Abstract
Study design
Longitudinal cohort study.
Objective
To explore the longitudinal association of baseline vitamin D levels with 1-year change in physical function outcomes in people with chronic spinal cord injury (SCI).
Setting
Rehabilitation institute.
Methods
Sixty-seven patients (44 men and 23 women) with chronic SCI admitted to a rehabilitation program were included. Functional independence in daily living activities (as evaluated by the Spinal Cord Independence Measure version III, SCIM III) and leisure time physical activity (LTPA) were assessed as measures of physical function at the admission and re-assessed 1-year later. Comorbidity was scored by Charlson comorbidity index (CCI).
Results
A 1-year worsening in SCIM and LTPA were registered in 44 and 40 patients (66% and 60% of the study population), respectively. They exhibited significantly lower baseline 25(OH)D levels, higher CCI, and shorter distance from the injury. At the multiple linear regression analyses, lower baseline 25(OH)D levels exhibited a significant independent association with higher percentages of 1-year worsening in both SCIM and LTPA. At ROC analysis, baseline 25(OH)D levels <18.6 and <18.2 ng/mL discriminated individuals with 1-year worsening in SCIM and LTPA, respectively. According to these cut-off points, at the multiple logistic regression analysis, patients with low baseline 25(OH)D levels exhibited an OR of worsening in SCIM and LTPA engagement 2.8- and 2.6-fold higher, after adjustment for CCI, distance from injury, and post-follow-up 25(OH)D levels.
Conclusions
In people with chronic SCI, a low 25(OH)D level may represent an independent predictor of worsening in physical function outcomes over time.
Similar content being viewed by others
Introduction
Growing evidence supports widespread pleiotropic activities of vitamin D beyond its pivotal role in bone health and calcium homeostasis. Vitamin D receptor (VDR) is almost ubiquitously expressed in human cells and regulates the expression of genes involved in several cell functions [1]. Accordingly, observational clinical studies have demonstrated significant associations between low 25-hydroxyvitamin D (25(OH)D) levels and a wide spectrum of chronic diseases.
Several lines of evidence, from both basic and clinical research, also support an involvement of vitamin D pathway in skeletal muscle health and function [2]. The VDR has been demonstrated in human myoblasts [3] and adult skeletal muscle [4], where its activation triggers signaling pathways and expression of genes regulating calcium homeostasis, proliferation, and differentiation of muscle cell [2]. Overall, these findings provide biological plausibility for the hypothesis of a direct activity of vitamin D in skeletal muscle physiology.
Most clinical studies exploring the relationship between vitamin D and muscle function have focused their attention on older populations, exhibiting a high risk of vitamin D deficiency. In the elderly, indeed, observational studies have demonstrated an independent association of low 25(OH)D levels with high risk for falls [5, 6], as a measure of global physical function. A causal link of hypovitaminosis D with poor muscle health and physical function has been suggested by meta-analyses of randomized intervention controlled trials, showing a significant, albeit small, improvement in muscle strength [7] and a decreased risk of falls [8] in older people with 25(OH)D deficiency, following vitamin D and calcium co-administration. Nevertheless, cross-sectional and longitudinal studies exploring the independent association of 25(OH)D levels with composite muscle performance outcomes have produced conflicting results [5, 9,10,11,12].
People with chronic spinal cord injury (SCI) are especially susceptible to vitamin D deficiency, even more than elderly population, because of a number of reasons, such as fat mass accumulation (which lowers the bioavailability of the fat-soluble vitamin D), inadequate sunlight exposure, coexisting illness, and intake of medications interfering with vitamin D metabolism [13]. Accordingly, a high prevalence of vitamin D deficiency has been reported in this population [13,14,15,16], where, however, the relationship between vitamin D and physical function remains poorly investigated. In a series of 100 individuals with chronic SCI, we recently demonstrated a cross-sectional independent association of 25(OH)D levels with both independence degree in performing activities of daily living (ADL) and leisure time physical activity (LTPA), as measures of physical function outcome after SCI [15]. Indeed, an interplay between vitamin D deficiency and physical function impairment could be particularly plausible in these patients. Disability and muscle wasting hinder the functional independence in ADL and the engagement in LTPA, leading to poor sunlight exposure and increased adiposity with subsequent inadequate biosynthesis of cholecalciferol in the skin and increased sequestration of 25(OH)D in the fat. The resultant vitamin D deficiency could in turn directly affect skeletal muscle health, thus contributing in worsening physical function. Obviously, a study with a cross-sectional design cannot ascertain the directionality of the associations under investigation.
In this study we explored, in a 1-year longitudinal analysis, whether low baseline 25(OH)D levels could be independent predictors of subsequent decline in functional independence degree in ADL and LTPA in people with chronic SCI.
Methods
Study population and design
Over a period of 2 years, all patients consecutively admitted to a rehabilitation program at the Spinal Unit of San Raffaele Institute of Sulmona were screened for inclusion criteria: (1) traumatic SCI, (2) duration of injury >1 year (chronic SCI), and (3) absence of acute or chronic severe coexisting illnesses and/or clinical instability hindering the rehabilitative program. Patients taking vitamin D supplements at the first admission (n = 7) were also excluded. Overall, we enrolled 100 patients representing the study population where we recently explored the cross-sectional association between 25(OH)D levels and physical function outcome [15]. All participants were requested to sign a written informed consent at the first admission, and the study was approved by the local ethics committee (ASL 1 Abruzzo). All applicable institutional and governmental regulations concerning the ethical use of human participants were followed during the course of the study.
Of the original cohort (n = 100), 67 patients (44 men and 23 women; mean age ± SD, 55.5 ± 33.5 years), who were re-assessed 1 year later (when they were admitted to their annual routine rehabilitation program), made up the analytical sample of the present longitudinal study, after the exclusion of 33 patients who did not come to our Institute again the year after (24 patients were admitted to a rehabilitation program elsewhere and 9 patients were unreachable).
At the first admission, patients underwent clinical–biochemical evaluations and assessment of physical function outcomes, as described below.
The presence and severity of significant medical comorbidity were also scored using a web-based calculator (http://www.pmidcalc.org/?sid=7722560&newtest=Y##ath) of the age-adjusted Charlson comorbidity index [17]: a weighted score was assigned to a number of medical diagnoses, based on the relative risk of 1-year mortality and according to the patient age; scores were summed to provide a weighted index of medical comorbidity. Charlson index diagnoses include cancer, liver diseases, diabetes, kidney diseases, ulcers, connective tissue disease, chronic pulmonary disease, dementia, cerebrovascular disease, heart diseases. Depressive symptoms were assessed using the interviewer-assisted self-report Beck Depression Inventory-II (BDI-II) [18].
Clinical examination
Clinical neurologic examination was performed according to the International Standards for Neurological Classification of SCI from the American Spinal Injury Association (ASIA) [19].
The presence and intensity of pain was assessed by the Numerical Rating Scale (NRS), in keeping with recommendations of the National Institute on Disability and Rehabilitation Research [20]. Patients verbally selected a number, ranging from 0 to 10, that best reflected the intensity of their pain (0 = no pain; 10 = worst possible pain).
Physical function outcome assessment
Functional independence degree in ADL and engagement in weekly LTPA were used as measures of overall physical function outcome, as previously described [15].
Functional independence was assessed by a physiatrist (G.F.) using the Spinal Cord Independence Measure, version III (SCIM III), which represents the primary outcome measure of functional recovery for people with SCI [21]. This scale weighs the independence degree in performing each ADL separately, providing a global score ranging from 0 (totally dependent) to 100 (totally independent).
LTPA includes physical activities that people with SCI choose to do in the free time: wheeling or walking, outdoor activities, sports. These activities were quantified by the LTPA Questionnaire (LTPAQ) for People with SCI [22]. This is a self-administered SCI-specific measure of minutes of LTPA performed at each intensity (mild, moderate, and heavy intensity LTPA) over the previous week. An intensity classification chart allows patients to quantify the intensity of their LTPA, based on the perceived psychophysical effort. Only total LTPA score was used for analyses, because of its strong correlation with mild, moderate, and heavy intensity sub-scores [23].
Biochemistry and hematology
Serum 25(OH)D levels were measured using a chemiluminescent immunoassay (LIAISON®, DiaSorin, Saluggia, VC, Italy) with an intra- and inter-assay coefficient of variation (CV) of 4.5% and 8.5%, respectively. Levels of 25(OH)D below 20 ng/mL indicated vitamin D deficiency. Standard methods and commercial kits (Instrumentation Laboratory Company, Lexington, MA, USA) were used for all of the other biochemical and hematologic measurements.
Statistical analysis
Statistical analysis was carried out with the R statistical software (version 3.0.3, 2014, The R Foundation for Statistical Computing, Vienna, Austria). According to the distribution of values, assessed by Shapiro–Wilk test, data were analyzed with Wilcoxon rank-sum test or unpaired two-sided Student's t-test, as appropriate. According to the size of comparison groups, proportional differences were assessed by Χ2 test or Fisher exact test. Multiple linear regression analyses of log-transformed values were carried out to assess independent associations of 1-year change (%Δ) in physical function outcomes (SCIM score and minutes of LTPA) with significant predictors selected by univariate analyses.
Receiver operating characteristics (ROC) analyses over baseline 25(OH)D levels were used to detect threshold values providing an accurate discriminating ability in predicting 1-year worsening in physical function outcomes. Multiple logistic regression analyses were carried out in order to calculate odds ratio (OR) of 1-year worsening in physical function outcomes in patients with baseline 25(OH)D levels below the ROC threshold values, after adjusting for confounders.
Results
A 1-year worsening in SCIM and LTPAQ scores were registered in 44 and 40 patients (66% and 60% of the study population), respectively. All patients with worsened LTPAQ scores also exhibited worsened SCIM scores; in 4 out the 44 patients who had worsened SCIM scores, the engagement in LTPA remained stable.
The baseline characteristics of the study population categorized according to the 1-year change in functional independence degree in ADL (as evaluated by SCIM) and LTPA are shown in Tables 1 and 2, respectively. Patients who worsened exhibited significantly lower baseline 25(OH)D levels, higher prevalence of vitamin D deficiency (25(OH)D <20 ng/mL), higher Charlson comorbidity index, and a shorter distance from injury than those who were stable or improved. The two groups did not significantly differ in terms of inflammatory status (C-reactive protein, CRP >5 mg/mL), level and completeness of the lesion, walking ability, baseline functional independence degree, and baseline LTPA engagement.
At the baseline, vitamin D deficiency was exhibited by 50 patients (75% of the study population). Although supplementation was offered at the first discharge to all vitamin D-deficient patients, the prescription adherence was extremely poor, resulting in a not significant increase in 25(OH)D levels after 1 year when compared to baseline: 25(OH)D levels ranged from 4.1 to 34.4 ng/mL (median: 12.9 ng/mL) at the baseline and from 4.1 to 32.0 ng/mL (median: 15.5 ng/mL) when re-assessed after 1 year (p = 0.36).
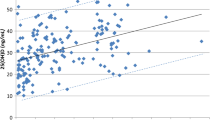
The three variables selected by the univariate analyses (baseline 25(OH)D levels, Charlson comorbidity index and distance from injury) were included in multiple linear regression analyses to reveal independent associations with the 1-year change in physical function outcomes. As shown in Fig. 1, lower baseline 25(OH)D levels exhibited a significant independent association with higher percentages of 1-year worsening in both functional independence degree (β = 62.2; 95%CI: 13.3, 111.0; Fig. 1a) and LTPA (β = 43.9; 95%CI: 14.3, 73.5; Fig. 1b). The only other independent predictor of worsened LTPA was a higher Charlson comorbidity index (Fig. 1b).
Multiple regression analyses of the relationship of putative predictors of worsened physical function (selected by univariate analyses) with 1-year change (%) in (a) functional independence degree in activities of daily living (ADL), as evaluated by the Spinal Cord Independence Measure (SCIM) III score and (b) leisure time physical activity (LTPA). Regression analyses were performed on log-transformed values. β-Coefficient: the standardized coefficient of regression, CI: confidence interval, R2: percentage of variability of each outcome variable explained by each independent variable
At ROC analysis, baseline 25(OH)D levels <18.6 ng/mL discriminated patients with 1-year worsening in functional independence with a sensitivity of 91.0% and a specificity of 65.0% (area under the curve, AUC: 80.1%; 95% CI: 68.8–91.4%; Fig. 2a) and levels <18.2 ng/mL discriminated patients with 1-year worsening in LTPA with a sensitivity of 92.5% and a specificity of 63.0% (AUC: 80.0%; 95% CI: 68.5–91.5%; Fig. 2b).
Receiver operating characteristics (ROC) analyses over baseline 25(OH)D levels showing threshold values which provide accurate discriminating ability in predicting 1-year worsening in (a) functional independence degree in activities of daily living (ADL), as evaluated by the Spinal Cord Independence Measure (SCIM) III score, and (b) leisure time physical activity (LTPA)
According to these cut-off points, at the multiple logistic regression analysis adjusted for Charlson comorbidity index, distance from injury, and also for post-follow up 25(OH)D levels, patients with baseline 25(OH)D levels <18.6 ng/mL exhibited an OR of worsening in functional independence degree 2.8-fold higher than those with baseline 25(OH)D levels ≥18.6 ng/mL (p = 0.0003); patients with baseline 25(OH)D levels <18.2 ng/mL exhibited an OR of worsening in LTPA 2.6-fold higher than those with baseline 25(OH)D levels ≥18.2 ng/mL (p = 0.001).
Discussion
In the present longitudinal study, lower baseline 25(OH)D levels independently predicted higher percentages of 1-year decline in both functional independence degree in ADL and weekly engagement in LTPA in people with chronic SCI. To our knowledge, this is the first longitudinal study investigating the association between 25(OH)D levels and changes in physical function outcomes in this population. According to previous reports [13,14,15,16], in our series, a high proportion of patients exhibited vitamin D deficiency. Although a 25(OH)D supplementation had been routinely offered to all patients with hypovitaminosis D at the first discharge, the 1-year medication compliance resulted to be extremely poor. This is not surprising: because of the risk to undergo calcium nephrolithiasis after acute SCI, people with chronic SCI often refuse dairy products intake and vitamin D supplementation [24]. Due to this poor adherence to supplementation, median 25(OH)D levels after 1 year were substantially unchanged with respect to the baseline. In any case, the independent longitudinal association between change in physical function outcomes and baseline 25(OH)D levels persisted even after adjustment for post-follow-up levels of 25(OH)D.
These findings contribute to clarify the directionality of the independent cross-sectional association between vitamin D levels and physical function outcomes which we recently demonstrated in people with chronic SCI [15].
When the relationship between 25(OH)D levels and physical function was explored in the elderly general population, observational cross-sectional studies produced controversial results, as an association was reported by some authors [10] but not by others [12]. Similarly, conflicting data arose from prospective studies assessing the independent association between 25(OH)D at the baseline and change in physical performance/muscle strength over time [5, 9, 11]. Randomized controlled trials of vitamin D supplementation also reported mixed effects on physical performance in older general population [25, 26]. Controversial findings might result from the use of different performance measures: as only a few tests were usually employed, results from most of the studies could not be representative of the overall physical function. Indeed, an independent association of low 25(OH)D levels with high risk for falls, which reflects a poor global physical function, has been more consistently reported [5, 6]. In people with chronic SCI, the assessment of both the independence degree in ADL and the engagement in LTPA, which is correlated with aerobic fitness and muscular strength, explores the ability in performing the entire spectrum of physical activities [22], thus representing a reliable measure of global physical function. The independent association between lower 25(OH)D levels and higher percentages of 1-year worsening in physical function outcomes, here demonstrated by using these proper endpoints, strongly suggests a causal link of vitamin D deficiency with impaired muscle functional activity. These findings would be supported by in vitro studies demonstrating an active role of muscular VDR in skeletal muscle physiology. It has been reported that this receptor modulates the activity of calcium pumps in muscle sarcoplasmic reticulum, thus regulating intracellular calcium and phosphate concentrations [27]. Muscular VDR activation also promotes cell phosphate uptake for adenosine triphosphate (ATP) generation [27] and protein synthesis [27], also preventing type 2 muscle fiber atrophy [28]. In this scenario, a poor vitamin D status could favor the loss of muscle fibers and the impoverishment in muscle protein and ATP content with an adverse impact on skeletal muscle performance, ultimately impairing the global physical function.
Actually, especially in SCI population, hypovitaminosis D and poor physical function share a number of risk factors, including disability and comorbidity status, which could mediate their association and interplay (see Introduction). Nevertheless, in our series, patients who underwent 1-year worsening in physical function outcomes did not exhibit at the baseline a lower independence degree in performing ADL, nor a poorer engagement in LTPA, when compared to patients who were stable or improved. Moreover, level and completeness of the lesion did not differ between the two groups. Finally, although comorbidity was significantly more prevalent in the worsened group, the significant association between lower baseline 25(OH)D levels and 1-year worsening in physical function outcomes persisted in multiple regression models where Charlson comorbidity index was included as an independent variable.
Some limitations of the present study should be mentioned. Firstly, the limited sample size and the short follow-up, which, however, did not hinder the demonstration of significant and independent associations. Secondly, although multiple regression models were adjusted for potential predictors selected by univariate analysis, other unmeasured confounders could have influenced the associations under investigation. For example, low 25(OH)D levels might reflect inadequate intakes of nutrients involved in skeletal muscle physiology (e.g. proteins), which in turn may negatively affect the outcomes under investigations; unfortunately, detailed information regarding dietary intakes was not available in our study population. Although more comprehensive and less biased analyses of confounders, such as directed acyclic graph approaches [29], have been proposed to identify key sets of covariates that need to be adjusted for and to explore the causal structure among the variables, their use is often hindered by the complexity of clinical issues and only randomized controlled trials could strengthen the cause–effect directionality of the associations under investigation. Finally, as another limitation, clinical peculiarities of people with SCI impose caution in extending our findings to the general able-bodied population.
In conclusion, in people with chronic SCI, low 25(OH)D levels represent independent predictors of physical function decline over time. Intervention studies are warranted to clarify whether vitamin D supplementation could maintain or improve muscle function and physical performance in these patients.
References
Bikle DD. Vitamin D: newly discovered actions require reconsideration of physiologic requirements. Trends Endocrinol Metab. 2010;21:375–84.
Girgis CM, Clifton-Bligh RJ, Hamrick MW, Holick MF, Gunton JE. The roles of vitamin D in skeletal muscle: form, function, and metabolism. Endocr Rev. 2013;34:33–83.
Olsson K, Saini A, Strömberg A, Alam S, Lilja M, Rullman E, et al. Evidence for vitamin D receptor expression and direct effects of 1α,25(OH)2D3 in human skeletal muscle precursor cells. Endocrinology. 2016;157:98–111.
Bischoff HA, Borchers M, Gudat F, Duermueller U, Theiler R, Stähelin HB, et al. In situ detection of 1,25-dihydroxyvitamin D3 receptor in human skeletal muscle tissue. Histochem J. 2001;33:19–24.
Faulkner KA, Cauley JA, Zmuda JM, Landsittel DP, Newman AB, Studenski SA, et al. Higher 1,25-dihydroxyvitamin D3 concentrations associated with lower fall rates in older community-dwelling women. Osteoporos Int. 2006;17:1318–28.
Snijder MB, van Schoor NM, Pluijm SM, van Dam RM, Visser M, Lips P. Vitamin D status in relation to one-year risk of recurrent falling in older men and women. J Clin Endocrinol Metab. 2006;91:2980–5.
Beaudart C, Buckinx F, Rabenda V, Gillain S, Cavalier E, Slomian J, et al. The effects of vitamin D on skeletal muscle strength, muscle mass and muscle power: a systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2014;99:4336–45.
Murad MH, Elamin KB, Abu Elnour NO, Elamin MB, Alkatib AA, Fatourechi MM, et al. Clinical review: the effect of vitamin D on falls: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:2997–3006.
Verreault R, Semba RD, Volpato S, Ferrucci L, Fried LP, Guralnik JM. Low serum vitamin D does not predict new disability or loss of muscle strength in older women. J Am Geriatr Soc. 2002;50:912–7.
Bischoff-Ferrari HA, Dietrich T, Orav EJ, Hu FB, Zhang Y, Karlson EW, et al. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged > or = 60 y. Am J Clin Nutr. 2004;80:752–8.
Wicherts IS, van Schoor NM, Boeke AJ, Visser M, Deeg DJ, Smit J, et al. Vitamin D status predicts physical performance and its decline in older persons. J Clin Endocrinol Metab. 2007;92:2058–65.
Ceglia L, Chiu GR, Harris SS, Araujo AB. Serum 25-hydroxyvitaminD concentration and physical function in adult men. Clin Endocrinol (Oxf). 2011;74:370–6.
Flueck JL, Perret C. Vitamin D deficiency in individuals with a spinal cord injury: a literature review. Spinal Cord. 2017;55:428–34.
Barbonetti A, Cavallo F, D’Andrea S, Muselli M, Felzani G, Francavilla S, et al. Lower vitamin D levels are associated with depression in people with chronic spinal cord injury. Arch Phys Med Rehabil. 2017;98:940–6.
Barbonetti A, Sperandio A, Micillo A, D’Andrea S, Pacca F, Felzani G, et al. Independent association of vitamin D with physical function in people with chronic spinal cord injury. Arch Phys Med Rehabil. 2016;97:726–32.
Barbonetti A, Vassallo MR, Felzani G, Francavilla S, Francavilla F. Association between 25(OH)-vitamin D and testosterone levels: evidence from men with chronic spinal cord injury. J Spinal Cord Med. 2016;39:246–52.
Charlson M, Szatrowsk TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–51.
Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71.
Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A, et al. International standards for neurological classification of spinal cord injury (Revised 2011). J Spinal Cord Med. 2011;34:535–46.
Bryce TN, Budh CN, Cardenas DD, Dijkers M, Felix ER, Finnerup NB, et al. Pain after spinal cord injury: an evidence-based review for clinical practice and research. J Spinal Cord Med. 2007;30:421–40.
Itzkovich M, Gelernter I, Biering-Sorensen F, Weeks C, Laramee MT, Craven BC, et al. The Spinal Cord Independence Measure (SCIM) version III: reliability and validity in a multi-center international study. Disabil Rehabil. 2007;29:1926–33.
Martin Ginis KA, Phang SH, Latimer AE, Arbour-Nicitopoulos KP. Reliability and validity tests of the leisure time physical activity questionnaire for people with spinal cord injury. Arch Phys Med Rehabil. 2012;93:677–82.
Barbonetti A, Vassallo MR, Pacca F, Cavallo F, Costanzo M, Felzani G, et al. Correlates of low testosterone in men with chronic spinal cord injury. Andrology. 2014;2:721–8.
Jiang SD, Dai LY, Jiang LS. Osteoporosis after spinal cord injury. Osteoporos Int. 2006;17:180–92.
Latham NK, Anderson CS, Reid IR. Effects of vitamin D supplementation on strength, physical performance, and falls in older persons: a systematic review. J Am Geriatr Soc. 2003;51:1219–26.
Annweiler C, Schott AM, Berrut G, Fantino B, Beauchet O. Vitamin D-related changes in physical performance: a systematic review. J Nutr Health Aging. 2009;13:893–8.
Ceglia L. Vitamin D and skeletal muscle tissue and function. Mol Asp Med. 2008;29:407–14.
Sato Y, Iwamoto J, Kanoko T, Satoh K. Low-dose vitamin D prevents muscular atrophy and reduces falls and hip fractures in women after stroke: a randomized controlled trial. Cerebrovasc Dis. 2005;20:187–92.
Shrier I, Platt RW. Reducing bias through directed acyclic graphs. BMC Med Res Methodol. 2008;8:70.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Barbonetti, A., D’Andrea, S., Martorella, A. et al. Low vitamin D levels are independent predictors of 1-year worsening in physical function in people with chronic spinal cord injury: a longitudinal study. Spinal Cord 56, 494–501 (2018). https://doi.org/10.1038/s41393-017-0058-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-017-0058-7
- Springer Nature Limited
This article is cited by
-
Independent association of hypovitaminosis d with non-alcoholic fatty liver disease in people with chronic spinal cord injury: a cross-sectional study
Journal of Endocrinological Investigation (2023)
-
Relationship of Vitamin D status with testosterone levels: a systematic review and meta-analysis
Endocrine (2021)
-
Comment on: Plasma vitamin D, past chest illness, and risk of future chest illness in chronic spinal cord injury (SCI): a longitudinal observational study
Spinal Cord (2020)
-
Testosterone, level of the lesion and age are independently associated with prostate volume in men with chronic spinal cord injury
Journal of Endocrinological Investigation (2020)
-
Can the positive association of osteocalcin with testosterone be unmasked when the preeminent hypothalamic–pituitary regulation of testosterone production is impaired? The model of spinal cord injury
Journal of Endocrinological Investigation (2019)