Abstract
Study design
Animal experimental study.
Objectives
Spinal cord injury (SCI) at or above the T6 level causes cardiovascular dysfunction. Maintaining cAMP levels with cAMP analogs can facilitate neurological recovery. In the present study, the effects of meglumine cyclic adenylate (MCA), a cAMP analog and approved cardiovascular drug, on cardiovascular and neurological recovery in acute T4-SCI in rats were investigated.
Setting
Hospital in Kunming, China.
Methods
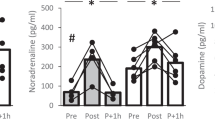
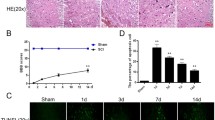
Eighty rats were randomly allocated to five groups, and groups A-D received SCI: (A) a group administered MCA at 2 mg/kg/d iv qd, (B) a group administered dopamine at 2.5 to 5 μg/kg/min iv to maintain mean arterial pressure above 85 mm Hg, (C) a group administered atropine at 1 mg/kg iv bid, (D) a group receiving an equal volume of saline iv qd for 3 weeks after SCI and (E) a group undergoing laminectomy only. The cardiovascular and behavioral parameters of the rats were examined, and spinal cord tissues were processed for hematoxylin and eosin staining, Nissl staining, electron microscopy, and analysis of cAMP levels.
Results
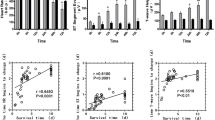
Compared with dopamine or atropine, MCA significantly reversed the decrease in cAMP levels in both myocardial cells and the injured spinal cord; improved hypotension, bradycardia and behavioral parameters at 6 weeks; and improved spinal cord blood flow and histological structure at 7 days post-SCI. The regression analysis suggested spinal cord motor-function improved as decreased heart rate and mean arterial pressure were stopped post-SCI.
Conclusions
MCA may be an effective treatment for acute SCI by sustaining cAMP-dependent reparative processes and improving post-SCI cardiovascular dysfunction.
Sponsorship
N/A.




Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Harman KA, States G, Wade A, Stepp C, Wainwright G, DeVeau K, et al. Temporal analysis of cardiovascular control and function following incomplete T3 and T10 spinal cord injury in rodents. Physiol Rep. 2018;6:e13634.
Sabharwal S. Addressing cardiometabolic risk in adults with spinal cord injury: acting now despite knowledge gaps. Spinal Cord Ser Cases. 2019;5:96.
Phillips AA, Krassioukov AV. Contemporary Cardiovascular Concerns after Spinal Cord Injury: Mechanisms, Maladaptations, and Management. J Neurotrauma. 2015;32:1927–42.
Popok DW, West CR, Hubli M, Currie KD, Krassioukov AV. Characterizing the Severity of Autonomic Cardiovascular Dysfunction after Spinal Cord Injury Using a Novel 24 h Ambulatory Blood Pressure Analysis Software. J Neurotrauma. 2017;34:559–66.
Karim F, Chang P, Garrison C, Steiner M. Role of Theophylline in Management of Bradycardia Secondary to High Cervical Spinal Cord Injury in a Seven-Year-Old Child: Case Report and a Review of Literature. Cureus. 2020;12:e10941.
Soubeyrand M, Dubory A, Laemmel E, Court C, Vicaut E, Duranteau J, et al. Effect of norepinephrine on spinal cord blood flow and parenchymal hemorrhage size in acute-phase experimental spinal cord injury. Eur Spine J. 2014;23:658–65.
Sadek MS, Cachorro E, El-Armouche A, Kämmerer S. Therapeutic Implications for PDE2 and cGMP/cAMP Mediated Crosstalk in Cardiovascular Diseases. Int J Mol Sci. 2020;21:7462.
Winslow RL, Walker MA, Greenstein JL. Modeling calcium regulation of contraction, energetics, signaling, and transcription in the cardiac myocyte. Wiley Interdiscip Rev Syst Biol Med. 2016;8:37–67.
McCabe KJ, Rangamani P. Computational modeling approaches to cAMP/PKA signaling in cardiomyocytes. J Mol Cell Cardiol. 2021;154:32–40.
Batty NJ, Fenrich KK, Fouad K. The role of cAMP and its downstream targets in neurite growth in the adult nervous system. Neurosci Lett. 2017;652:56–63.
Siddiq MM, Hannila SS. Looking downstream: the role of cyclic AMP-regulated genes in axonal regeneration. Front Mol Neurosci. 2015;8:26.
Bavencoffe A, Li Y, Wu Z, Yang Q, Herrera J, Kennedy EJ, et al. Persistent Electrical Activity in Primary Nociceptors after Spinal Cord Injury Is Maintained by Scaffolded Adenylyl Cyclase and Protein Kinase A and Is Associated with Altered Adenylyl Cyclase Regulation. J Neurosci. 2016;36:1660–8.
Blesch A, Lu P, Tsukada S, Alto LT, Roet K, Coppola G, et al. Conditioning lesions before or after spinal cord injury recruit broad genetic mechanisms that sustain axonal regeneration: superiority to camp-mediated effects. Exp Neurol. 2012;235:162–73.
Boomkamp SD, McGrath MA, Houslay MD, Barnett SC. Epac and the high affinity rolipram binding conformer of PDE4 modulate neurite outgrowth and myelination using an in vitro spinal cord injury model. Br J Pharm. 2014;171:2385–98.
Xia T, Huang B, Ni S, Gao L, Wang J, Wang J, et al. The combination of db-cAMP and ChABC with poly(propylene carbonate) microfibers promote axonal regenerative sprouting and functional recovery after spinal cord hemisection injury. Biomed Pharmacother. 2017;86:354–62.
Sierksma AS, Van den Hove DL, Pfau F, Philippens M, Bruno O, Fedele E, et al. Improvement of spatial memory function in APPswe/PS1dE9 mice after chronic inhibition of phosphodiesterase type 4D. Neuropharmacology. 2014;77:120–30.
Wu D, Zhao Y, Yang Y, Wang W, Xu R. A multicenter clinical study on clinical effects of meglumine cyclic adenylate in treating patients with chronic pulmonary heart disease. Zhonghua Nei Ke Za Zhi. 2001;40:467–70.
Feng L, Lai Y, Bu SZ. The efficacy of Meglumine adenosine cyclophosphate vs Amrinone in the treatment of congestive heart failure. Foreing Med Sci Sect Cardiovusc Dis. 2002;29:41–3.
Ding D, Ding J, Jin Z, Qin X, Guan L, Cui X, et al. Evaluation of curative effect of meglumine cyclic adenylate combined with recombinant human brain natriuretic peptide in treatment of coronary artery disease patients with heart failure. J Jilin Univ. 2011;37:723–6.
LI QF, Huang LJ, Wei S. Therapeutic effect of meglumine cyclic adenosine monophosphate on chronic heart failure in patients with type 2 diabetes mellitus and coronary heart disease. Chin Foreign Med Res. 2014;12:9–11.
Liao J, Xie J, Lin D, Lu N, Guo L, Li W, et al. Meglumine cyclic adenylate improves neurological function following acute spinal cord injury in rats. Mol Med Rep. 2014;10:1225–30.
Arifin WN, Zahiruddin WM. Sample Size Calculation in Animal Studies Using Resource Equation Approach[J]. Malaysian J Med Sci. 2017;24:101–5.
Wrathall JR, Pettegrew RK, Harvey F. Spinal cord contusion in the rat: production of graded, reproducible, injury groups. Exp Neurol. 1985;88:108–22.
Ploumis A, Yadlapalli N, Fehlings MG, Kwon BK, Vaccaro AR. A systematic review of the evidence supporting a role for vasopressor support in acute SCI. Spinal Cord. 2010;48:356–62.
Hawryluk G, Whetstone W, Saigal R, Ferguson A, Talbott J, Bresnahan J, et al. Mean Arterial Blood Pressure Correlates with Neurological Recovery after Human Spinal Cord Injury: Analysis of High Frequency Physiologic Data. J Neurotrauma. 2015;32:1958–67.
Aigbe F, Adeyemi O, Zubaid M, Rathore H, Sofidiya M. Effect of the aqueous root extract of aristolochiaringens and its fractions on haemodynamic parameters in a rodent model of essential hypertension. basic Clin Pharmacol Toxicol. 2014;115:21–21.
Merrick A, Hadley WM, Holcslaw TL. The effect of large doses of atropine sulfate on heart rate and blood pressure in rats. Res Commun Chem Pathol Pharm. 1979;25:13–22.
Walters BC, Hadley MN, Hurlbert RJ, Aarabi B, Dhall SS, Gelb DE, et al. Guidelines for the management of acute cervical spine and spinal cord injuries: 2013 update. Neurosurgery. 2013;60:82–91.
Evans LT, Lollis SS, Ball PA. Management of acute spinal cord injury in the neurocritical care unit. Neurosurg Clin N Am. 2013;24:339–47.
Scheff SW, Saucier DA, Cain ME. A statistical method for analyzing rating scale data: the BBB locomotor score. J Neurotrauma. 2002;19:1251–60.
Rivlin AS, Tator CH. Objective clinical assessment of motor function after experimental spinal cord injury in the rat. J Neurosurg. 1977;47:577–81.
Buñag RD, Butterfield J. Tail-cuff blood pressure measurement without external preheating in awake rats. Hypertension 1982;4:898–903.
Cawthon DF, Senter HJ, Stewart WB. Comparison of hydrogen clearance and 14C-antipyrine autoradiography in the measurement of spinal cord blood flow after severe impact injury. J Neurosurg. 1980;52:801–7.
Augulis V, Sepinwall J. Brazilin-toluidine blue O and hematoxylin-darrow red methods for brain and spinal cord. Stain Technol. 1969;44:131–7.
Schleicher A, Zilles K. A quantitative approach to cytoarchitectonics: analysis of structural inhomogeneities in nervous tissue using an image analyser. J Microsc. 1990;157:367–81.
Dykstra M, Reuss L. Biological Electron Microscopy: Theory, Techniques, and Troubleshooting. Springer Science & Business Med. 2003; 2003: 182–95.
Oh YM, Eun JP. Cardiovascular dysfunction due to sympathetic hypoactivity after complete cervical spinal cord injury: A case report and literature review. Med (Baltim). 2015;94:e686.
Hou S, Saltos TM, Mironets E, Trueblood CT, Connors TM, Tom VJ, et al. Grafting Embryonic Raphe Neurons Reestablishes Serotonergic Regulation of Sympathetic Activity to Improve Cardiovascular Function after Spinal Cord Injury. J Neurosci. 2020;40:1248–64.
Laird AS, Carrive P, Waite PM. Cardiovascular and temperature changes in spinal cord injured rats at rest and during autonomic dysreflexia. J Physiol. 2006;577:539–48.
Iorio-Morin C, Noonan VK, White B, Noreau L, Leblond J, Dumont FS, et al. Quality of Life and Health Utility Scores Among Canadians Living With Traumatic Spinal Cord Injury - A National Cross-Sectional Study. Spine (Philos Pa 1976). 2018;43:999–1006.
Inskip JA, Ramer LM, Ramer MS, Krassioukov AV, Claydon VE. Spectral analyses of cardiovascular control in rodents with spinal cord injury. J Neurotrauma. 2012;29:1638–49.
Ventura AM, Shieh HH, Bousso A, Góes PF, de Cássia FOFI, de Souza DC, et al. Double-Blind Prospective Randomized Controlled Trial of Dopamine Versus Epinephrine as First-Line Vasoactive Drugs in Pediatric Septic Shock. Crit Care Med. 2015;43:2292–302.
Bobin P, Belacel-Ouari M, Bedioune I, Zhang L, Leroy J, Leblais V, et al. Cyclic nucleotide phosphodiesterases in heart and vessels: A therapeutic perspective. Arch Cardiovasc Dis. 2016;109:431–43.
Motiejunaite J, Amar L, Vidal-Petiot E. Adrenergic receptors and cardiovascular effects of catecholamines. Ann Endocrinol (Paris). 2021;82:193–7.
Nait Taleb Ali H, Morel MP, Doulazmi M, Scotto-Lomassese S, Gaspar P, Dusart I, et al. Lack of adenylate cyclase 1 (AC1): consequences on corticospinal tract development and on locomotor recovery after spinal cord injury. Brain Res. 2014;1549:1–10.
Omura T, Omura K, Tedeschi A, Riva P, Painter MW, Rojas L, et al. Robust Axonal Regeneration Occurs in the Injured CAST/Ei Mouse CNS. Neuron. 2015;86:1215–27.
Nikulina E, Tidwell JL, Dai HN, Bregman BS, Filbin MT. The phosphodiesterase inhibitor rolipram delivered after a spinal cord lesion promotes axonal regeneration and functional recovery. Proc Natl Acad Sci USA. 2004;101:8786–90.
Pearse DD, Pereira FC, Marcillo AE, Bates ML, Berrocal YA, Filbin MT, et al. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat Med. 2004;10:610–6.
Wilson JR, Cadotte DW, Fehlings MG. Clinical predictors of neurological outcome, functional status, and survival after traumatic spinal cord injury: a systematic review[J]. J Neurosurg: Spine. 2012;17:11–26.
Alizadeh A, Dyck SM, Karimi-Abdolrezaee S. Traumatic spinal cord injury: an overview of pathophysiology, models and acute injury mechanisms[J]. Front Neurol. 2019;10:282.
Mputu PM, Beauséjour M, Richard-Denis A, Mac-Thiong JM. Early predictors of neurological outcomes after traumatic spinal cord injury: a systematic review and proposal of a conceptual framework[J]. Am J Phys Med Rehab. 2021;100:700–11.
Mccawley LJ, Li S, Benavidez M, Halbleib J, Wattenberg EV, Hudson LG. Elevation of intracellular cAMP inhibits growth factor-mediated matrix metalloproteinase-9 induction and keratinocyte migration.[J]. Mol Pharmacol. 2000;58:145–51.
Szabo-Fresnais N, Blondeau JP, Pomérance M. Activation of the cAMP pathway synergistically increases IL-1-induced IL-6 gene expression in FRTL-5 thyroid cells: involvement of AP-1 transcription factors[J]. Mol Cell Endocrinol. 2008;284:28–37.
Garcia-Gil M, Camici M, Allegrini S, Pesi R, Tozzi MG. Metabolic aspects of adenosine functions in the brain[J]. Front Pharmacol. 2021;12:672182.
Tate DG, Wheeler T, Lane GI, Forchheimer M, Anderson KD, Biering-Sorensen F, et al. Recommendations for evaluation of neurogenic bladder and bowel dysfunction after spinal cord injury and/or disease[J]. J Spinal Cord Med. 2020;43:141–64.
Bhattacharyya S, Dinda A, Vishnubhatla S, Anwar MF, Jain S. A combinatorial approach to modulate microenvironment toward regeneration and repair after spinal cord injury in rats[J]. Neurosci Lett. 2021;741:135500.
Acknowledgements
We would like to thank Wang Bang Biochemical Pharmaceutical Co., Ltd for their generous gift of MCA. We are also grateful to Xuexu Liu and Qiongying Li for their technical assistance. We would also like to thank Yinghua Zhong, Wu Li, Malin Li, Meiqiong Li, and Yu Wei for their constructive criticism of the research, critical review of the manuscript and sincere help.
Author information
Authors and Affiliations
Contributions
YS and JL conceived and designed research. JL and LG conducted experiments. JL wrote the manuscript. The other authors contributed new reagents, analytical tools, or analyzed data. All authors have read and approved the manuscript, and all data were generated in-house and that no paper mill was used. YS and LG contributed equally to this work.
Corresponding author
Ethics declarations
Ethical approval
All experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of Sichuan University (approved animal protocol number 20096029 A) and met the ''Guide for the Regulations on the administration of laboratory animals'' and ''Guiding opinions on treating experimental animals well'' of China.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Song, Y., Guo, L., Jiang, X. et al. Meglumine cyclic adenylate improves cardiovascular hemodynamics and motor-function in a rat model of acute T4 thoracic spinal cord injury. Spinal Cord 61, 422–429 (2023). https://doi.org/10.1038/s41393-023-00909-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-023-00909-y
- Springer Nature Limited