Abstract
Background
Nowadays a tool able to predict the risk of lymph-node invasion (LNI) in patients underwent target biopsy (TB) only before radical prostatectomy (RP) is still lacking. Our aim is to develop a model based on mp-MRI and target biopsy (TB) alone able to predict the risk of LNI.
Methods
We retrospectively extracted data of patients with preoperative positive mp-MRI and TB only who underwent RARP with ePLND from April 2014 to March 2020. A logistic regression model was performed to evaluate the impact of pre- and intra-operative factors on the risk of LNI. Model discrimination was assessed using an area under (AUC) the ROC curve. A nomogram, and its calibration plot, to predict the risk of LNI were generated based on the logistic model. A validation of the model was done using a similar cohort.
Results
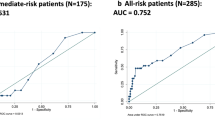
461 patients were included, of which 52 (11.27) had LNI. After logistic regression analysis and multivariable model DRE, PI-RADS, seminal vesicle invasion, PSA and worst GS at I and II target lesions were significant predictors of LNI. The AUC was 0.74 [0.67–0.81] 95% CI. The calibration plot shows that our model is very close to the ideal one which is in the 95% CI. After the creation of a visual nomogram, the cut-off to discriminate between the risk or not of LNI was set with Youden index at 60 points that correspond to a risk of LNI of 7%. The model applied on a similar cohort shown a LH+ of 2.58 [2.17–2.98] 95% CI.
Conclusions
Our nomogram for patients undergoing MRI-TB only takes into account clinical stage, SVI at MRI, biopsy Gleason pattern and PSA and it is able to identify patients with risk of LNI when a score higher than 7% is achieved.


Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author, EC, upon reasonable request
References
Checcucci E, Amparore D, De Luca S, Autorino R, Fiori C, Porpiglia F. Precision prostate cancer surgery: an overview of new technologies and techniques. Minerva Urol Nefrol. 2019;71:487–501. https://doi.org/10.23736/S0393-2249.19.03365-4.
Onol FF, Bhat S, Moschovas M, Rogers T, Albala D, Patel V. The ongoing dilemma in pelvic lymph node dissection during radical prostatectomy: who should decide and in which patients? J Robot Surg. 2020;14:549–58. https://doi.org/10.1007/s11701-019-01041-x.
Fossati N, Willemse PM, Van den Broeck T, van den Bergh RCN, Yuan CY, Briers E, et al. The benefits and harms of different extents of lymph node dissection during radical prostatectomy for prostate cancer: a systematic review. Eur Urol. 2017;72:84–109. https://doi.org/10.1016/j.eururo.2016.12.003.
Tyritzis SI, Kalampokis N, Grivas N, van der Poel H, Wiklund NP. Robot-assisted extended lymphadenectomy in prostate cancer. Minerva Chir. 2019;74:88–96. https://doi.org/10.23736/S0026-4733.18.07780-5.
Mottet N, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer-2020 update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2021;79:243–62. https://doi.org/10.1016/j.eururo.2020.09.042.
Sebben M, Tafuri A, Pirozzi M, Processali T, Rizzetto R, Amigoni N, et al. Open approach, extended pelvic lymph node dissection, and seminal vesicle invasion are independent predictors of hospital readmission after prostate cancer surgery: a large retrospective study. Minerva Urol Nefrol. 2020;72:72–81. https://doi.org/10.23736/S0393-2249.19.03586-0.
Bandini M, Marchioni M, Pompe RS, Tian Z, Gandaglia G, Fossati N, et al. First North American validation and head-to-head comparison of four preoperative nomograms for prediction of lymph node invasion before radical prostatectomy. BJU Int. 2018;121:592–9. https://doi.org/10.1111/bju.14074.
Gandaglia G, Ploussard G, Valerio M, Mattei A, Fiori C, Fossati N, et al. A novel nomogram to identify candidates for extended pelvic lymph node dissection among patients with clinically localized prostate cancer diagnosed with magnetic resonance imaging-targeted and systematic biopsies. Eur Urol. 2019;75:506–14. https://doi.org/10.1016/j.eururo.2018.10.012.
Porpiglia F, Manfredi M, Mele F, Bertolo R, Bollito E, Gned D, et al. Indication to pelvic lymph nodes dissection for prostate cancer: the role of multiparametric magnetic resonance imaging when the risk of lymph nodes invasion according to Briganti updated nomogram is <5. Prostate Cancer Prostatic Dis. 2018;21:85–91. https://doi.org/10.1038/s41391-017-0026-5.
Porpiglia F, Manfredi M, Mele F, Cossu M, Bollito E, Veltri A, et al. Diagnostic pathway with multiparametric magnetic resonance imaging versus standard pathway: results from a randomized prospective study in biopsy-naïve patients with suspected prostate cancer. Eur Urol. 2017;72:282–8. https://doi.org/10.1016/j.eururo.2016.08.041.
Checcucci E, De Cillis S, Piramide F, Amparore D, Kasivisvanathan V, Giganti F, et al. The role of additional standard biopsy in the MRI-targeted biopsy era. Minerva Urol Nefrol. 2020;72:637–9. https://doi.org/10.23736/S0393-2249.20.03958-2.
Checcucci E, De Cillis S, Amparore D, Garrou D, Aimar R, Piana A et al. Naive patients with suspicious prostate cancer and positive multiparametric magnetic resonance imaging (mp-MRI): is it time for fusion target biopsy alone? J Clin Urol. 2021. https://doi.org/10.1177/20514158211023713.
Miah S, Hosking-Jervis F, Connor MJ, Eldred-Evans D, Shah TT, Arya M, et al. A multicentre analysis of the detection of clinically significant prostate cancer following transperineal image-fusion targeted and nontargeted systematic prostate biopsy in men at risk. Eur Urol Oncol. 2020;3:262–9. https://doi.org/10.1016/j.euo.2019.03.005.
Goldberg H, Ahmad AE, Chandrasekar T, Klotz L, Emberton M, Haider MA, et al. Comparison of magnetic resonance imaging and transrectal ultrasound informed prostate biopsy for prostate cancer diagnosis in biopsy naïve men: a systematic review and meta-analysis. J Urol. 2020;203:1085–93. https://doi.org/10.1097/JU.0000000000000595.
Connor MJ, Eldred-Evans D, van Son M, Hosking-Jervis F, Bertoncelli Tanaka M, Reddy D, et al. A multicenter study of the clinical utility of nontargeted systematic transperineal prostate biopsies in patients undergoing pre-biopsy multiparametric magnetic resonance imaging. J Urol. 2020;204:1195–201. https://doi.org/10.1097/JU.0000000000001184.
Hou Y, Jiang KW, Zhang J, Bao ML, Shi HB, Qu JR et al. A clinical available decision support scheme for optimizing prostate biopsy based on mpMRI. Prostate Cancer Prostatic Dis. 2022. https://doi.org/10.1038/s41391-021-00489-z.
Bass EJ, Pantovic A, Connor MJ, Loeb S, Rastinehad AR, Winkler M, et al. Diagnostic accuracy of magnetic resonance imaging targeted biopsy techniques compared to transrectal ultrasound guided biopsy of the prostate: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2021. https://doi.org/10.1038/s41391-021-00449-7.
Porpiglia F, Bertolo R, Manfredi M, De Luca S, Checcucci E, Morra I, et al. Total anatomical reconstruction during robot-assisted radical prostatectomy: implications on early recovery of urinary continence. Eur Urol 2016;69:485–95. https://doi.org/10.1016/j.eururo.2015.08.005.
Manfredi M, Checcucci E, Fiori C, Garrou D, Aimar R, Amparore D, et al. Total anatomical reconstruction during robot-assisted radical prostatectomy: focus on urinary continence recovery and related complications after 1000 procedures. BJU Int. 2019;124:477–86. https://doi.org/10.1111/bju.14716.
Barentsz JO, Richenberg J, Clements R, Choyke P, Verma S, Villeirs G, et al. European Society of Urogenital Radiology. ESUR prostate MR guidelines 2012. Eur Radiol. 2012;22:746–57. https://doi.org/10.1007/s00330-011-2377-y.
Barentsz JO, Weinreb JC, Verma S, Thoeny HC, Tempany CM, Shtern F, et al. Synopsis of the PI-RADS v2 guidelines for multiparametric prostate magnetic resonance imaging and recommendations for use. Eur Urol. 2016;69:41–9. https://doi.org/10.1016/j.eururo.2015.08.038.
Porpiglia F, De Luca S, Passera R, De Pascale A, Amparore D, Cattaneo G, et al. Multiparametric magnetic resonance/ultrasound fusion prostate biopsy: number and spatial distribution of cores for better index tumor detection and characterization. J Urol. 2017;198:58–64. https://doi.org/10.1016/j.juro.2017.01.036.
Moore CM, Kasivisvanathan V, Eggener S, Emberton M, Fütterer JJ, Gill IS, et al. Standards of reporting for MRI-targeted biopsy studies (START) of the prostate: recommendations from an International Working Group. Eur Urol. 2013;64:544–52. https://doi.org/10.1016/j.eururo.2013.03.030.
Montironi R, Lopez-Beltran A, Mazzucchelli R, Scarpelli M, Bollito E. Assessment of radical prostatectomy specimens and diagnostic reporting of pathological findings. Pathologica. 2001;93:226–32.
Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–9. https://doi.org/10.1016/s0895-4356(96)00236-3.
Long JS. Regression Models for categorical and limited dependent variables. Thousand Oaks, CA: Sage Publications; 1997.
Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Mak. 2006;26:565–74. https://doi.org/10.1177/0272989X06295361.
Peirce CS. The numerical measure of the success of predictions. Science. 1884;4:453–4. https://doi.org/10.1126/science.ns-4.93.453-a.
Ruopp MD, Perkins NJ, Whitcomb BW, Schisterman EF. Youden Index and optimal cut-point estimated from observations affected by a lower limit of detection. Biom J. 2008;50:419–30. https://doi.org/10.1002/bimj.200710415.
Van Calster B, Nieboer D, Vergouwe Y, De Cock B, Pencina MJ, Steyerberg EW. A calibration hierarchy for risk models was defined: from utopia to empirical data. J Clin Epidemiol. 2016;74:167–76. https://doi.org/10.1016/j.jclinepi.2015.12.005.
Cimino S, Reale G, Castelli T, Favilla V, Giardina R, Russo GI, et al. Comparison between Briganti, Partin and MSKCC tools in predicting positive lymph nodes in prostate cancer: a systematic review and meta-analysis. Scand J Urol. 2017;51:345–50. https://doi.org/10.1080/21681805.2017.1332680.
De Nunzio C, Lombardo R, Baldassarri V, Cindolo L, Bertolo R, Minervini A, et al. Rotterdam mobile phone app including MRI data for the prediction of prostate cancer: a multicenter external validation. Eur J Surg Oncol. 2021;47:2640–5. https://doi.org/10.1016/j.ejso.2021.04.033.
Gallagher KM, Christopher E, Cameron AJ, Little S, Innes A, Davis G, et al. Four-year outcomes from a multiparametric magnetic resonance imaging (MRI)-based active surveillance programme: PSA dynamics and serial MRI scans allow omission of protocol biopsies. BJU Int. 2019;123:429–38. https://doi.org/10.1111/bju.14513.
Klotz L, Chin J, Black PC, Finelli A, Anidjar M, Bladou F, et al. Comparison of multiparametric magnetic resonance imaging-targeted biopsy with systematic transrectal ultrasonography biopsy for biopsy-naive men at risk for prostate cancer: a phase 3 randomized clinical trial. JAMA Oncol. 2021. https://doi.org/10.1001/jamaoncol.2020.7589.
Briganti A, Larcher A, Abdollah F, Capitanio U, Gallina A, Suardi N, et al. Updated nomogram predicting lymph node invasion in patients with prostate cancer undergoing extended pelvic lymph node dissection: the essential importance of percentage of positive cores. Eur Urol. 2012;61:480–7.
Gandaglia G, Fossati N, Zaffuto E, Bandini M, Dell’Oglio P, Bravi CA, et al. Development and internal validation of a novel model to identify the candidates for extended pelvic lymph node dissection in prostate cancer. Eur Urol. 2017;72:632–40.
Memorial Sloan Kettering Cancer Center. Dynamic prostate cancer nomogram: coefficients. www.mskcc.org/nomograms/prostate/pre-op/coefficients.
De Luca S, Passera R, Fiori C, Garrou D, Manfredi M, Aimar R, et al. The role of side-specific biopsy and dominant tumor location at radical prostatectomy in predicting the side of nodal metastases in organ confined prostate cancer: is lymphatic spread really unpredictable? Minerva Urol Nefrol. 2019;71:146–53. https://doi.org/10.23736/S0393-2249.18.03286-1.
Morozov A, Barret E, Veneziano D, Grigoryan V, Salomon G, Fokin I, In collaboration with ESUT-YAUWP Group, et al. A systematic review of nerve-sparing surgery for high-risk prostate cancer. Minerva Urol Nefrol. 2021. https://doi.org/10.23736/S0393-2249.20.04178-8.
Author information
Authors and Affiliations
Contributions
CF: Conceptualization, Methodology, Review. EC: Data Analysis, Manuscript Writing. IS: Methodology, Statistical Analysis. DA: Manuscript writing, Data Analysis. SDC: Data Collection. AP: Data Collection. SG: Data Collection. GV: Data Collection, Language review. MS: Data Collection. FP: Data Collection. PV: Data Collection, Language review. MM: Review, Supervision. SDL: Supervision. RA: Review. GM: Methodology, Statistical Analysis. FP: Conceptualization, Methodology, Review
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Fiori, C., Checcucci, E., Stura, I. et al. Development of a novel nomogram to identify the candidate to extended pelvic lymph node dissection in patients who underwent mpMRI and target biopsy only. Prostate Cancer Prostatic Dis 26, 388–394 (2023). https://doi.org/10.1038/s41391-022-00565-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-022-00565-y
- Springer Nature Limited
This article is cited by
-
Analysis of biopsy pathology and risk factors of lymph node metastasis in prostate cancer
International Urology and Nephrology (2024)
-
The predictive value of machine learning and nomograms for lymph node metastasis of prostate cancer: a systematic review and meta-analysis
Prostate Cancer and Prostatic Diseases (2023)
-
Impact of peritoneal reconfiguration on lymphocele formation after robot-assisted radical prostatectomy with pelvic lymph node dissection: a systematic review and meta-analysis of randomized controlled trials
Prostate Cancer and Prostatic Diseases (2023)
-
Nomograms in PCa: where do we stand
Prostate Cancer and Prostatic Diseases (2023)
-
Relative impact of lymph-node metastasis and seminal vesical invasion on oncologic outcomes following radical prostatectomy
Prostate Cancer and Prostatic Diseases (2023)