
All studies assessed symptoms related to depression. Random effects analyses showed a beneficial main effect of 0 ∙ 98 (Hedges’s g; 95% CI = 0 ∙ 54 to 1 ∙ 41; Z-value = 4 ∙ 43; P = 0 ∙ 00; Supplementary Fig. 4).
Obsessive-compulsive symptoms
All studies assessed obsessive-compulsive symptoms. Random effects analyses showed a beneficial main effect of 0 ∙ 72 (Hedges’s g; 95% CI = 0 ∙ 39 to 1 ∙ 06; Z-value = 4 ∙ 19; P = 0 ∙ 00; Supplementary Fig. 5).
Symptoms of anxiety
Three non-randomized non-controlled clinical trials [4, 15, 25] assessed symptoms of anxiety. Random effects analyses showed a beneficial main effect of 0 ∙ 85 (Hedges’s g; 95% CI = 0 ∙ 38 to 1 ∙ 31; Z-value = 3 ∙ 55; P = 0 ∙ 00; Supplementary Fig. 6).
Secondary analysis: quality of life
Three non-randomized non-controlled clinical trials [15, 25, 33] assessed eating disorder symptoms. Random effects analyses showed a beneficial main effect of 0 ∙ 86 (Hedges’s g; 95% CI = 0 ∙ 44 to 1 ∙ 28; Z-value = 4 ∙ 01; P = 0 ∙ 00; Supplementary Fig. 7).
Adverse events
The most frequently occurring adverse events in the four studies were related to surgery or procedure; pain at incision site within <4 days (n = 22), pain at incision site after >4 days (n = 5), cutaneous complications (n = 4), and to stimulation procedure; hypomanic symptoms (n = 3), seizure (n = 3), and auto-intoxication (n = 3). Supplementary Table 2 lists all reported adverse events, including those possibly but not probably related to the intervention [15]. Other reported adverse events were either defined as unrelated to the intervention and attributed to underlying illness, or had no cause specified. Short-term side-effects like flush and sweating were observed during the programming of the DBS device, but were relieved with adjustment of the parameters.
Risk of bias and quality of evidence
Risk of bias summary is shown in Supplementary fig. 1. The risk of bias of the objective (BMI) and subjective (psychological outcomes) measurements of the included studies are respectively shown in supplementary Fig. 1a, b. Overall, the risk of bias was moderate for objective outcome measurements and serious for subjective outcome measurements. This was mainly caused by the lack of a control group in the included studies, potentially leading to biases. Furthermore, the studies did not report blinding of both the participants and the researchers, or assessment of the subjective outcomes by an independent party, leading to serious risk of potential bias in measurement of subjective psychological outcomes. No other clear evidence for biases was found.
Supplementary Table 3 shows details of the assessment of quality of the resulting evidence using GRADE. For the more objective outcome BMI, the quality of evidence that deep brain stimulation improves BMI in refractory-treatment AN-patients is considered moderate, whereas for subjective outcomes psychiatric symptoms and quality of life the quality of evidence is deemed low.
Publication bias
The classic fail-safe N was 40, Orwin’s fail-safe N was 42 with criterion for a ‘trivial’ standardized difference in means as 0 ∙ 1. This suggested that at least 40 studies without any effect must be reported to decrease the overall effect to a trivial effect. Concerning the Begg and Mazumbar rank correlation test, Kendall’s tau’s with as well as without continuity correction was 0 ∙ 00 (P2-sided = 1 ∙ 00), suggesting no publication bias. Egger’s regression intercept was 0 ∙ 23 (95% confidence interval: −3 ∙ 47 to 3 ∙ 94; P2-sided = 0 ∙ 81), also indicating no publication bias.
Duval and Tweedie’s trim-and-fill method used the random-effects model looking for missing studies to the left of the mean, meaning a less favourable effect of deep brain stimulation, showed one study that needed to be trimmed. This resulted in an effect size of 0 ∙ 91 (Q-value; 95% CI = 0 ∙ 79 to 1 ∙ 43). Fig. 3 shows the values of the Duval and Tweedie’s trim-and-fill method. Using a fixed effect model, the resulting point estimate did not change.
Sensitivity analyses
Using the pre-operative BMI instead of the reference BMI for the study of Villalba et al. resulted in a comparable overall effect of DBS on primary outcome BMI change after DBS, with a large effect size (Hedges’s g = 0 ∙ 90; 95% CI = 0 ∙ 58 to 1 ∙ 21; Z-value = 5 ∙ 60; P < 0 ∙ 001).
Discussion
This first meta-analysis combined all available trials on the effect of DBS in AN. Four non-randomized non-controlled studies provided a total of 56 participants. Random effects meta-analysis showed an improvement in primary outcome BMI, with a large effect size of 1 ∙ 13 and no heterogeneity. Moreover, meta-analysis showed a beneficial effect on overall psychiatric symptom severity, with a comparable large effect size of 0 ∙ 89. All four symptom domains showed a large effect: depressive symptoms (Hedges’s g = 0 ∙ 98), obsessive-compulsive symptoms (Hedges’s g = 0 ∙ 72), symptoms of anxiety (Hedges’s g = 0 ∙ 85), and eating disorder symptoms (Hedges’s g = 0 ∙ 98). Furthermore, DBS also improved the quality of life, with a large effect size (Hedges’s g = 0 ∙ 86). There was limited evidence for publication bias. The quality of evidence was considered moderate for the more objective primary outcome BMI, and low for the subjective secondary psychological outcome measurements. All in all, results suggest that DBS has statistically large beneficial effects in severe and life-threatening treatment-refractory AN.
Although no statistical heterogeneity was found between included studies, some differences are worth mentioning. It is important to note that the study of Villalba et al. had a relatively short period of follow-up of 6 months. This is particularly noteworthy in comparison to the study of Oudijn et al., where an optimization period was included prior to the maintenance phase leading to a maximal post-operative follow-up of 91 weeks. This shorter follow-up may explain the relatively low post-intervention BMI in Villalba et al., although this did not result in statistical heterogeneity between studies. However, the studies used different targets for DBS, possibly resulting in clinical heterogeneity. Another important difference is that only one study reported psychiatric adverse events related to DBS [15]. Other studies either experienced no psychological adverse events or did not report them systematically.
The risk of bias was considered moderate for objective outcome measurements (BMI) and serious for subjective secondary outcome measurements. This was caused by the lack of a control group, which ethically speaking would be difficult to implement in such a treatment-refractory patient group. A placebo effect could therefore not be excluded. Independent outcome assessors blinded for treatment status could theoretically ameliorate this bias. Nevertheless, we did not find any clear evidence for other biases.
The present meta-analysis showed statistically large effects of DBS in treatment-refractory AN for weight gain, whereas effect sizes otherwise found in literature for pharmacological and psychological treatment seem to be respectively moderate [35, 36] and low [37]. The effects of DBS in AN are in line with effects of DBS in other psychiatric disorders including depression and OCD [38].
The beneficial clinical effects of DBS in this meta-analyses may also improve insight in the pathophysiology of AN. It is hypothesised that DBS creates a reversible lesion to the stimulated area [6, 39]. However, recent evidence suggests that DBS modulates widespread brain network activity, e.g. normalizing neuronal firing in reward circuitry. Due to the uncertainties in the pathophysiology of AN and the working mechanisms of DBS, included trials used diverse stimulation sites, including the NAcc, SCC, and vALIC. However, the effects of the studies were comparable with low heterogeneity. This shows that DBS may be effective at diverse targets, potentially suggesting diverse inroads to normalize aberrant activity in comparable brain circuits. This is in line with the concept of connectomic DBS, where different implementation targets all relate to similar pathophysiologically relevant white matter tracts [40]. Moreover, these effects may be shared with other psychiatric disorders, where similar targets resulted in transdiagnostic beneficial effects [34, 41].
Literature on the biological effects of DBS in AN is sparse. Zhang et al. used FDG-PET to image glucose metabolism in the brain after DBS to the NAcc to identify which brain regions would be affected by DBS in AN [42], and noted a reduction of (hyper)metabolism in the lentiform nucleus, hippocampus, and frontal lobe. Further evidence comes from research other disorders. DBS to the NAcc in treatment-resistant depression showed metabolic decreases in prefrontal subregions, subgenual cingulate region, posterior cingulate cortex, thalamus and caudate nucleus. DBS of the anterior limb of the internal capsule in patients suffering from OCD, resulted in long-term changes in metabolic activity [43]. A decrease of frontal metabolism is a fundamental component of NAcc-DBS mechanism [44]. Moreover, Fridgeirsson et al. found an association in OCD between improvement in mood and anxiety with decreased functional connectivity between the amygdala and insula due to DBS [45]. These circuitries are also involved in AN, presumably, though the precise mechanisms of action of DBS remains to be determined.
Limitations and strengths
The main limitation of this study was the inability to rule out the presence of a placebo effect. The nature of DBS and particularly AN make it difficult to apply a double-blind study design. Nevertheless, effects, including on the objective outcome BMI, in this severe and extremely treatment-refractory population maintained over a study follow-up of up to 24 months, which argues for consistency and durability. Another limitation is the variation in stimulation targets between the studies. Nevertheless, heterogeneity was low, suggesting that the different stimulation sites have comparable effects. Furthermore, the overall risk of bias of the included studies was moderate to serious. This could be improved by adding an independent assessor to the study. Also the quality of evidence was considered moderate for the more objective primary outcome, and low for the subjective outcome measurements. The pooled results showed statistically large effects suggesting clinical relevance, however more parameters should be taken into account to allow the conclusion of relevant clinical functional improvement. It is important to note that our meta-analysis showed a large beneficial effect of DBS on BMI, however not all patients reached BMI in a normal range at the end of the follow-up. Also, three included studies reported only somatic adverse events related to surgery or stimulation. Only one study reported psychiatric adverse events related to DBS [15]. Finally, no international consensus has been reached on the definition of response to treatment or remission in anorexia nervosa in general, and of response to DBS in AN in particular. The included studies used heterogeneous definitions of response to DBS, therefore responder rates could not be determined. This study again emphasizes the need for such a consensus, as this would allow clinicians to assess the efficacy of this procedure.
A major strength of this study was that it is the first meta-analysis of the effect of DBS in treatment-refractory AN. Thereby, we provide an overall estimate of the size of the effect and the strength and quality of the evidence for the efficacy of DBS in treatment-refractory AN.
Research implications
Results from this meta-analyse provide several new inroads for future research. A first point of focus are the differences and similarities of the diverse DBS targets that have been applied. Studies might focus on clinical and biological differences in effects, e.g. by applying advanced clinical phenotyping and diverse biological assessments including advanced (connectomic) neuroimaging.
A second issue is prediction of response to improve patient selection. It would be helpful to identify factors that may predict response to DBS treatment in AN, and test whether the predictive value is strong enough for clinical implementation [46].
A third aspect is the combination with psychotherapy. DBS studies in psychiatry for other indications suggested that DBS may improve the response to psychotherapeutic interventions. It would be worthwhile to test whether this is also the case for AN-patients, and whether psychotherapy may increase the effectiveness of DBS.
An international database, including up-to-date naturalistic data from all AN-patients that are treated with DBS worldwide, may substantially increase power to test for subgroup effects.
Clinical implications
It is striking to note that deep brain stimulation, being effective in several psychiatric disorders, is relatively understudied in anorexia nervosa, being the world’s most lethal psychiatric disorder. However, it needs to be noted that, as of yet, both the mechanism of action of DBS and the pathophysiology of AN are not fully understood. To be able to understand and treat AN, more insight is needed in the complex dynamics in which this psychiatric disorder comes to expression. Using DBS targeting different brain areas gets us closer to this understanding or even finding the root of this complex disorder.
Of note, other forms of neuromodulation (i.e. electro-convulsive therapy) also show promising results in anorexia nervosa, particularly on symptoms of depression [47].
Our results suggest that DBS can be an effective last-resort treatment option in severe treatment-refractory AN. Despite the invasive nature of the procedure and the risk of side-effects, we suggest that there is a clinical indication for DBS in selected cases of AN, under strict monitoring and scientific evaluation of effects. Target selection should be based on the available experience of the involved neurosurgeon and psychiatric treatment team. After DBS, the potential effect of concomitant psychological and/or pharmacological therapy should be re-evaluated.
Conclusion
This meta-analysis demonstrated a statistically large beneficial effect of DBS on weight restoration, quality of life, and psychiatric symptoms severity in patients with treatment-refractory AN. Adverse effects were related to surgery and stimulation. The size of the beneficial effects suggest potential clinical relevance as a last-resort treatment option in severe and life-threatening AN. Future, more extensive, naturalistic research could strengthen conclusions regarding clinical relevance and incorporation in guidelines, also in relation to other forms of neuromodulation. These promising outcomes form new inspiration for future research, and may provide a more hopeful perspective for patients that did not yet respond sufficiently to other forms of therapy.
Registration and protocol
The systematic review and meta-analysis protocol was preregistered in PROSPERO under study’s registration number: CRD42022295712.
References
Arcelus J, Mitchell AJ, Wales J, Nielsen S. Mortality rates in patients with anorexia nervosa and other eating disorders. A meta-analysis of 36 studies. Arch Gen Psychiatry. 2011;68:724–31.
van Hoeken D, Hoek HW. Review of the burden of eating disorders: mortality, disability, costs, quality of life, and family burden. Curr Opin Psychiatry. 2020;33:521–7.
Hayes DJ, Lipsman N, Chen DQ, Woodside DB, Davis KD, Lozano AM, et al. Subcallosal cingulate connectivity in anorexia nervosa patients differs from healthy controls: a multi-tensor tractography study. Brain Stimul. 2015;8:758–68.
Liu W, Zhan S, Li D, Lin Z, Zhang C, Wang T, et al. Deep brain stimulation of the nucleus accumbens for treatment-refractory anorexia nervosa: a long-term follow-up study. Brain Stimul. 2020;13:643–9.
Benninger D, Schüpbach M. [Deep brain stimulation]. Ther Umsch. 2018;75:425–31.
Aum DJ, Tierney TS. Deep brain stimulation: foundations and future trends. Front Biosci (Landmark Ed). 2018;23:162–82.
Salanova V. Deep brain stimulation for epilepsy. Epilepsy Behav. 2018;88s:21–4.
Blomstedt P, Naesström M, Bodlund O. Deep brain stimulation in the bed nucleus of the stria terminalis and medial forebrain bundle in a patient with major depressive disorder and anorexia nervosa. Clin Case Rep. 2017;5:679–84.
Israël M, Steiger H, Kolivakis T, McGregor L, Sadikot AF. Deep brain stimulation in the subgenual cingulate cortex for an intractable eating disorder. Biol Psychiatry. 2010;67:e53–4.
Barbier J, Gabriëls L, van Laere K, Nuttin B. Successful anterior capsulotomy in comorbid anorexia nervosa and obsessive-compulsive disorder: case report. Neurosurgery. 2011;69:E745–51.
McLaughlin NC, Didie ER, Machado AG, Haber SN, Eskandar EN, Greenberg BD. Improvements in anorexia symptoms after deep brain stimulation for intractable obsessive-compulsive disorder. Biol Psychiatry. 2013;73:e29–31.
O’Hara CB, Campbell IC, Schmidt U. A reward-centred model of anorexia nervosa: a focussed narrative review of the neurological and psychophysiological literature. Neurosci Biobehav Rev. 2015;52:131–52.
Kaye WH, Fudge JL, Paulus M. New insights into symptoms and neurocircuit function of anorexia nervosa. Nat Rev Neurosci. 2009;10:573–84.
Lipsman N, Woodside B, Lozano AM. Evaluating the potential of deep brain stimulation for treatment-resistant anorexia nervosa. Handb Clin Neurol. 2013;116:271–6.
Oudijn MS, Mocking RJT, Wijnker RR, Lok A, Schuurman PR, van den Munckhof P, et al. Deep brain stimulation of the ventral anterior limb of the capsula interna in patients with treatment-refractory anorexia nervosa. Brain Stimul. 2021;14:1528–30.
Steward T, Menchon JM, Jiménez-Murcia S, Soriano-Mas C, Fernandez-Aranda F. Neural network alterations across eating disorders: a narrative review of fMRI studies. Curr Neuropharmacol. 2018;16:1150–63.
Compan V, Walsh BT, Kaye W, Geliebter A. How does the brain implement adaptive decision making to eat? J Neurosci. 2015;35:13868–78.
Bergfeld IO, Dijkstra E, Graat I, de Koning P, van den Boom BJG, Arbab T, et al. Invasive and non-invasive neurostimulation for OCD. Curr Top Behav Neurosci. 2021;49:399–436.
Graat I, Mocking R, Figee M, Vulink N, de Koning P, Ooms P, et al. Long-term outcome of deep brain stimulation of the ventral part of the anterior limb of the internal capsule in a cohort of 50 patients with treatment-refractory obsessive-compulsive disorder. Biol Psychiatry. 2020;90:714–20.
Bergfeld IO, Mantione M, Hoogendoorn ML, Ruhé HG, Notten P, van Laarhoven J, et al. Deep brain stimulation of the ventral anterior limb of the internal capsule for treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2016;73:456–64.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700.
NIHR. PROSPERO. CRD. 2021. https://www.crd.york.ac.uk/prospero/. Accessed 30 Sept 2020.
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919.
Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490.
Villalba Martínez G, Justicia A, Salgado P, Ginés JM, Guardiola R, Cedrón C, et al. A randomized trial of deep brain stimulation to the subcallosal cingulate and nucleus accumbens in patients with treatment-refractory, chronic, and severe anorexia nervosa: initial results at 6 months of follow up. J Clin Med. 2020;9:1946.
Sunday SR, Halmi KA, Einhorn A. The Yale-Brown-Cornell eating disorder scale: a new scale to assess eating disorder symptomatology. Int J Eat Disord. 1995;18:237–45.
Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, et al. The Yale-Brown obsessive compulsive scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46:1006–11.
Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–5.
Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62.
Burckhardt CS, Anderson KL. The Quality of Life Scale (QOLS): reliability, validity, and utilization. Health Qual Life Outcomes. 2003;1:60.
Lins L, Carvalho FM. SF-36 total score as a single measure of health-related quality of life: scoping review. SAGE Open Med. 2016;4:2050312116671725.
Engel SG, Wittrock DA, Crosby RD, Wonderlich SA, Mitchell JE, Kolotkin RL. Development and psychometric validation of an eating disorder-specific health-related quality of life instrument. Int J Eat Disord. 2006;39:62–71.
Lipsman N, Lam E, Volpini M, Sutandar K, Twose R, Giacobbe P, et al. Deep brain stimulation of the subcallosal cingulate for treatment-refractory anorexia nervosa: 1 year follow-up of an open-label trial. Lancet Psychiatry. 2017;4:285–94.
Lipsman N, Woodside DB, Giacobbe P, Hamani C, Carter JC, Norwood SJ, et al. Subcallosal cingulate deep brain stimulation for treatment-refractory anorexia nervosa: a phase 1 pilot trial. Lancet 2013;381:1361–70.
de Vos J, Houtzager L, Katsaragaki G, van de Berg E, Cuijpers P, Dekker J. Meta analysis on the efficacy of pharmacotherapy versus placebo on anorexia nervosa. J Eat Disord. 2014;2:27.
Solmi M, Wade TD, Byrne S, Del Giovane C, Fairburn CG, Ostinelli EG, et al. Comparative efficacy and acceptability of psychological interventions for the treatment of adult outpatients with anorexia nervosa: a systematic review and network meta-analysis. Lancet Psychiatry. 2021;8:215–24.
van den Berg E, Houtzager L, de Vos J, Daemen I, Katsaragaki G, Karyotaki E, et al. Meta-analysis on the efficacy of psychological treatments for anorexia nervosa. Eur Eat Disord Rev. 2019;27:331–51.
Zhou C, Zhang H, Qin Y, Tian T, Xu B, Chen J, et al. A systematic review and meta-analysis of deep brain stimulation in treatment-resistant depression. Prog Neuropsychopharmacol Biol Psychiatry. 2018;82:224–32.
Wang X, Wang J, Zhao H, Li N, Ge S, Chen L, et al. Clinical analysis and treatment of symptomatic intracranial hemorrhage after deep brain stimulation surgery. Br J Neurosurg. 2017;31:217–22.
Horn A. Connectomic deep brain stimulation. Berlin, Germany: Elsevier Science Publishing Co Inc; 2021.
Mavridis IN. How deep brain stimulation of the nucleus accumbens affects the cingulate gyrus and vice versa. Brain Sci. 2019;9:5.
Zhang HW, Li DY, Zhao J, Guan YH, Sun BM, Zuo CT. Metabolic imaging of deep brain stimulation in anorexia nervosa: a 18F-FDG PET/CT study. Clin Nucl Med. 2013;38:943–8.
Bewernick BH, Hurlemann R, Matusch A, Kayser S, Grubert C, Hadrysiewicz B, et al. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol Psychiatry. 2010;67:110–6.
Lee DJ, Lozano CS, Dallapiazza RF, Lozano AM. Current and future directions of deep brain stimulation for neurological and psychiatric disorders. J Neurosurg. 2019;131:333–42.
Fridgeirsson EA, Figee M, Luigjes J, van den Munckhof P, Schuurman PR, van Wingen G, et al. Deep brain stimulation modulates directional limbic connectivity in obsessive-compulsive disorder. Brain 2020;143:1603–12.
Graat I, Mocking RJT, de Koning P, Vulink N, Figee M, van den Munckhof P, et al. Predicting response to vALIC deep brain stimulation for refractory obsessive-compulsive disorder. J Clin Psychiatry. 2021;82:20m13754.
Pacilio RM, Livingston RK, Gordon MR. The use of electroconvulsive therapy in bating disorders: a systematic literature review and case report. J ect. 2019;35:272–8.
Funding
RJTM is supported by an unrestricted ABC talent grant. The funder had no role in the study design, data collection, data analysis, data interpretation, writing of the report, or in the decision to submit for publication. RM is supported by an unrestricted ABC talent grant.
Author information
Authors and Affiliations
Contributions
DK: conceptualisation, data curation, formal analysis, investigation, methodology, project administration, supervision, validation, visualisation, writing—original draft, and writing—review and editing. PC: conceptualisation, data curation, formal analysis, investigation, methodology, project administration, writing—original draft. MO: investigation, supervision, validation, writing—review and editing. RM: conceptualisation, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, software, supervision, validation, visualisation, writing—original draft, and writing—review and editing. AL: investigation, writing—review and editing. AvE: investigation, writing—review and editing. DD: investigation, writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Potential conflicts of interest: all authors have no conflicts to disclose.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Karaszewska, D., Cleintuar, P., Oudijn, M. et al. Efficacy and safety of deep brain stimulation for treatment-refractory anorexia nervosa: a systematic review and meta-analysis. Transl Psychiatry 12, 333 (2022). https://doi.org/10.1038/s41398-022-02102-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-022-02102-w
- Springer Nature Limited
This article is cited by
-
Neural effects of deep brain stimulation on reward and loss anticipation and food viewing in anorexia nervosa: a pilot study
Journal of Eating Disorders (2023)