Abstract
Schizophrenia is a debilitating neuropsychiatric illness that is characterized by positive, negative, and cognitive symptoms. Research over the past two decades suggests that the nociceptin receptor system may be involved in domains affected in schizophrenia, based on evidence aligning it with hallmark features of the disorder. First, aberrant glutamatergic and striatal dopaminergic function are associated with psychotic symptoms, and the nociceptin receptor system has been shown to regulate dopamine and glutamate transmission. Second, stress is a critical risk factor for first break and relapse in schizophrenia, and evidence suggests that the nociceptin receptor system is also directly involved in stress modulation. Third, cognitive deficits are prevalent in schizophrenia, and the nociceptin receptor system has significant impact on learning and working memory. Last, reward processing is disrupted in schizophrenia, and nociceptin signaling has been shown to regulate reward cue salience. These findings provide the foundation for the involvement of the nociceptin receptor system in the pathophysiology of schizophrenia and outline the need for future research into this system.
Similar content being viewed by others
Introduction
Schizophrenia is a debilitating disorder characterized by positive symptoms, such as delusions and hallucinations, and negative symptoms, such as a flat affect, alogia, and anhedonia, as well as deficits in cognition and reward modulation1,2,3. Some of the psychopathology of schizophrenia is characterized by dopaminergic and glutamatergic dysregulation, increased stress vulnerability via hypothalamic–pituitary–adrenal (HPA) axis dysregulation, cognitive deficits, which include alterations in the cholinergic system, and deficits in reward modulation2,3,4,5,6,7.
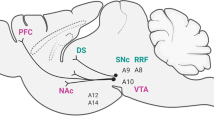
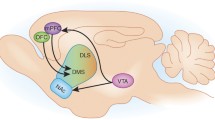
The nociceptin receptor (NOPr) is a G protein-coupled receptor identified in 1994, and was initially classified as a member of the opioid receptor family based on structural homology to the opioid receptors8. However, it was later reclassified as a non-opioid member of the opioid system, because endogenous ligands for other opioid receptors, such as the mu, kappa, and delta receptors, showed little affinity for it. The endogenous peptide, now known as nociceptin/orphanin FQ (N/OFQ), was identified in 1995, and is a heptadecapeptide with pro-nociceptive properties9,10. In vitro receptor autoradiography in rats and post-mortem studies in humans have shown NOPr to be widely distributed, with greater density in cortical regions and the human striatum11,12. Positron emission tomography (PET) studies using the ligand [11C]NOP-1A have corroborated these findings in vivo, with high concentrations of NOPr observed in the cerebral cortex and the striatum13. Given this widespread expression, it is well positioned to interact with multiple receptor systems in the brain and be involved in several functions.
In investigating the NOPr system, the majority of the literature is a result of preclinical work. This highlights a need for more research into this system as it could have potential in elucidating and treating psychiatric disorders. With regard to schizophrenia, the NOPr system may indeed have an impact given its involvement in important neurotransmitter systems and symptom clusters particularly relevant for the disorder.
Methods
The goal of this systematic review is to describe the role of the NOPr system in specific systems and symptoms that are relevant to schizophrenia. Thus, in this review we searched for the involvement of the NOPr system in each of the aforementioned domains relevant to schizophrenia. A search was conducted on the MEDLINE database for all research articles from 1994 onward using the Boolean string “(nociceptin receptor OR orl1 OR N/OFQ OR nociceptin/orphanin FQ OR orphanin FQ) AND (stress OR hypothalamic–pituitary–adrenal OR HPA OR immune system OR immune cells OR cytokines OR reward OR place preference OR cognition OR learning OR memory OR acetylcholine OR potentiation OR dopamine OR glutamate OR behavior OR locomotor OR schizophrenia OR psychosis OR post mortem OR microdialysis)”. The most recent search was conducted on October 3rd 2017. The abstracts for each of the articles in the search results were then screened using the following inclusion criteria: (a) studies investigating NOPr system involvement in cognition, stress, reward, cholinergic modulation, dopamine modulation, or glutamate modulation, and (b) that could be related to the symptoms underlying schizophrenia. Exclusion criteria were as follows: (a) studies that investigated novel ligands for NOPr investigation; (b) studies investigating pain or pain mechanisms; (c) studies that were unrelated to any of the four domains mentioned above or that could not be related to schizophrenia symptomatology; and (e) general review articles. The flowchart for this process is depicted in Fig. 1. We also want to note that, while there is evidence for NOPr system involvement in serotonin, β-endorphin, and norepinephrine signaling, these were excluded from this review in favor of focus on well-supported systems in psychosis.
Results
A total of 743 articles were obtained through the search, which were then screened. Following screening of abstracts, 123 articles were identified as potentially relevant to this review, and of these, 119 were included in this systematic review. Our discussion will thus focus on results from four areas of research relevant to psychosis: (1) NOPr system involvement in dopamine and glutamate transmission; (2) NOPr system involvement in stress and HPA modulation; (3) NOPr system involvement in cognition; and (4) NOPr system involvement in reward modulation.
Involvement of the NOPr system in dopamine transmission
A common pathology in schizophrenia is characterized by the dopamine hypothesis, which suggests an increased striatal dopamine transmission in these individuals4. Evidence suggests that the NOPr system could play a role. An initial study by Norton et al. revealed the presence of NOPr on cell bodies of dopamine neurons in the midbrain and a co-localization of NOP mRNA with tyrosine hydroxylase (TH) neurons, with mRNA also present on tegmental and nigral dopaminergic neurons14. This localization of NOP mRNA was later confirmed with an experiment using 6-hydroxydopamine lesions in rats, in which a large loss of TH neurons led to a reduction of N/OFQ and NOPr mRNA in the caudate putamen15. TH is an enzyme involved in the synthesis of the dopamine precursor L-DOPA, which plays a critical role in dopamine synthesis16. Olianas et al. furthered these findings by demonstrating an inhibitory effect of N/OFQ on TH phosphorylation, which inhibited dopamine transmission presynaptically17. They also observed a selective post-synaptic downregulation of dopamine D1 receptor signaling in the nucleus accumbens and striatum after N/OFQ administration. An involvement with dopamine D2 receptor signaling is also noted given that administration of a D2 antagonist prevented the improvements in motor performance with NOPr antagonists18. In the same study, genetic knockout of the D2 receptor erased the motor facilitating effect of a low dose of N/OFQ, indicating that the NOPr system could also exert effects on dopamine transmission through this receptor, potentially via a presynaptic mechanism as suggested by the authors.
Early research showed intracerebroventricular (ICV) administration of N/OFQ to induce a reduction in locomotor activity in mice at comparatively high doses (1–10 nmol), which is a finding that was later corroborated in rats and with additional studies in mice at the same doses10,19,20,21. These effects were then theorized to occur indirectly via their actions on dopaminergic neurons. Indeed, N/OFQ inhibits dopamine transmission in striatal brain slices22. In another study, injection of N/OFQ resulted in regulation of motor performance in rats, with injection of a NOPr antagonist producing the opposite effect and leading to an increase in excitability of the motor cortex23. This motor behavior may be regulated by effects on cortical afferents produced by subcortical NOPr. Liu et al. demonstrated, in vitro, an inhibitory effect of a low dose of N/OFQ on the dopamine transporter, which inhibits dopaminergic activity24. They theorized this to be a potential mechanism for the decrease in locomotor activity seen in earlier studies. However, given the presence of NOPr on dopamine neurons and NOP mRNA in TH neurons, and the additional evidence of a decrease in motor cortex excitability, the NOPr system could also inhibit dopamine transmission via a direct impact on dopamine synthesis.
Di Giannuario et al. reported a reduction in morphine-induced dopamine release induced by treatment with N/OFQ in vivo25. On a similar stream, antagonism of NOPr has also been repeatedly shown to enhance dopamine transmission26,27. Marti et al. supported the in vitro evidence by demonstrating an inhibitory effect of N/OFQ administration on dopamine transmission in the striatum in vivo26. Marti et al. demonstrated these effects in a Parkinsonian model by showing improvements in Parkinsonian symptomatology following antagonism of NOPr in the nigrostriatal pathway, furthering the notion of an inhibitory effect of NOPr on dopamine transmission27. This evidence is further supported by additional preclinical investigations using Parkinsonian models28,29,30. Viaro et al26. demonstrated an attenuation of Parkinsonism in MPTP-treated mice with a NOPr antagonist, and a synergistic effect when this was employed with L-DOPA, indicating that the NOPr system was exerting its effects via dopamine transmission (also supported by Marti et al.29)28,31.
More recently, neuroprotective effects of NOPr downregulation on dopamine neurons were demonstrated by Arcuri et al.32. They observed a significantly greater (50%) amount of nigral dopamine neurons spared in mice following acute administration of MPTP. These findings led the authors to conclude that NOP-N/OFQ signaling contributes to dopamine neuron loss in Parkinson’s, speculated to be due to glutamate-mediated excitotoxic mechanisms, and provide support to previous findings33,34. Although the aforementioned evidence conveys a definite impact of the NOPr system on dopamine transmission, the exact mechanism by which this occurs is still unclear.
Ces et al.33 investigated NOPr signaling with pre-pulse inhibition (PPI), a validated model for schizophrenia and demonstrated an impairment of visual PPI with a NOPr agonist35,36. Authors also found that co-administration of haloperidol and the NOPr agonist attenuated PPI deficits, leading them to conclude that there is a functional cooperation between N/OFQ and dopamine. This evidence furthers the notion of the possibility of a role for NOPr signaling in schizophrenia.
Involvement of the NOPr system in glutamate transmission
The glutamate hypothesis of schizophrenia is also well accepted, demonstrating hypofunction of the N-methyl-d-aspartate receptor (NMDAr), leading to a downregulation of glutamate5. In light of the significant impact of NOPr signaling on neurotransmission, glutamate transmission has also been studied. Nicol et al. showed decreased K+-evoked glutamate release in rat cerebrocortical, cerebellar, and brainstem slices in response to N/OFQ administration (see also Meis and Pape)37,38,39. Gompf et al. also showed N/OFQ to inhibit glutamate release in the retinohypothalamic tract and suprachiasmatic nucleus, and accorded this to be the result of presynaptic mechanisms by reducing Ca2+ (presynaptic release machinery)40. This was corroborated more recently by Kallupi et al., who demonstrated N/OFQ decreased glutamate release in the rat central amygdala41. Conversely, Marti et al. reported decreased glutamate release after NOPr antagonism in rats27. The difference in evidence may be reconciled by the consideration of the effects of NOPr on GABAergic signaling42,43,44,45,46,47.
Marti et al. reported a stimulatory effect of N/OFQ on nigral glutamate in vivo, and proposed this to be mediated via either dopaminergic or GABAergic mechanisms; this is because a GABAA receptor antagonist was found to counter the effects of N/OFQ48. The GABAergic system is involved in the pathophysiology of psychosis, as has been demonstrated by post-mortem studies showing abnormal GABAergic interneurons (see review: Taylor and Tso and also Wassef et al.)47,49. Gavioli et al. showed NOPr signaling to be involved in anxiety through the GABAA receptor, indicating through in vivo data the existence of effects of the NOPr system on GABAergic signaling43. Collectively, the data indicate a role of NOPr signaling in glutamate, as well as GABA transmission, with more evidence required to define the effects and exact mechanisms involved. A summary of these and additional findings is provided in Tables 1 and 2.
Together, the evidence of NOPr involvement in dopamine and glutamate signaling, given the localization patterns and modulatory roles, suggests considerable potential for NOPr signaling in the pathophysiology of schizophrenia.
Involvement of the NOPr system in the HPA axis
Patients with schizophrenia present with an increased vulnerability to stress, which is thought to be a result of HPA axis dysregulation6. The NOPr system may be a critical mediator in the stress response via the HPA axis through its effects on adrenocorticotropic hormone and corticosterone. The collective findings are summarized in Table 3. N/OFQ increased corticosterone and adrenocorticotropic hormone in non-stressed rats and in mildly stressed rats, indicating its ability to activate the HPA axis50. In a similar manner, stress decreased N/OFQ content in the basal forebrain51. Leggett et al. also observed increased plasma adrenocorticotropic hormone and corticotropin-releasing factor mRNA in the paraventricular nucleus, known to be instrumental in HPA axis activity, in response to N/OFQ, leading the authors to conclude that N/OFQ mediates HPA axis activation52. Limbic system involvement is also evident, as acute restraint stress increased N/OFQ expression in hippocampal subfields and was associated with concentration of glucocorticoids53. However, HPA axis activity effects appear to occur through additive actions in multiple brain regions, since ICV injection of N/OFQ also resulted in elevated corticosterone levels54.
Genetic knockout of N/OFQ reduced the adaptability of mice to stress, but resulted in an elevated level of plasma corticosterone, demonstrating that the effect of NOPr on the HPA axis may be inhibitory55. In another study, N/OFQ knockout mice had impaired adaptation to stress, furthering the theory of NOPr involvement in stress adaptability56. In this study, exposure to repeated stress by way of a forced-swim test failed to produce adaptability in knockout mice, while an increase in anxiety-like behavior was also noted.
Similarly, Le Cudennec et al. showed N/OFQ to decrease corticosterone levels following stress, indicating anti-stress effects of this neurotransmitter and also an inhibitory effect on the HPA axis57. These differential findings may arise due to species differences, but may also indicate a dynamic role of NOPr modulation of stress reactivity. Social stress (in different forms) increased N/OFQ and NOPr mRNA in the hippocampus, central amygdala, paraventricular nucleus, and in the nucleus accumbens shell58,59,60,61.
Differential results do exist, such as those obtained by Prince-Zullig et al.62 They reported no difference in basal corticosterone levels between N/OFQ knockout mice and wild-type controls, in direct contrast with those of Koster et al.56 Additionally, they found N/OFQ administration to have no significant impact on corticosterone levels compared to saline-injected controls, contradicting prior evidence and suggesting a role of environmental stressors or the injection procedure itself in producing these elevated responses. Such effects have also been noted previously (see review: Gavioli et al.63). These differences may arise from the NOPr system’s significant involvement in pain, as one study showed a lack of HPA activation in a neuropathic pain model, thus implicating pain in HPA axis activation64.
We also note that the NOPr system plays a role in the production of cytokines, with several studies demonstrating its peripheral impact on the immune system (see review: Bodera et al.)65,66,67. This could also account for HPA axis activation. Nonetheless, the accumulation of this evidence thus far aligns itself with the findings of a dysregulated HPA axis in schizophrenia patients6, given the apparently modulatory role of NOPr on the HPA axis.
Involvement of the NOPr system in cognition
Cognitive deficits are a prevalent finding in the schizophrenia population, with deficits in working memory being commonplace2. The NOPr system has been shown to play a role in cognition, based on evidence from preclinical studies. These findings are summarized in Table 4. Initial evidence demonstrated spatial learning deficits after N/OFQ injection into the rat hippocampus, and blocking of these effects by NOPr antagonism68,69,70. Higgins et al. also observed improved performance in N/OFQ knockout rats, with dose-dependent reductions in swim speed, demonstrating effects on locomotion70. Similarly, Sandin et al. demonstrated a dose-dependent biphasic effect of N/OFQ on spatial learning, with low doses improving learning and higher doses impairing it71. In contrast, Kuzmin et al. replicated these findings in mice with ICV administration of N/OFQ and observed no biphasic effect, suggesting potential species differences72. Questions regarding the mechanism of NOPr signaling effects on cognition do exist, as Koster et al. demonstrated N/OFQ knockout mice to have no difference in spatial learning compared to controls56.
Moreover, working memory impairments have been noted via insufficiencies in passive avoidance in animals following NOPr activation through administration of agonists or N/OFQ70,73,74. NOPr activation impaired long-term memory formation as measured through recognition memory. The mechanism for this is potentially via the suppression of glutamatergic function at the NMDA receptor75,76,77,78. Reiss et al. demonstrated selective impairment of recognition memory in mice following co-administration of a NOPr agonist and NMDA receptor antagonist, further demonstrating NOPr system modulation of memory formation via glutamatergic receptors79. These findings have since found additional support with deficits in recognition memory and fear learning in mice following increases in NOPr activity mediated via suppression of glutamate transmission80,81. Furthermore, a negative impact of NOPr signaling on long-term potentiation (LTP) in the hippocampus has also been observed, as NOPr-deficient mice had improved LTP, gauged through NOPr and N/OFQ gene expression in the hippocampus70,75,76,82.
Acetylcholine (ACh) signaling is posited to play a role in the cognitive deficits observed in schizophrenia7, and the NOPr system is also implicated with this neurotransmitter. Initial in vivo evidence showed N/OFQ to decrease ACh release in the striatum in rats83. This was later corroborated with similar evidence in cortical and hippocampal regions, thus further demonstrating effects of the NOPr system on cognition84. Uezu et al. reported specific findings in the hippocampus, with knockout mice having greater amounts of ACh, leading the authors to speculate an impact of NOPr signaling on memory function85. Findings by Hiramatsu et al. indicate a dose-dependent effect of N/OFQ on ACh signaling, as they found high doses to decrease it, while lower doses countered antagonist-induced ACh signaling decrease86. Additional research is necessary in order to further elucidate the mechanisms by which these effects occur.
Collectively, these results confirm an involvement of NOPr signaling on cognition including working memory deficits, spatial working memory deficits, and impairments in LTP. Dopaminergic dysfunction, glutamatergic hypofunction, and effects on cholinergic transmission have been outlined as mechanisms that may underlie these deficits87,88,89, and hence, aberrant NOPr signaling may play a crucial role in the cognitive deficits of schizophrenia.
Involvement of the NOPr system in reward modulation
Deficits in reward processing and motivation are a common finding in schizophrenia3. Through investigations into the rewarding properties of drugs of abuse, the NOPr system could be involved in reward modulation, particularly since a moderate-to-high concentration of NOPr in regions associated with reward is observed, including the ventral tegmental area, medial prefrontal cortex, amygdala, and the bed nucleus of the stria terminalis11. The findings are summarized in Table 5. Conditioned place preference (CPP) is a valid method for the study of motivational effects and reward in different paradigms90. Thus, studies with NOPr signaling in CPP can aid understanding of the role of the NOPr system in reward. Treatment with N/OFQ resulted in an inhibition of reward salience, as measured via CPP, and these findings were replicated with multiple drugs of abuse, including morphine, cocaine, and amphetamines91,92,93,94,95. Similarly, NOPr antagonism or knockout results in an increase in CPP with drugs of abuse96,97,98. However, the literature is still inconsistent as an increase in CPP has also been observed with NOPr agonism98,99. Endogenous N/OFQ does not have any reinforcing effects, indicating that it in itself does not have any effects on CPP98. Generally, activation of the NOPr system could be involved in negative reinforcement, as agonism has been shown to decrease self-administration of ethanol100.
This discrepancy is highlighted by more recent findings regarding the NOPr system in reward and motivation. ICV administration of N/OFQ increased bouts of licking for sucrose (a sweet solution) in mice, which led to the suggestion that activation of this system increases the motivation associated with appetite101. Conversely, NOPr knockout rats in another study did not differ from wild-type controls in their preference for saccharin, although these rats did have a significantly reduced proclivity for self-administration of cocaine, heroin, and alcohol102. A similar finding was observed in a comparison between cocaine and sweetened condensed milk103. These results suggest a role for the NOPr system in drug reward specifically, but are also inconsistent with previous findings of a role in motivation in general. These differences could be a result of inherent species differences as well as a difference in methodology (i.e., licking microstructure analysis versus fixed-ratio self-administration).
Another recent study demonstrated negative correlations between reward learning and N/OFQ peptide mRNA levels in the cingulate gyrus and with NOPr mRNA levels in the ventral tegmental area61. Overall, the results align with the reward system disruptions noted in schizophrenia, in which the existence of deficits is well supported by the literature3. The NOPr system may thus play a role in these deficits, further potentiating its involvement in the pathophysiology of schizophrenia.
Conclusion and future directions
In summary, the literature indicates a role of the NOPr system in dopamine and glutamate regulation, with NOPr activation generally decreasing dopamine and glutamate transmission, although this requires further elucidation. Activation of NOPr is also associated with HPA axis regulation, implicating a role for it in the modulation of stress. Cognition is generally negatively impacted with NOPr activation. While reports of the system’s impact on the reward system are mixed, they nonetheless point to the existence of an effect. Indeed, the NOPr system has potential in other psychiatric illnesses, such as depression, in which antagonism is demonstrated to have promising effectiveness104.
Due to the multi-faceted effects of the NOPr system in the brain, how exactly it may be altered in psychosis remains to be elucidated. This preclinical and in vitro evidence presented in conjunction with the well-replicated findings in schizophrenia clearly implicate a plausible contribution of the NOPr system in the pathophysiology of schizophrenia89,105. The literature we have presented in this review indicates the paucity in this field and thus highlights the need for further research. The development and validation of the novel PET tracer [11C]NOP-1A now makes this possible to investigate in clinical populations13,106.
In conclusion, we present here a novel approach to a complex neuropsychiatric illness and demonstrate that the literature suggests a potential role of the NOPr system in schizophrenia, with ramifications in the development of better treatment and interventions, and possibly even prevention.
References
Schultz, S. H., North, S. W. & Shields, C. G. Schizophrenia: a review. Am. Fam. Physician 75, 1821–1829 (2007).
Barch, D. M. & Ceaser, A. Cognition in schizophrenia: core psychological and neural mechanisms. Trends Cogn. Sci. 16, 27–34 (2012).
Strauss, G. P., Waltz, J. A. & Gold, J. M. A review of reward processing and motivational impairment in schizophrenia. Schizophr. Bull. 40(Suppl 2), S107–S116 (2014).
Grace, A. A. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat. Rev. Neurosci. 17, 524–532 (2016).
Moghaddam, B. & Javitt, D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacol 37, 4–15 (2012).
Walker, E., Mittal, V. & Tessner, K. Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annu. Rev. Clin. Psychol. 4, 189–216 (2008).
Freedman, R. Alpha7-nicotinic acetylcholine receptor agonists for cognitive enhancement in schizophrenia. Annu. Rev. Med. 65, 245–261 (2014).
Mollereau, C. et al. ORL1, a novel member of the opioid receptor family. FEBS Lett. 341, 33–38 (1994).
Meunier, J. et al. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature 377, 532–535 (1995).
Reinscheid, R. K. et al. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science 270, 792–794 (1995).
Neal, C. R. et al. Opioid receptor-like (ORL1) receptor distribution in the rat central nervous system: comparison of ORL1 receptor mRNA expression with125I-[14Tyr]-orphanin FQ binding. J. Comp. Neurol. 412, 563–605 (1999).
Berthele, A. et al. [3h]-nociceptin ligand-binding and nociceptin opioid receptor mrna expression in the human brain. Neuroscience 121, 629–640 (2003).
Lohith, T. G. et al. Brain and whole-body imaging of nociceptin/orphanin FQ peptide receptor in humans using the PET ligand 11C-NOP-1A. J. Nucl. Med. 53, 385–392 (2012).
Norton, C. S., Neal, C. R., Kumar, S., Akil, H. & Watson, S. J. Nociceptin/orphanin FQ and opioid receptor-like receptor mRNA expression in dopamine systems. J. Comp. Neurol. 444, 358–368 (2002).
Di Benedetto, M. et al Alterations of N/OFQ and NOP receptor gene expression in the substantia nigra and caudate putamen of MPP+ and 6-OHDA lesioned rats. Neuropharmacology 56, 761–767 (2009).
Nagatsu, T. Tyrosine hydroxylase: human isoforms, structure and regulation in physiology and pathology. Essays Biochem. 30, 15–35 (1995).
Olianas, M. C., Dedoni, S., Boi, M. & Onali, P. Activation of nociceptin/orphanin FQ-NOP receptor system inhibits tyrosine hydroxylase phosphorylation, dopamine synthesis, and dopamine D(1) receptor signaling in rat nucleus accumbens and dorsal striatum. J. Neurochem. 107, 544–556 (2008).
Viaro, R., Calcagno, M., Marti, M., Borrelli, E. & Morari, M. Pharmacological and genetic evidence for pre- and postsynaptic D2 receptor involvement in motor responses to nociceptin/orphanin FQ receptor ligands. Neuropharmacology 72, 126–138 (2013).
Devine, D. P. et al. Rats rapidly develop tolerance to the locomotor-inhibiting effects of the novel neuropeptide orphanin FQ. Neurochem. Res. 21, 1387–1396 (1996).
Noble, F. & Roques, B. P. Association of aminopeptidase N and endopeptidase 24.15 inhibitors potentiate behavioral effects mediated by nociceptin/orphanin FQ in mice. FEBS Lett. 401, 227–229 (1997).
Rizzi, A. et al. Characterization of the locomotor activity-inhibiting effect of nociceptin/orphanin FQ in mice. N. S. Arch. Pharmacol. 363, 161–165 (2001).
Flau, K., Redmer, A., Liedtke, S., Kathmann, M. & Schlicker, E. Inhibition of striatal and retinal dopamine release via nociceptin/orphanin FQ receptors. Br. J. Pharmacol. 137, 1355–1361 (2002).
Marti, M., Viaro, R., Guerrini, R., Franchi, G. & Morari, M. Nociceptin/orphanin FQ modulates motor behavior and primary motor cortex output through receptors located in substantia nigra reticulata. Neuropsychopharmacol 34, 341–355 (2009).
Liu, Z. et al. Orphanin FQ: an endogenous antagonist of rat brain dopamine transporter. Neuroreport 12, 699–702 (2001).
Di Giannuario, A., Pieretti, S., Catalani, A. & Loizzo, A. Orphanin FQ reduces morphine-induced dopamine release in the nucleus accumbens: a microdialysis study in rats. Neurosci. Lett. 272, 183–186 (1999).
Marti, M. et al. Blockade of nociceptin/orphanin FQ receptor signaling in rat substantia nigra pars reticulata stimulates nigrostriataldopaminergic transmission and motor behavior. J. Neurosci. 24, 6659–6666 (2004).
Marti, M. et al. Blockade of nociceptin/orphanin FQ transmission attenuates symptoms and neurodegeneration associated with Parkinson’s disease. J. Neurosci. 25, 9591–9601 (2005).
Marti, M., Trapella, C. & Morari, M. The novel nociceptin/orphanin FQ receptor antagonist Trap-101 alleviates experimental parkinsonism through inhibition of the nigro-thalamic pathway: positive interaction with L-DOPA. J. Neurochem. 107, 1683–1696 (2008).
Viaro, R. et al. Nociceptin/orphanin FQ receptor blockade attenuates MPTP-induced parkinsonism. Neurobiol. Dis. 30, 430–438 (2008).
Volta, M., Viaro, R., Trapella, C., Marti, M. & Morari, M. Dopamine–nociceptin/orphanin FQ interactions in the substantia nigra reticulata of hemiparkinsonian rats: involvement of D2/D3 receptors and impact on nigro-thalamic neurons and motor activity. Exp. Neurol. 228, 126–137 (2011).
Viaro, R., Marti, M. & Morari, M. Dual motor response to l-dopa and nociceptin/orphanin FQ receptor antagonists in 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine (MPTP) treated mice: paradoxical inhibition is relieved by D2/D3 receptor blockade. Exp. Neurol. 223, 473–484 (2010).
Arcuri, L. et al. Genetic and pharmacological evidence that endogenous nociceptin/orphanin FQ contributes to dopamine cell loss in Parkinson’s disease. Neurobiol. Dis. 59, 55–64 (2016).
Brown, J. M., Gouty, S., Iyer, V., Rosenberger, J. & Cox, B. M. Differential protection against MPTP or methamphetamine toxicity in dopamine neurons by deletion of ppN/OFQ expression. J. Neurochem. 98, 495–505 (2006).
Sakoori, K. & Murphy, N. P. Reduced degeneration of dopaminergic terminals and accentuated astrocyte activation by high dose methamphetamine administration in nociceptin receptor knock out mice. Neurosci. Lett. 469, 309–313 (2010).
Ces, A. et al. Activation of nociceptin/orphanin FQ peptide receptors disrupts visual but not auditory sensorimotor gating in BALB/cByJ mice: comparison to dopamine receptor agonists. Neuropsychopharmacol 37, 378–389 (2012).
Koch, M. Clinical relevance of animal models of schizophrenia. Suppl. Clin. Neurophys. 62, 113–120 (2013).
Nicol, B., Lambert, D. G., Rowbotham, D. J., Smart, D. & Mcknight, A. T. Nociceptin induced inhibition of K evoked glutamate release from rat cerebrocortical slices. Br. J. Pharmacol. 199, 1081–1083 (1996).
Nicol, B., Rowbotham, D. & Lambert, D. Nociceptin/orphanin FQ inhibits glutamate release from rat cerebellar and brain stem slices. Neurosci. Lett. 326, 85–88 (2002).
Meis, S. & Pape, H. Control of glutamate and GABA release by nociceptin/orphanin FQ in the rat lateral amygdala. J. Physiol. 532, 701–712 (2001).
Gompf, H. S., Moldavan, M. G., Irwin, R. P. & Allen, C. N. Nociceptin/orphanin FQ (N/OFQ) inhibits excitatory and inhibitory synaptic signaling in the suprachiasmatic nucleus (SCN). Neuroscience 132, 955–965 (2005).
Kallupi, M. et al. Nociceptin/orphanin FQ decreases glutamate transmission and blocks ethanol-induced effects in the central amygdala of naive and ethanol-dependent rats. Neuropsychopharmacol 39, 1081–1092 (2014).
Mabrouk, O. S., Marti, M. & Morari, M. Endogenous nociceptin/orphanin FQ (N/OFQ) contributes to haloperidol-induced changes of nigral amino acid transmission and parkinsonism: a combined microdialysis and behavioral study in naive and nociceptin/orphanin FQ receptor knockout mice. Neuroscience 166, 40–48 (2010).
Gavioli, E. C. et al. GABA(A) signalling is involved in N/OFQ anxiolytic-like effects but not in nocistatin anxiogenic-like action as evaluated in the mouse elevated plus maze. Peptides 29, 1404–1412 (2008).
Mabrouk, O. S. et al. Stimulation of opioid receptor and blockade of nociceptin/orphanin FQ receptor synergistically attenuate Parkinsonism. J. Neurosci. 34, 12953–12962 (2014).
Marti, M., Trapella, C., Viaro, R. & Morari, M. The nociceptin/orphanin FQ receptor antagonist J-113397 and L-DOPA additively attenuate experimental Parkinsonism through overinhibition of the nigrothalamic pathway. J. Neurosci. 27, 1297–1307 (2007).
Murphy, N. P. & Maidment, N. T. Orphanin FQ/nociceptin modulation of mesolimbic dopamine transmission determined by microdialysis. J. Neurochem. 73, 179–186 (1999).
Taylor, T. F. & Tso, I. F. GABA abnormalities in schizophrenia: a methodological review of in vivo studies. Schizophr. Res. 167, 84–90 (2015).
Marti, M., Guerrini, R., Beani, L., Bianchi, C. & Morari, M. Nociceptin/orphanin FQ receptors modulate glutamate extracellular levels in the substantia nigra pars reticulata. A microdialysis study in the awake freely moving rat. Neuroscience 112, 153–160 (2002).
Wassef, A., Baker, J. & Kochan, L. D. GABA and schizophrenia: a review of basic science and clinical studies. J. Clin. Psychopharmacol. 23, 601–640 (2003).
Devine, D., Watson, S. & Akil, H. Nociceptin/orphanin FQ regulates neuroendocrine function of the limbic–hypothalamic–pituitary–adrenal axis. Neuroscience 102, 541–553 (2001).
Devine, D., Hoversten, M. T., Ueda, Y. & Akil, H. Nociceptin/orphanin FQ content is decreased in forebrain neurones during acute stress. J. Neuroendocrinol. 15, 69–74 (2003).
Leggett, J., Harbuz, M., Jessop, D. & Fulford, A. The nociceptin receptor antagonist [Nphe1,Arg14,Lys15]nociceptin/orphanin FQ-NH2 blocks the stimulatory effects of nociceptin/orphanin FQ on the HPA axis in rats. Neuroscience 141, 2051–2057 (2006).
Nativio, P., Pascale, E., Maffei, A., Scaccianoce, S. & Passarelli, F. Effect of stress on hippocampal nociceptin expression in the rat. Stress 15, 378–384 (2012).
Green, M. K., Barbieri, E. V., Brown, B. D., Chen, K. & Devine, D. Roles of the bed nucleus of stria terminalis and of the amygdala in N/OFQ-mediated anxiety and HPA axis activation. Neuropeptides 41, 399–410 (2007).
Jenck, F. et al. Orphanin FQ acts as an anxiolytic to attenuate behavioral responses to stress. Proc. Natl. Acad. Sci. USA 94, 14854–14858 (1997).
Koster, A. et al. Targeted disruption of the orphanin FQ/nociceptin gene increases stress susceptibility and impairs stress adaptation in mice. Proc. Natl. Acad. Sci. USA 96, 10444–10449 (1999).
Le Cudennec, C., Naudin, B., Do Rego, J. D. & Costentin, J. Nociceptin/orphanin FQ and related peptides reduce the increase in plasma corticosterone elicited in mice by an intracerebroventricular injection. Life Sci. 72, 163–171 (2002).
Green, M. K. & Devine, D. P. Nociceptin/orphanin FQ and NOP receptor gene regulation after acute or repeated social defeat stress. Neuropeptides 43, 507–514 (2009).
Reiss, D., Wolter-Sutter, A., Krezel, W. & Ouagazzal, A. M. Effects of social crowding on emotionality and expression of hippocampal nociceptin/orphanin FQ system transcripts in mice. Behav. Brain Res. 184, 167–173 (2007).
Ciccocioppo, R. et al. Restraint stress alters nociceptin/orphanin FQ and CRF systems in the rat central amygdala: significance for anxiety-like behaviors. J. Neurosci. 34, 363–372 (2014).
Der-Avakian, A. et al. Social defeat disrupts reward learning and potentiates striatal nociceptin/orphanin FQ mRNA in rats. Psychopharmacology 234, 1603–1614 (2017).
Prince-Zullig, K. L., Murphree, E., Reinscheid, R. K., Janik, J. & Callahan, P. Effect of Nociceptin/orphanin FQ (N/OFQ) and isoflurane on the corticosterone secretory response in mice lacking the N/OFQ prepropeptide (ppN/OFQ−/−). Neuropeptides 43, 201–205 (2009).
Gavioli, E. C., de Medeiros, I. U., Monteiro, M. C., Calo, G. & Romao, P. R. Nociceptin/orphanin FQ-NOP receptor system in inflammatory and immune-mediated diseases. Vitam. Horm. 97, 241–266 (2015).
Palmisano, M. et al. N/OFQ system in brain areas of nerve-injured mice: its role in different aspects of neuropathic pain. Genes. Brain Behav. 16, 537–545 (2017).
Fu, X., Zhu, Z. H., Wang, Y. Q. & Wu, G. C. Regulation of proinflammatory cytokines gene expression by nociceptin/orphanin FQ in the spinal cord and the cultured astrocytes. Neuroscience 144, 275–285 (2007).
Finley, M. J., Happel, C. M., Kaminsky, D. E. & Rogers, T. J. Opioid and nociceptin receptors regulate cytokine and cytokine receptor expression. Cell. Immunol. 252, 146–154 (2008).
Bodera, P., Stankiewicz, W. & Kocik, J. Interactions of orphanin FQ/nociceptin (OFQ/N) system with immune system factors and hypothalamic-pituitary-adrenal (HPA) axis. Pharmacol. Rep. 66, 288–291 (2014).
Sandin, J., Georgieva, J., Schött, P. A., Ögren, S. O. & Terenius, L. Nociceptin/orphanin FQ microinjected into hippocampus impairs spatial learning in rats. Eur. J. Neurosci. 9, 194–197 (1997).
Redrobe, J. P., Calo, G., Guerrini, R., Regoli, D. & Quirion, R. [Nphe1]-Nociceptin (1-13)-NH2, a nociceptin receptor antagonist, reverses nociceptin-induced spatial memory impairments in the Morris water maze task in rats. Br. J. Pharmacol. 131, 1379–1384 (2000).
Higgins, G. A. et al. A combined pharmacological and genetic approach to investigate the role of orphanin FQ in learning and memory. Eur. J. Neurosci. 15, 911–922 (2002).
Sandin, J., Ögren, S. O. & Terenius, L. Nociceptin/orphanin FQ modulates spatial learning via ORL-1 receptors in the dorsal hippocampus of the rat. Brain Res. 997, 222–233 (2004).
Kuzmin, A., Madjid, N., Johansson, B., Terenius, L. & Ögren, S. O. The nociceptin system and hippocampal cognition in mice: a pharmacological and genetic analysis. Brain Res. 1305, S7–S19 (2009).
Mamiya, T., Noda, Y., Nishi, M., Takeshima, H. & Nabeshima, T. Nociceptin system plays a role in the memory retention. Neuroreport 10, 1171–1175 (1999).
Hiramatsu, M. & Inoue, K. Nociceptin/orphanin FQ and nocistatin on learning and memory impairment induced by scopolamine in mice. Br. J. Pharmacol. 127, 655–660 (1999).
Yu, T. & Xie, C. Orphanin FQ/nociceptin inhibits synaptic transmission and long-term potentiation in rat dentate gyrus through postsynaptic mechanisms. J. Neurophysiol. 80, 1277–1284 (1998).
Manabe, T. et al. Facilitation of long-term potentiation and memory in mice lacking nociceptin receptors. Nature 394, 577–581 (1998).
Wei, W. & Xie, C. Orphanin FQ suppresses NMDA receptor-dependent long-term depression and depotentiation in hippocampal dentate gyrus. Learn. Mem. 6, 467–477 (1999).
Bongsebandhu-Phubhakdi, S. & Manabe, T. The neuropeptide nociceptin is a synaptically released endogenous inhibitor of hippocampal long-term potentiation. J. Neurosci. 27, 4850–4858 (2007).
Reiss, D., Prinssen, E. P., Wichmann, J., Kieffer, B. L. & Ouagazzal, A. The nociceptin orphanin FQ peptide receptor agonist, Ro64-6198, impairs recognition memory formation through interaction with glutamatergic but not cholinergic receptor antagonists. Neurobiol. Learn. Mem. 98, 254–260 (2012).
Goeldner, C. et al. Nociceptin receptor impairs recognition memory via interaction with NMDA receptor-dependent mitogen-activated protein kinase/extracellular signal-regulated kinase signaling in the hippocampus. J. Neurosci. 28, 2190–2198 (2008).
Goeldner, C., Reiss, D., Wichmann, J., Kieffer, B. L. & Ouagazzal, A. Activation of nociceptin opioid peptide (NOP) receptor impairs contextual fear learning in mice through glutamatergic mechanisms. Neurobiol. Learn. Mem. 91, 393–401 (2009).
Yu, T. et al. Inhibits synaptic transmission and long‐term potentiation in rat hippocampus. Hippocampus 7, 88–94 (1997).
Itoh, K., Konya, H., Takai, E., Masuda, H. & Nagai, K. Modification of acetylcholine release by nociceptin in conscious rat striatum. Brain Res. 845, 242–245 (1999).
Cavallini, S., Marino, S., Beani, L., Bianchi, C. & Siniscalchi, A. Nociceptin inhibition of acetylcholine efflux from different brain areas. Neuroreport 14, 2167–2170 (2003).
Uezu, K. et al. Enhanced hippocampal acetylcholine release in nociceptin-receptor knockout mice. Brain Res. 1050, 118–123 (2005).
Hiramatsu, M., Miwa, M., Hashimoto, K., Kawai, S. & Nomura, N. Nociceptin/orphanin FQ reverses mecamylamine-induced learning and memory impairment as well as decrease in hippocampal acetylcholine release in the rat. Brain Res. 1195, 96–103 (2008).
Akil, M. et al. Lamina-specific alterations in the dopamine innervation of the prefrontal cortex in schizophrenic subjects. Am. J. Psychiatry 156, 1580–1589 (1999).
Anticevic, A. et al. NMDA receptor function in large-scale anticorrelated neural systems with implications for cognition and schizophrenia. Proc. Natl. Acad. Sci. USA 109, 16720–16725 (2012).
Cassidy, C. M. et al. Dynamic connectivity between brain networks supports working memory: relationships to dopamine release and schizophrenia. J. Neurosci. 36, 4377–4388 (2016).
Tzschentke, T. M. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict. Biol. 12, 227–462 (2007).
Rutten, K., De Vry, J., Bruckmann, W. & Tzschentke, T. M. Pharmacological blockade or genetic knockout of the NOP receptor potentiates the rewarding effect of morphine in rats. Drug. Alcohol Depend. 114, 253–256 (2011).
Vazquez-Derose, J. et al. Retrodialysis of N/OFQ into the nucleus accumbens shell blocks cocaine-induced increases in extracellular dopamine and locomotor activity. Eur. J. Pharmacol. 699, 200–206 (2013).
Ciccocioppo, R., Angeletti, S., Sanna, P. P., Weiss, F. & Massi, M. Effect of nociceptin/orphanin FQ on the rewarding properties of morphine. Eur. J. Pharmacol. 404, 153–159 (2000).
Sakoori, K. & Murphy, N. P. Central administration of nociceptin/orphanin FQ blocks the acquisition of conditioned place preference to morphine and cocaine, but not conditioned place aversion to naloxone in mice. Psychopharmacology 172, 129–136 (2004).
Kotlinska, J. et al. Nociceptin inhibits acquisition of amphetamine-induced place preference and sensitization to stereotypy in rats. Eur. J. Pharmacol. 474, 233–239 (2003).
Marquez, P., Nguyen, A. T., Hamid, A. & Lutfy, K. The endogenous OFQ/N/ORL-1 receptor system regulates the rewarding effects of acute cocaine. Neuropharmacology 54, 564–568 (2008).
Sakoori, K. & Murphy, N. P. Endogenous nociceptin (orphanin FQ) suppresses basal hedonic state and acute reward responses to methamphetamine and ethanol, but facilitates chronic responses. Neuropsychopharmacol 33, 877–891 (2008).
Rutten, K., De Vry, J., Bruckmann, W. & Tzschentke, T. M. Effects of the NOP receptor agonist Ro65-6570 on the acquisition of opiate- and psychostimulant-induced conditioned place preference in rats. Eur. J. Pharmacol. 645, 119–126 (2010).
Kotlinska, J., Wichmann, J., Legowska, A., Rolka, K. & Silberring, J. Orphanin FQ/nociceptin but not Ro 65-6570 inhibits the expression of cocaine-induced conditioned place preference. Behav. Pharmacol. 13, 229–235 (2002).
Kuzmin, A., Kreek, M. J., Bakalkin, G. & Liljequist, S. The nociceptin/orphanin FQ receptor agonist Ro 64-6198 reduces alcohol self-administration and prevents relapse-like alcohol drinking. Neuropsychopharmacol 32, 902–910 (2007).
Mendez, I. A., Maidment, N. T. & Murphy, N. P. Parsing the hedonic and motivational influences of nociceptin on feeding using licking microstructure analysis in mice. Behav. Pharmacol. 27, 516–527 (2016).
Kallupi, M. et al. Genetic deletion of the nociceptin/orphanin FQ receptor in the rat confers resilience to the development of drug addiction. Neuropsychopharmacol 42, 695–706 (2017).
de Guglielmo, G. et al. Cebranopadol blocks the escalation of cocaine intake and conditioned reinstatement of cocaine seeking in rats. J. Pharmacol. Exp. Ther. 362, 378–384 (2017).
Post, A. et al. A selective nociceptin receptor antagonist to treat depression: evidence from preclinical and clinical studies. Neuropsychopharmacol 41, 2624 (2016).
Slifstein, M. et al. Deficits in prefrontal cortical and extrastriatal dopamine release in schizophrenia. JAMA Psychiatry 72, 316 (2015).
Narendran R. et al. Nociceptin receptors in alcohol use disorders: a positron emission tomography study using [11C]NOP-1A. Biol. Psychiatry, (2017). https://doi.org/10.1016/j.biopsych.2017.05.019.
Florin, S., Suaudeau, C., Meunier, J. C. & Costentin, J. Nociceptin stimulates locomotion and exploratory behaviour in mice. Eur. J. Pharmacol. 317, 9–13 (1996).
Devine, D. P., Reinscheid, R. K., Monsma, F. J. Jr., Civelli, O. & Akil, H. The novel neuropeptide orphanin FQ fails to produce conditioned place preference or aversion. Brain Res. 727, 225–229 (1996).
Narayanan, S., Lam, H., Carroll, F. I. & Lutfy, K. Orphanin FQ/nociceptin suppresses motor activity through an action along the mesoaccumbens axis in rats. J. Psychiatry Neurosci. 29, 116–123 (2004).
Chesnokova, E. A. et al. The effects of new nociceptin analogs on the behavior of white rats. Dokl. Biol. Sci. 449, 85–88 (2013).
Rizzi, A., Molinari, S., Marti, M., Marzola, G. & Calo, G. Nociceptin/orphanin FQ receptor knockout rats: in vitro and in vivo studies. Neuropharmacology 60, 572–579 (2011).
Maidment, N. T., Chen, Y., Tan, A. M., Murphy, N. P. & Leslie, F. M. Rat ventral midbrain dopamine neurons express the orphanin FQ/nociceptin receptor ORL-1. Neuroreport 13, 1137–1140 (2002).
Murphy, N. P., Ly, H. T. & Maidment, N. T. Intracerebroventricular orphanin FQ/nociceptin suppresses dopamine release in the nucleus accumbens of anaesthetized rats. Neuroscience 75, 1–4 (1996).
Zheng, F., Grandy, D. K. & Johnson, S. W. Actions of orphanin FQ/nociceptin on rat ventral tegmental area neurons in vitro. Br. J. Pharmacol. 136, 1065–1071 (2002).
Murphy, N. P., Tan, A. M., Lam, H. A. & Maidment, N. T. Nociceptin/orphanin FQ modulation of rat midbrain dopamine neurons in primary culture. Neuroscience 127, 929–940 (2004).
Lutfy, K., Do, T. & Maidment, N. T. Orphanin FQ/nociceptin attenuates motor stimulation and changes in nucleus accumbens extracellular dopamine induced by cocaine in rats. Psychopharmacology 154, 1–7 (2001).
Koizumi, M., Midorikawa, N., Takeshima, H. & Murphy, N. P. Exogenous, but not endogenous nociceptin modulates mesolimbic dopamine release in mice. J. Neurochem. 89, 257–263 (2004).
Koizumi, M., Sakoori, K., Midorikawa, N. & Murphy, N. P. The NOP (ORL1) receptor antagonist compound B stimulates mesolimbic dopamine release and is rewarding in mice by a non-NOP-receptor-mediated mechanism. Br. J. Pharmacol. 143, 53–62 (2004).
Konya, H., Masuda, H., Itoh, K., Nagai, K., Kakishita, E. & Matsuoka, A. Modification of dopamine release by nociceptin in conscious rat striatum. Brain Res. 788, 341–344 (1998).
Marti, M. et al. Blockade of nociceptin/orphanin FQ transmission in rat substantia nigra reverses haloperidol-induced akinesia and normalizes nigral glutamate release. J. Neurochem. 91, 1501–1504 (2004).
Marti, M. et al. Nociceptin/orphanin FQ receptor agonists attenuate L-DOPA-induced dyskinesias. J. Neurosci. 32, 16106–16119 (2012).
Marti, M. et al. Brain interstitial nociceptin/orphanin FQ levels are elevated in Parkinson’s disease. Mov. Disord. 25, 1723–1732 (2010).
Gouty, S., Brown, J. M., Rosenberger, J. & Cox, B. M. MPTP treatment increases expression of pre-pro-nociceptin/orphanin FQ mRNA in a subset of substantia nigra reticulata neurons. Neuroscience 169, 269–278 (2010).
Mamiya, T. et al. Neuronal mechanism of nociceptin-induced modulation of learning and memory: involvement of N-methyl-D-aspartate receptors. Mol. Psychiatry 8, 752–765 (2003).
Griebel, G., Perrault, G. & Sanger, D. J. Orphanin FQ, a novel neuropeptide with anti-stress-like activity. Brain Res. 836, 221–224 (1999).
Leggett, J., Jessop, D. & Fulford, A. The nociceptin/orphanin FQ antagonist UFP-101 differentially modulates the glucocorticoid response to restraint stress in rats during the peak and nadir phases of the hypothalamo–pituitary–adrenal axis circadian rhythm. Neuroscience 147, 757–764 (2007).
Leggett, J. D., Dawe, K. L., Jessop, D. S. & Fulford, A. J. Endogenous nociceptin / orphanin FQ system involvement in hypothalamic-pituitary-adrenal axis responses: relevance to models of inflammation. J. Neuroendocrinol. 21, 888–897 (2009).
Delaney, G. et al. Role of nociceptin/orphanin FQ and NOP receptors in the response to acute and repeated restraint stress in rats. J. Neuroendocrinol. 24, 1527–1541 (2012).
Jinsmaa, Y., Takahashi, M., Fukunaga, H. & Yoshikawa, M. Retro-nociceptin methylester, a peptide with analgesic and memory-enhancing activity. Life Sci. 67, 3095–3101 (2000).
Noda, Y. [Possible mechanisms in latent learning formation investigated by using mutant mice]. Folia Pharmacol. Jpn. 117, 169–176 (2001).
Mamiya, T., Noda, Y., Nishi, M., Takeshima, H. & Nabeshima, T. Enhancement of spatial attention in nociceptin/orphanin FQ receptor-knockout mice. Brain Res. 783, 236–240 (1998).
Liu, E. H., Lee, T. L., Nishiuchi, Y., Kimura, T. & Tachibana, S. Nocistatin and its derivatives antagonize the impairment of short-term acquisition induced by nociceptin. Neurosci. Lett. 416, 155–159 (2007).
Nagai, J., Kurokawa, M., Takeshima, H., Kieffer, B. L. & Ueda, H. Circadian-dependent learning and memory enhancement in nociceptin receptor-deficient mice with a novel KUROBOX apparatus using stress-free positive cue task. J. Pharm. Exp. Ther. 321, 195–201 (2007).
Noda, Y., Mamiya, T. & Nabeshima, T. Behavioral pharmacological characterization of mice lacking the nociceptin receptor. Nihon Shinkei Seishin Yakurigaku Zasshi 19, 73–78 (1999).
Taverna, F. A. et al. Defective place cell activity in nociceptin receptor knockout mice with elevated NMDA receptor-dependent long-term potentiation. J. Physiol. 565(Pt 2), 579–591 (2005).
Miwa, M. et al. Nociceptin and its metabolite attenuate U0126-induced memory impairment through a nociceptin opioid peptide (NOP) receptor-independent mechanism. Neurobiol. Learn. Mem. 93, 396–405 (2010).
Murphy, N. P., Lee, Y. & Maidment, N. T. Orphanin FQ/nociceptin blocks acquisition of morphine place preference. Brain Res. 832, 168–170 (1999).
Kotlinska, J. et al. Is the nociceptin (NOP) receptor involved in attenuation of the expression of sensitization to morphine-induced hyperlocomotion in mice? Behav. Pharmacol. 16, 101–106 (2005).
Zhao, R. J. et al. Orphanin FQ/nociceptin blocks methamphetamine place preference in rats. Neuroreport 14, 2383–2385 (2003).
Acknowledgements
This work has been supported by the Centre for Addiction and Mental Health Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khan, M.S., Boileau, I., Kolla, N. et al. A systematic review of the role of the nociceptin receptor system in stress, cognition, and reward: relevance to schizophrenia. Transl Psychiatry 8, 38 (2018). https://doi.org/10.1038/s41398-017-0080-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-017-0080-8
- Springer Nature Limited
This article is cited by
-
Development of a genetically encoded sensor for probing endogenous nociceptin opioid peptide release
Nature Communications (2024)
-
Differences in olfactory dysfunction and its relationship with cognitive function in schizophrenia patients with and without auditory verbal hallucinations
European Archives of Psychiatry and Clinical Neuroscience (2023)
-
Regulation of N-type calcium channels by nociceptin receptors and its possible role in neurological disorders
Molecular Brain (2022)
-
Nicotine in action: cigarette smoking modulated homotopic functional connectivity in schizophrenia
Brain Imaging and Behavior (2019)