Abstract
Neurocognitive impairment is commonly associated with functional disability in established depressive, bipolar and psychotic disorders. However, little is known about the longer-term functional implications of these impairments in early phase transdiagnostic cohorts. We aimed to examine associations between neurocognition and functioning at baseline and over time. We used mixed effects models to investigate associations between neurocognitive test scores and longitudinal social and occupational functioning (“Social and Occupational Functioning Assessment Scale”) at 1–7 timepoints over five-years in 767 individuals accessing youth mental health services. Analyses were adjusted for age, sex, premorbid IQ, and symptom severity. Lower baseline functioning was associated with male sex (coefficient −3.78, 95% CI −5.22 to −2.34 p < 0.001), poorer verbal memory (coefficient 0.90, 95% CI 0.42 to 1.38, p < 0.001), more severe depressive (coefficient −0.28, 95% CI −0.41 to −0.15, p < 0.001), negative (coefficient −0.49, 95% CI −0.74 to −0.25, p < 0.001), and positive symptoms (coefficient −0.25, 95% CI −0.41 to −0.09, p = 0.002) and lower premorbid IQ (coefficient 0.13, 95% CI 0.07 to 0.19, p < 0.001). The rate of change in functioning over time varied among patients depending on their sex (male; coefficient 0.73, 95% CI 0.49 to 0.98, p < 0.001) and baseline level of cognitive flexibility (coefficient 0.14, 95% CI 0.06 to 0.22, p < 0.001), such that patients with the lowest scores had the least improvement in functioning. Impaired cognitive flexibility is common and may represent a meaningful and transdiagnostic target for cognitive remediation in youth mental health settings. Future studies should pilot cognitive remediation targeting cognitive flexibility while monitoring changes in functioning.
Similar content being viewed by others
Introduction
Reducing the burden of disability attributable to mental disorders is a global health priority. Mental, neurological, and substance use disorders are the world’s leading contributors to years lived with disability and the third-ranked cause of disability-adjusted life years1,2. This disability burden is particularly heavy for young people. For example, disability-adjusted life years related to common mental disorders reach their peak between ages 10–29 years2, and depression, bipolar disorder, and schizophrenia are three of the four most burdensome conditions in those aged 10–24 years3. As 50% of mental disorders emerge before the middle-teens and 75% by the mid-twenties4, it is likely that the burden of disability in early and later adulthood represents an extension of problems originating premorbidly or in early phases of illness. These early phases are characterized by changes in social behaviors and difficulties participating in work and study5, both of which are reflected in the high rates of functional impairment at presentation to youth-specific services6,7. As functional trajectories are heterogeneous and often persistently impaired6, there is a critical need for identification of factors associated with functional course which may guide interventions to those at risk of enduring and lifelong functional disability8.
Despite clinical remission, many individuals with mental disorders fail to reach their expected or premorbid levels of social and occupational functioning, suggesting the presence of enduring factors which limit functional recovery. One such factor is neurocognitive impairment, which is common from early in the course of depressive9, bipolar10, and psychotic disorders10,11. While there is good evidence to suggest that neurocognitive impairments represent an enduring and trait-like feature of neurodevelopmental disorders such as schizophrenia12, the state-trait distinction is less clear for other mental disorders. However, some evidence suggests that attentional and other executive impairments commonly persist despite remission of symptoms in major depression9,13, while domains such as memory, verbal fluency, and processing speed are more strongly influenced by mood state9,13. Likewise, impairments in memory, processing speed, and executive functions are common in individuals with bipolar disorder across mood episodes14,15, notwithstanding common inter-episode syndromal and sub-syndromal symptoms 16.
The functional consequences of neurocognitive impairments in people with mental disorders are increasingly clear. Research over the last two decades has demonstrated that neurocognition is a strong and prospective determinant of functioning in schizophrenia17, with impairments in domains including processing speed, executive functions, and memory18 limiting patients’ capacity to acquire, retain, and relearn skills required for adaptive functioning17. More recently, similar impacts have been appreciated for a wider range of mental disorders. Longitudinal studies of people with bipolar disorder have demonstrated associations between impairments in executive functions14,19, processing speed14,20, and verbal learning and memory14,19,20 and poorer social and occupational outcome. While a less developed literature, poorer memory and executive functions have also been linked to worse follow-up social and occupational outcome in major depression21,22.
Importantly, there is a developing notion that neurocognition may represent a continuum cutting across diagnostic boundaries10,23, with the National Institute of Mental Health’s Research Domain Criteria endorsing a dimensional framework to the study of neurocognition in mental disorders24. Our group has demonstrated the utility of a transdiagnostic approach to examining neurocognition and functioning across the major mental disorders. We have reported strong cross-sectional25 and longitudinal23,26 relationships between general neurocognition and social and occupational functioning in a cohort of young people presenting to mental health services with a range of mood, anxiety, and psychotic syndromes. Moreover, we have shown that changes in neurocognition map onto changes in functioning when statistically adjusting for diagnosis and symptom severity, supporting a meaningful and robust link between neurocognition and functioning across mental disorders27. Limited work however has examined relationships between specific neurocognitive domains and the course of functioning in young transdiagnostic samples.
Accordingly, we aimed to test several questions regarding the links between neurocognition and functioning and their broader implications across mental disorders in a cohort of adolescents and young adults accessing mental health services. First, we aimed to examine associations between neurocognitive test scores across nine domains and functioning at baseline and change in functioning over time. Second, we aimed to determine whether associations between neurocognition and functioning at baseline and change in functioning over time would be robust to adjustment for confounding factors (age, sex, premorbid IQ, and symptom severity). Based on our work23,26 and the wider literature19,20,28, we hypothesized that baseline executive functions, processing speed, and verbal learning and memory would be uniquely associated with baseline functioning and change in functioning longitudinally.
Materials and methods
Human ethics
The study and consent procedure were approved by the University of Sydney Human Research Ethics Committee (project numbers 2012/1626 and 2012/1631) and conducted in accordance with the revised Declaration of Helsinki. All participants aged 16 and older provided written informed consent and parental or guardian consent was obtained for participants aged under 16 years.
Participants
Participants were drawn from a cohort of 6743 consecutive referrals (aged 12–30) presenting to youth mental health clinics at the Brain and Mind Center in Sydney, Australia, who were recruited to a research register of adolescents and young adults with mental disorders between 2008–2018. These clinics (e.g., headspace) aim to provide youth-friendly and highly accessible early intervention services for young people with emerging mental and substance use disorders29. Headspace consists of an integrated mix of primary-level services and more specialized services (e.g., drug and alcohol) and primarily attracts young people with a wide range of mental health problems (typically anxiety, mood and/or psychotic syndromes). All participants were receiving ongoing clinician-based case management and relevant social, psychological and/or medical treatments as part of standard care, which may have involved contact with a psychiatrist, psychologist, occupational therapist, support worker or hospitalization for those whose need exceeded the capacity of the primary care services.
Eligibility criteria
Inclusion criteria for this study were: (a) a baseline neurocognitive assessment with the majority of test scores available; (b) aged 12–30 at the neurocognitive assessment; (c) an available proforma assessment (see below) within three-months of the neurocognitive assessment; and (d) willing and able to provide written informed consent (or parental/guardian consent was obtained). Exclusion criteria were: (i) history of neurological disease; (ii) medical illness known to impact brain function (e.g., cancer, epilepsy); (iii) electroconvulsive therapy in three-months prior to neurocognitive assessment; (iv) clinically-evident intellectual disability; and/or (v) insufficient understanding of the English language to allow participation in verbal assessments or testing.
Data collection (baseline)
A subset of the wider cohort participated in detailed clinical and neurocognitive assessments between 2008–2015. A board-certified neuropsychologist, research psychologist or supervised doctoral student administered a neurocognitive battery with the domains chosen on the basis of sound validity and reliability30, relevance to the diagnoses under study9,11,31, and overlap with instruments used in the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) initiative32. The following domains were assessed: processing speed (Trail Making Test, part A)33, cognitive flexibility (Trail Making Test, part B)33, verbal learning (sum of trials 1–5 of the Rey Auditory Verbal Learning Test; RAVLT)34, verbal memory (20-min delayed recall of the RAVLT)34, sustained attention (A’ Prime subtest of the Rapid Visual Information Processing Test)35, set-shifting (Intra-Extra Dimensional Set Shift)35, visuospatial learning (Paired Associates Learning Task)35 and working memory (Spatial Span Task)35. Premorbid IQ was estimated using the Wechsler Test of Adult Reading36 or the Wide Range Achievement Test37 (for participants younger than 16). Neurocognitive scores were standardized to age-matched and sex-matched norms (z-scores) using established norms38,39. To limit the impact of extreme scores and minimize data transformation, z-scores were curtailed at a maximum of ±5.0, with fewer than 3% of scores curtailed for each test. Symptom type and severity were determined using the Brief Psychiatric Rating Scale, with four dimensions empirically derived (depressive, negative, positive, and manic)40.
Data collection (longitudinal)
A standardized clinical proforma was used to retrospectively extract demographic, clinical, and functioning data from clinical and research files across eight predetermined timepoints (baseline, 3 months, 6 months, 1 year, 2 years, 3 years, 4 years, and 5 years)41. A “time-last-seen” entry was also recorded; however, this was not included in the current study. The proforma captures information at each timepoint regarding the current presentation and illness course, including: (a) demographics; (b) socio-occupational functioning; (c) clinical presentation (including clinical diagnosis according to DSM-542); (d) self-harm and suicidal thoughts and behaviors; (e) alcohol and other substance use; and (f) physical health comorbidities.
The proforma provided the primary outcome measure of socio-occupational functioning as assessed by a trained clinician using the Social and Occupational Functioning Assessment Scale (SOFAS). The SOFAS is a 100-point scale (with higher scores denoting better functioning) which improves on other measures of global functioning in its instruction to the rater to avoid confounding the rating with symptoms. A score of 60–70 is indicative of moderate difficulty in social, occupational, or school functioning. The SOFAS is widely used and has good construct validity43, inter-rater reliability43, and predictive validity 44.
As neurocognition was the primary baseline predictor for this study, we used the nearest proforma assessment occurring within a three-month interval of the neurocognitive assessment as the participants’ baseline timepoint (T1), with remaining proforma timepoints recoded if necessary. As we allowed a three-month interval for recoding, we subsequently excluded the three-month proforma timepoint from further analysis. The number of proforma assessments at each timepoint and the number of participants with one or more proforma assessments over time are presented in Supplementary Tables 1, 2, respectively.
Statistical analyses
Analyses were conducted in RStudio (version 1.0.143). Linear mixed-effects models with random-intercepts were constructed using the “lme4” package (version 1.1–18–145). Full-information maximum-likelihood estimation was used to handle missing follow-up data (as loss to follow-up was uncontrolled). The mixed-effects framework is recommended for longitudinal designs as it tolerates: (a) repeated-measures within participants (i.e., non-independence); (b) unbalanced assessment intervals; and (c) missing follow-up data. The continuous SOFAS rating at each timepoint represented the outcome variable, and participants could contribute one or multiple assessments over time (i.e., assessments nested within participants) (see Supplementary Table 2). To model associations with the rate of change in SOFAS over time, a “Time” variable was used which represented the timepoint of each assessment and was linearly coded. All baseline predictor variables were continuous (except for sex).
The literature describing relationships between neurocognitive test performance and aspects of functioning in major mental disorders report associations between a large variety of neurocognitive tests and various measures of global functioning and specific subdomains of functioning (e.g., relationship impairment, work impairment, and independent living)14,18,19,20,21,22. Relatedly, most studies have focussed on specific diagnostic groups (e.g., schizophrenia) and there is very limited research regarding specific neurocognition-functioning associations in early-phase, transdiagnostic cohorts. Accordingly, we chose to use a data-driven, backward elimination statistical approach to identify associations between individual neurocognitive test scores and social and occupational functioning in our cohort. Modeling proceeded in three stages. First, we examined unadjusted associations between all baseline predictors and variation in SOFAS scores at baseline as well as the rate of change in SOFAS over time. Second, we examined associations between all baseline predictors and variation in baseline SOFAS scores, using backward elimination to iteratively remove the least significant variable until only significant predictors remained (α = 0.05). Third, we examined associations between the rate of change in SOFAS longitudinally and all predictor variables that had significant associations with variation in SOFAS at baseline, using backward elimination to reduce the full model.
Normality of residuals was inspected with Q–Q plots, with an approximate normal distribution evident. Multicollinearity was assessed with the variation inflation factor (VIF), with no predictors exceeding a VIF of 3.0. Parameter-specific p-values were calculated using Satterthwaite’s approximation for degrees of freedom in the “lmerTest” package (version 1.046). Missing baseline neurocognitive and clinical data were imputed using multiple imputation by chained equations in the “mice” package (version 3.3.047). Missing data patterns were consistent with a missing-at-random mechanism and fewer than 10% of each neurocognitive domain and fewer than 12% of BPRS scores were missing (see Supplementary Table 3 for numbers and proportions of missing values for each predictor variable). Following recommendations, we multiply imputed 100 datasets using predictive mean matching (which makes use of all available data), modeled each imputed dataset separately, and pooled the coefficients, test statistics, and p-values47,48,49.
Role of the funding source
This study was partially funded by an Australian Government Research Training Program Scholarship (awarded to J.J.C.), a National Health & Medical Research Council Center of Research Excellence Grant (No. 1061043) and an Australia Fellowship (No. 511921) (awarded to I.B.H.). The funders of this study had no involvement in the: study design; collection, analysis and reporting of the data; writing of the report; or decision to submit the paper for publication.
Results
Sample characteristics
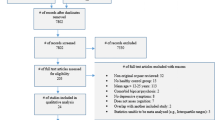
Participants were drawn from a cohort of 6743 young people who were recruited to a research register50. Of these, 2767 participants had an available baseline clinical proforma assessment, and a total of 767 participants met all eligibility criteria and were included in the final analysis.
Sample characteristics are presented in Table 1. At baseline, the sample consisted of 767 participants (409/767 female; 53.3%) with a median age of 19 years (IQR = 6). Baseline SOFAS ratings ranged from 30 to 90 with a mean in the moderate-impairment range (mean [SD] 60.19 [10.05]) (mean SOFAS scores at each timepoint are reported in Supplementary Table 4). Numbers and proportions of participants with SOFAS outcome data at follow-up timepoints were: 6 months (N = 247, 32.2%); 1 year (N = 275, 35.5%); 2 years (N = 236, 30.8%); 3 years (N = 170, 22.2%); 4 years (N = 112, 14.6%); and 5 years (N = 59, 7.7%). Around two-thirds of the sample had two or more timepoints (N = 465, 60.6%) and over one-third had three or more timepoints (N = 36.9%) (see Supplementary Table 2).
Presenting primary mental health diagnoses are shown in Table 2. The majority of patients presented with a primary mood (depressive or bipolar) or anxiety disorder (N = 523/767, 68.2%). Level of symptoms on the BPRS were in the “very mild” to “mild” range across the four dimensions (depressive, negative, positive, and manic). The means for each neurocognitive domain were within normal limits. Only scores for cognitive flexibility (mean [SD] −0.65 [1.56]) and sustained attention (mean [SD] −0.70 [1.35]) exceeded −0.5 SD below the norm, while all other domains fell within 0 and −0.5 SD of the norm: processing speed (mean [SD] −0.07 [1.14]), verbal learning (mean [SD] −0.31 [1.33]), verbal memory (mean [SD] −0.30 [1.35]), verbal fluency (mean [SD] −0.35 [1.12]), visuospatial learning (mean [SD] −0.26 [1.32]), set-shifting (mean [SD] −0.41 [1.46]) and working memory (mean [SD] 0.00 [1.14]). Clinically significant impairment (i.e., −1.5 SD or greater below the norm) was common across all domains: working memory (8.6%), processing speed (10.2%), visuospatial learning (11.5%), verbal fluency (14.3%), set-shifting (16.5%), verbal learning (16.9%), verbal memory (20.6%), cognitive flexibility (23.0%), and sustained attention (27.4%).
Unadjusted associations with baseline functioning and change in functioning over time
We first modeled associations between all baseline predictors and variation in SOFAS at baseline and variation in the rate of SOFAS change longitudinally. As presented in Supplementary Table 5, all variables (except for age at baseline) in the unadjusted models were significantly associated with baseline functioning. Significant positive associations with baseline functioning were observed for all nine neurocognitive domains and premorbid IQ, while significant negative associations were observed for male sex and depressive, negative, positive, and manic symptoms. There were significant and positive associations with the rate of SOFAS change longitudinally for cognitive flexibility, verbal learning, verbal memory, working memory, processing speed, male sex, baseline age, and depressive, positive, and manic symptoms.
Associations with baseline functioning and change in functioning over time, adjusted for socio-demographics and type and severity of symptoms
We next used backward elimination to reduce the full model including all predictor variables down to a final model in which only variables significantly associated with SOFAS at baseline remained. This model is presented in Table 3. There were positive associations with baseline functioning for verbal memory (coefficient 0.82, 95% CI 0.33 to 1.32, p = 0.001), cognitive flexibility (coefficient 0.65, 95% CI 0.25 to 1.06, p = 0.002), and premorbid IQ (coefficient 0.12, 95% CI 0.06 to 0.18, p < 0.001), and negative associations for male sex (coefficient −1.87, 95% CI −3.18 to −0.57, p = 0.004) and depressive (coefficient −0.29, 95% CI −0.42 to −0.16, p < 0.001), negative (coefficient −0.48, 95% CI −0.73 to −0.24, p < 0.001), and positive symptoms (coefficient −0.23, 95% CI −0.39 to −0.07, p = 0.004).
In a second model also including associations with the rate of SOFAS change over time, there were associations with baseline functioning for verbal memory (coefficient 0.90, 95% CI 0.42 to 1.38, p < 0.001), premorbid IQ (coefficient 0.13, 95% CI 0.07 to 0.19, p < 0.001), male sex (coefficient −3.78, 95% CI −5.22 to −2.34, p < 0.001) and depressive (coefficient −0.28, 95% CI −0.41 to −0.15, p < 0.001), negative (coefficient −0.49, 95% CI −0.74 to −0.25, p < 0.001), and positive symptoms (coefficient −0.25, 95% CI −0.41 to −0.09, p = 0.002). The rate of change in functioning over time varied among patients depending on their sex (male; coefficient 0.73, 95% CI 0.49 to 0.98, p < 0.001), indicating that males had a greater rate of improvement in functioning than females, and the baseline level of cognitive flexibility (coefficient 0.14, 95% CI 0.06 to 0.22, p < 0.001), indicating that patients with the lowest scores had the least improvement in functioning.
Discussion
This study is the first to model unique associations between neurocognitive test scores and longer-term social and occupational functioning in a transdiagnostic clinical cohort of adolescents and young adults accessing youth mental health services. Of note, we observed a novel link between scores on a measure of “cognitive flexibility” (TMT-B) and the rate of improvement in social and occupational functioning over time, which was statistically independent of socio-demographics and level of symptom severity. This approach aligns with the dimensional framework endorsed by the National Institute of Mental Health’s Research Domain Criteria initiative24, and importantly extends diagnosis-specific links between executive functions and socio-occupational functioning17,19,21 to a broader transdiagnostic context. Our results expand the evidence base to suggest that cognitive flexibility may represent a meaningful and transdiagnostic target for cognitive remediation protocols in youth mental health settings.
Consistent with previous studies, we observed specific associations between baseline functioning and scores on measures of verbal memory and cognitive flexibility, clinically significant impairments of which were common and experienced by 20.6% and 23.0% of the cohort, respectively. The mechanisms underlying the associations between these domains and functioning in a transdiagnostic context are not well understood but may involve both direct (e.g., difficulty remembering instructions or inflexible decision-making) and indirect effects (e.g., mediated by social cognition or self-efficacy), as observed in schizophrenia51,52. Critically, cognitive flexibility also had a robust association with the rate of improvement in functioning longitudinally, such that impaired flexibility was associated with a lower rate of functional recovery over time in contact with clinical services. The independence from level of symptom severity provides clues to an enduring executive impairment linked to functioning. The measure of cognitive flexibility used in this study—the TMT-B—is thought to index higher-order skills such as the ability to flexibly switch between different task demands, in addition to other lower-order abilities such as visual search and processing speed53. In general populations, greater cognitive flexibility predicts a range of favorable outcomes across the life course, including better reading ability in children54, trait resilience to emotional events in adults55, and better health-related quality of life in older adults56. Moreover, studies in patients with mental disorders such as bipolar disorder have reported associations between impairments on the TMT-B and poorer functioning cross-sectionally and longitudinally14,57. Neuroimaging studies in healthy adults have revealed a distributed network of frontoparietal regions supporting cognitive flexibility58, a number of which are commonly altered in individuals with mental disorders. Thus, the link between cognitive flexibility and functioning observed in general populations may be amplified in individuals who have a mental disorder and neurocognitive impairment, as in our cohort wherein almost one-quarter had a clinically significant impairment in cognitive flexibility.
The link between cognitive flexibility and functional improvement may have important treatment implications. Cognitive remediation is increasingly being incorporated into treatment plans for individuals with mental disorders, with evidence that real-world functional gains are greatest when cognitive training is combined with supplemental functional skills training59 or other vocational interventions. Moreover, some preliminary animal modeling suggests that the adolescent brain may be better able to learn from cognitive training60 as a function of the unique neurobiology of adolescence (e.g., reward hypersensitivity). Further, accumulating evidence suggests that cognitive remediation in early phases of illness may yield greater than when applied in chronic phases61,62. Our results suggest that cognitive flexibility may represent a meaningful and transdiagnostic target for cognitive remediation, which may be enhanced when offered to young people early in the course of illness alongside other interventions targeting social and occupational functioning.
Limitations
Several limitations need mention. First, we relied on a baseline neurocognitive assessment, which is less informative than tracking neurocognitive and functional change dynamically over time. Second, we relied on a single-item index of functioning, potentially missing specific associations with sub-domains of functioning (e.g., relating interpersonally vs. vocational performance). Third, the age range studied spans a dynamic phase of neurocognitive development. Age-related test heterogeneity may therefore have obscured age-specific effects, and our results may not be generalizable to all age groups. However, more than 80% of the sample was aged 15–25, and some research suggests that while cognitive flexibility peaks in early adulthood, it is relatively mature by later childhood63. Fourth, as a result of the naturalistic design of this cohort study, sample attrition over time was uncontrolled and may have biased our model estimates. For example, the number of participants retained at the 5-year follow-up timepoint was limited, and it is possible that those remaining in care for longer durations have more severe illnesses which require greater attention from clinical services. Unfortunately, we did not collect data regarding specific patterns of treatment usage (e.g., number of sessions with a psychologist). However, the naturalistic design of this study may in fact better reflect the real-world patterns of service usage and functioning. Fifth, studies in schizophrenia consistently report statistical mediation of the path from neurocognition to functional outcome by several factors which were unmeasured here, including social cognition and intrinsic motivation51—they are likely relevant to other major mental disorders. Finally, cognitive flexibility and set-shifting are related neurocognitive functions, but we did not observe an association between functioning and set-shifting in our final model. One possible explanation for this discrepancy is that the test used to measure cognitive flexibility (TMT-B) additionally recruits functions including visual search, processing speed, and working memory and may therefore be inherently more difficult53.
Conclusions
In summary, we demonstrate for the first time a robust association between performance on a measure of cognitive flexibility and the rate of functional recovery over time in a transdiagnostic cohort of adolescents and young adults. Our results may have particular relevance for young people accessing broadly-based youth mental health services for whom impairments in cognitive flexibility may represent a treatment target for cognitive remediation in isolation or alongside functional interventions. Future studies should attempt to replicate our observations and determine the efficacy of cognitive remediation or functional interventions in individuals with impaired cognitive flexibility.
References
Whiteford, H. A. et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet 382, 1575–1586 (2013).
Whiteford, H. A., Ferrari, A. J., Degenhardt, L., Feigin, V. & Vos, T. The global burden of mental, neurological and substance use disorders: an analysis from the Global Burden of Disease Study 2010. PLoS ONE 10, e0116820 (2015).
Gore, F. M. et al. Global burden of disease in young people aged 10–24 years: a systematic analysis. Lancet 377, 2093–2102 (2011).
Kessler, R. C. et al. Age of onset of mental disorders: a review of recent literature. Curr. Opin. Psychiatry 20, 359–364 (2007).
Slade, M. & Longden, E. Empirical evidence about recovery and mental health. BMC Psychiatry 15, 285 (2015).
Iorfino, F. et al. Delineating the trajectories of social and occupational functioning of young people attending early intervention mental health services in Australia: a longitudinal study. BMJ Open 8, e020678 (2018).
Scott, J. et al. Functional impairment in adolescents and young adults with emerging mood disorders. Br. J. Psychiatry 205, 362–368 (2014).
Hickie, I. B. et al. Right care, first time: a highly personalised and measurement-based care model to manage youth mental health. Med. J. Aust. 211, S3–S46 (2019).
Lee, R. S., Hermens, D. F., Porter, M. A. & Redoblado-Hodge, M. A. A meta-analysis of cognitive deficits in first-episode major depressive disorder. J. Affect. Disord. 140, 113–124 (2012).
Bora, E. & Pantelis, C. Meta-analysis of cognitive impairment in first-episode bipolar disorder: comparison with first-episode schizophrenia and healthy controls. Schizophr. Bull. 41, 1095–1104 (2015).
Mesholam-Gately, R. I., Giuliano, A. J., Goff, K. P., Faraone, S. V. & Seidman, L. J. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology 23, 315–336 (2009).
Reichenberg, A. et al. Static and dynamic cognitive deficits in childhood preceding adult schizophrenia: a 30-year study. Am. J. Psychiatry 167, 160–169 (2010).
Douglas, K. M. & Porter, R. J. Longitudinal assessment of neuropsychological function in major depression. Aust. N. Z. J. Psychiatry 43, 1105–1117 (2009).
Mora, E., Portella, M. J., Forcada, I., Vieta, E. & Mur, M. Persistence of cognitive impairment and its negative impact on psychosocial functioning in lithium-treated, euthymic bipolar patients: a 6-year follow-up study. Psychol. Med. 43, 1187–1196 (2013).
Martinez-Aran, A. et al. Cognitive function across manic or hypomanic, depressed, and euthymic states in bipolar disorder. Am. J. Psychiatry 161, 262–270 (2004).
Paykel, E. S., Abbott, R., Morriss, R., Hayhurst, H. & Scott, J. Sub-syndromal and syndromal symptoms in the longitudinal course of bipolar disorder. Br. J. Psychiatry 189, 118–123 (2006).
Green, M. F., Llerena, K. & Kern, R. S. The “Right Stuff” revisited: what have we learned about the determinants of daily functioning in schizophrenia? Schizophr. Bull. 41, 781–785 (2015).
Milev, P., Ho, B. C., Arndt, S. & Andreasen, N. C. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: a longitudinal first-episode study with 7-year follow-up. Am. J. Psychiatry 162, 495–506 (2005).
Bonnin, C. M. et al. Clinical and neurocognitive predictors of functional outcome in bipolar euthymic patients: a long-term, follow-up study. J. Affect. Disord. 121, 156–160 (2010).
Burdick, K. E., Goldberg, J. F. & Harrow, M. Neurocognitive dysfunction and psychosocial outcome in patients with bipolar I disorder at 15-year follow-up. Acta Psychiatr. Scand. 122, 499–506 (2010).
Withall, A., Harris, L. M. & Cumming, S. R. The relationship between cognitive function and clinical and functional outcomes in major depressive disorder. Psychol. Med. 39, 393–402 (2009).
Cambridge, O. R., Knight, M. J., Mills, N. & Baune, B. T. The clinical relationship between cognitive impairment and psychosocial functioning in major depressive disorder: a systematic review. Psychiatry Res. 269, 157–171 (2018).
Lee, R. S. et al. Neuropsychological and socio-occupational functioning in young psychiatric outpatients: a longitudinal investigation. PLoS ONE 8, e58176 (2013).
Insel, T. et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am. J. Psychiatry 167, 748–751 (2010).
Lee, R. S. C. et al. Clinical, neurocognitive and demographic factors associated with functional impairment in the Australian Brain and Mind Youth Cohort Study (2008–2016). BMJ Open 8, e022659 (2018).
Lee, R. S. C. et al. A transdiagnostic study of education, employment, and training outcomes in young people with mental illness. Psychol. Med. 47, 2061–2070 (2017).
Lee, R. S. C. et al. Neuropsychological and functional outcomes in recent-onset major depression, bipolar disorder and schizophrenia-spectrum disorders: a longitudinal cohort study. Transl. Psychiatry 5, e555 (2015).
Green, M. F. What are the functional consequences of neurocognitive deficits in schizophrenia? Am. J. Psychiatry 153, 321–330 (1996).
McGorry, P. D. et al. headspace: Australia’s National Youth Mental Health Foundation-where young minds come first. Med. J. Aust. 187, S68–S70 (2007).
Strauss, E., Sherman, E. M. S. & Spreen, O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. 3rd edn. (Oxford University Press, New York, NY, 2006).
Lee, R. S. et al. A meta-analysis of neuropsychological functioning in first-episode bipolar disorders. J. Psychiatr. Res. 57, 1–11 (2014).
Nuechterlein, K. H. et al. The MATRICS consensus cognitive battery, part 1: test selection, reliability, and validity. Am. J. Psychiatry 165, 203–213 (2008).
Franzen, M. & Paul, D. G. P. Alternate form Reliability of Trails A, B, C and D. Ninth Annual Convention of The National Academy of Neuropsychology (Reno, NY,1990).
Taylor, E. M. Psychological Appraisal of Children with Cerebral Deficits. (Harvard University Press, Cambridge, MA, 1959).
Sahakian, B. J. & Owen, A. M. Computerized assessment in neuropsychiatry using CANTAB: discussion paper. J. R. Soc. Med. 85, 399–402 (1992).
Wechsler, D. Wechsler Test of Adult Reading (WTAR) (The Psychological Corporation, San Antonio, TX, 2001).
Wilkinson, G. & Robertson, G. Wide Range Achievement Test. 4th edn. (Psychological Assessment Resources, Lutz, FL, 2006).
Rickert, P. & Senior, G. WMS-III List Learning Test and the Rey Auditory Verbal Learning Test: Comparisons and Australian Normative Data. 4th Annual Conference of the College of Clinical Neuropsychologists. Lorne, Victoria (1998).
Tombaugh, T. in A Compendium of Neuropsychological Tests: Administration, Norms and Commentary. 2nd edn (eds Spreen, O. & Strauss, E.). (Oxford University Press, New York, 1998).
Dingemans, P. M., Linszen, D. H., Lenior, M. E. & Smeets, R. M. Component structure of the expanded Brief Psychiatric Rating Scale (BPRS-E). Psychopharmacology 122, 263–267 (1995).
Iorfino, F. et al. Clinical stage transitions in persons aged 12 and 25 years presenting to early intervention mental health services with anxiety, mood, and psychotic disorders. JAMA Psychiatry 76, 1167–1175 (2019).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders 5th edn. (American Psychiatric Association, Arlington, VA, 2013).
Hilsenroth, M. J. et al. Reliability and validity of DSM-IV axis V. Am. J. Psychiatry 157, 1858–1863 (2000).
Hay, P., Katsikitis, M., Begg, J., Da Costa, J. & Blumenfeld, N. A two-year follow-up study and prospective evaluation of the DSM-IV axis V. Psychiatr. Serv. 54, 1028–1030 (2003).
Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015).
Kuznetsova, A. K., Brockhoff, P. B. & Christensen, R. H. B. lmerTest Package: tests in linear mixed effects models. J. Stat. Softw. 82, 1–26 (2017).
van Buuren, S. & Groothius-Oudshoorn, K. Mice: multivariate imputation by chained equations in R. J. Stat. Softw. 45, 1–67 (2011).
Rubin, D. B. Multiple Imputation for Nonresponse in Surveys. (Wiley, New York, 1987).
van Buuren, S. Flexible Imputation of Missing Data 2nd edn. (Chapman & Hall/CRC, New York, 2018).
Scott, E. M. et al. Targeted primary care-based mental health services for young Australians. Med. J. Aust. 196, 136–140 (2012).
Galderisi, S. et al. The influence of illness-related variables, personal resources and context-related factors on real-life functioning of people with schizophrenia. World Psychiatry 13, 275–287 (2014).
Schmidt, S. J., Mueller, D. R. & Roder, V. Social cognition as a mediator variable between neurocognition and functional outcome in schizophrenia: empirical review and new results by structural equation modeling. Schizophr. Bull. 37, S41–S54 (2011).
Bowie, C. R. & Harvey, P. D. Administration and interpretation of the trail making test. Nat. Protoc. 1, 2277 (2006).
Engel de Abreu, P. M. J. et al. Executive functioning and reading achievement in school: a study of Brazilian children assessed by their teachers as “poor readers”. Front. Psychol. 5, 550 (2014).
Genet, J. J. & Siemer, M. Flexible control in processing affective and non-affective material predicts individual differences in trait resilience. Cogn. Emot. 25, 380–388 (2011).
Davis, J. C., Marra, C. A., Najafzadeh, M. & Liu-Ambrose, T. The independent contribution of executive functions to health related quality of life in older women. BMC Geriatrics 10, 16 (2010).
Torrent, C. et al. Cognitive impairment in bipolar II disorder. Br. J. Psychiatry 189, 254–259 (2006).
Kim, C., Cilles, S. E., Johnson, N. F. & Gold, B. T. Domain general and domain preferential brain regions associated with different types of task switching: a meta-analysis. Hum. Brain Mapp. 33, 130–142 (2012).
Bowie, C. R., McGurk, S. R., Mausbach, B., Patterson, T. L. & Harvey, P. D. Combined cognitive remediation and functional skills training for schizophrenia: effects on cognition, functional competence, and real-world behavior. Am. J. Psychiatry 169, 710–718 (2012).
Lee, H. et al. Early cognitive experience prevents adult deficits in a neurodevelopmental schizophrenia model. Neuron 75, 714–724 (2012).
Bowie, C. R., Grossman, M., Gupta, M., Oyewumi, L. K. & Harvey, P. D. Cognitive remediation in schizophrenia: efficacy and effectiveness in patients with early versus long-term course of illness. Early Interv. Psychiatry 8, 32–38 (2014).
Deste, G. et al. Effectiveness of cognitive remediation in early versus chronic schizophrenia: a preliminary report. Front. Psychiatry 10, 236 (2019).
Anderson, P. Assessment and development of executive function (EF) during childhood. Child Neuropsychol. 8, 71–82 (2002).
Acknowledgements
The authors gratefully thank the young people who have contributed to our research and to financial support from the Australian Government (Research Training Program Scholarship awarded to J.J.C.) and the National Health & Medical Research Council (Center of Research Excellence grant: No. 1061043; Australia Fellowship awarded to IBH: No. 511921).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The current study was supported by the following: an Australian Government Research Training Program Scholarship (awarded to J.J.C.), National Health & Medical Research Council Center of Research Excellence Grant (No. 1061043) and an Australia Fellowship (No. 511921) (awarded to I.B.H.). Mr. Jacob J. Crouse has nothing to disclose. Dr. Kate M. Chitty has nothing to disclose. Dr. Frank Iorfino has nothing to disclose. Dr. Joanne S. Carpenter has nothing to disclose. Mr. Django White has nothing to disclose. Ms. Alissa Nichles has nothing to disclose. Ms. Natalia Zmicerevska has nothing to disclose. Prof. Adam J. Guastella has nothing to disclose. Associate Prof. Elizabeth M. Scott reports personal fees from St. Vincent’s Private Hospital, grants from Servier, personal fees from Servier, personal fees from Eli-Lilly, personal fees from Pfizer, outside the submitted work. A/Prof. Elizabeth Scott is the Medical Director, Young Adult Mental Health Unit, St. Vincent’s Hospital Darlinghurst, Discipline Leader of Adult Mental Health, School of Medicine, University of Notre Dame, Research Affiliate, The University of Sydney and Consultant Psychiatrist. She has received honoraria for educational seminars related to the clinical management of depressive disorders supported by Servier and Eli-Lilly pharmaceuticals. She has participated in a national advisory board for the antidepressant compound Pristiq, manufactured by Pfizer. She was the National Coordinator of an antidepressant trial sponsored by Servier. Dr. Rico S. C. Lee has nothing to disclose. Prof. Sharon L. Naismith has nothing to disclose. Prof. Jan Scott has nothing to disclose. Prof. Daniel F. Hermens has nothing to disclose. Prof. Ian B. Hickie reports personal fees from National Mental Health Commission, personal fees from Medibank Clinical Reference Group, non-financial support from Psychosis Australia Trust, grants from NHMRC, grants from Innowell Pty LTD, grants from NHMRC, grants from NHMRC, outside the submitted work. Professor Ian Hickie was an inaugural Commissioner on Australia’s National Mental Health Commission (2012–2018). He is the Co-Director, Health and Policy at the Brain and Mind Center (BMC) University of Sydney. The BMC operates an early-intervention youth services at Camperdown under contract to headspace. Professor Hickie has previously led community-based and pharmaceutical industry-supported (Wyeth, Eli Lily, Servier, Pfizer, AstraZeneca) projects focused on the identification and better management of anxiety and depression. He was a member of the Medical Advisory Panel for Medibank Private until October 2017, a Board Member of Psychosis Australia Trust and a member of Veterans Mental Health Clinical Reference group. He is the Chief Scientific Advisor to, and an equity shareholder in, Innowell. Innowell has been formed by the University of Sydney and PwC to deliver the $30 m Australian Government-funded “Project Synergy”. Project Synergy is a three-year program for the transformation of mental health services through the use of innovative technologies.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Crouse, J.J., Chitty, K.M., Iorfino, F. et al. Modelling associations between neurocognition and functional course in young people with emerging mental disorders: a longitudinal cohort study. Transl Psychiatry 10, 22 (2020). https://doi.org/10.1038/s41398-020-0726-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-020-0726-9
- Springer Nature Limited
This article is cited by
-
Building a neurocognitive profile of suicidal risk in severe mental disorders
BMC Psychiatry (2022)
-
Days out of role and somatic, anxious-depressive, hypo-manic, and psychotic-like symptom dimensions in a community sample of young adults
Translational Psychiatry (2021)