Abstract
Background
The association between prenatal metal exposure and congenital anomalies is unclear. We aimed to examine the association between exposure to cadmium, lead, mercury, selenium, and manganese and physical abnormalities.
Methods
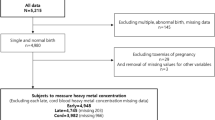
Data from 89,887 pregnant women with singleton pregnancies who participated in the Japan Environment and Children’s Study (JECS) were used. The correlation between maternal blood metal concentrations and physical abnormalities during the second or third trimester was investigated using logistic regression models. Physical anomalies included those observed at birth or at 1 month, primarily from ICD-10 Chapter 17, particularly congenital anomalies associated with environmental factors (e.g., hypospadias, cryptorchidism, cleft lip and palate, digestive tract atresia, congenital heart disease, and chromosomal abnormalities) and minor abnormalities.
Results
After adjusting for covariates, the OR (95% CIs) of physical abnormalities for a one-unit rise in Mn concentrations in all individuals were 1.26 (1.08, 1.48). The OR (95% CIs) of physical abnormalities in the 4th quartile (≥18.7 ng/g) were 1.06 (1.01, 1.13) (p-value for the trend = 0.034) compared with those in the 1st quartile (≤12.5 ng/g).
Conclusion
In Japan, maternal blood Mn concentrations above threshold during pregnancy may slightly increase the incidence of physical abnormalities.
Impact
-
Physical abnormalities (including minor anomalies and congenital anomalies) are associated with prenatal manganese concentrations.
-
They are not associated with cadmium, lead, mercury, and selenium concentrations.


Similar content being viewed by others
Data availability
Regarding Data of the Paper Publication http://www.env.go.jp/chemi/ceh/en/index.html. Data are unsuitable for public deposition owing to ethical restrictions and legal framework of Japan. It is prohibited by the Act on the Protection of Personal Information (Act No. 57 of 30 May 2003, amendment on 9 September 2015) to publicly deposit the data containing personal information. Ethical Guidelines for Medical and Health Research Involving Human Subjects enforced by the Japan Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labor and Welfare also restrict the open sharing of epidemiologic data. All inquiries about access to data should be sent to: jecs-en@nies.go.jp. The person responsible for handling enquiries sent to this e-mail address is Dr Shoji F. Nakayama, JECS Program Office, National Institute for Environmental Studies.
Change history
08 March 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41390-024-03099-2
References
Hanaoka, T. et al. Prevalence and risk of birth defects observed in a prospective cohort study: the Hokkaido study on environment and children’s health. J. Epidemiol. 28, 125–132 (2018).
World Health Organization Congenital anomalies. https://www.who.int/en/news-room/fact-sheets/detail/congenital-anomalies.
Mai, C. T. et al. National population-based estimates for major birth defects, 2010-2014. Birth Defects Res. 111, 1420–1435 (2019).
Feldkamp, M. L. et al. Etiology and clinical presentation of birth defects: population based study. BMJ 357, j2249 (2017).
Oliveira, C. I. & Fett-Conte, A. C. Birth defects: risk factors and consequences. J. Pediatr. Genet. 2, 85–90 (2013).
Caserta, D. et al. Heavy metals and placental fetal-maternal barrier: a mini-review on the major concerns. Eur. Rev. Med. Pharmacol. Sci. 17, 2198–2206 (2013).
Mistry, H. D., Broughton Pipkin, F., Redman, C. W. & Poston, L. Selenium in reproductive health. Am. J. Obstet. Gynecol. 206, 21–30 (2012).
Krachler, M., Rossipal, E. & Micetic-Turk, D. Trace element transfer from the mother to the newborn-investigations on triplets of colostrum, maternal and umbilical cord sera. Eur. J. Clin. Nutr. 53, 486–494 (1999).
Ciesielski, T. et al. Cadmium exposure and neurodevelopmental outcomes in U.S. children. Environ. Health Perspect. 120, 758–763 (2012).
Murata, K., Iwata, T., Dakeishi, M. & Karita, K. Lead toxicity: does the critical level of lead resulting in adverse effects differ between adults and children? J. Occup. Health 51, 1–12 (2009).
Bose-O’Reilly, S., McCarty, K. M., Steckling, N. & Lettmeier, B. Mercury exposure and children’s health. Curr. Probl. Pediatr. Adolesc. Health Care 40, 186–215 (2010).
Claus Henn, B. et al. Maternal and cord blood manganese concentrations and early childhood neurodevelopment among residents near a mining-impacted Superfund site. Environ. Health Perspect. 125, 067020 (2017).
Gunier, R. B. et al. Manganese in teeth and neurodevelopment in young Mexican-American children. Environ. Res. 142, 688–695 (2015).
Lin, C. C. et al. In utero exposure to environmental lead and manganese and neurodevelopment at 2 years of age. Environ. Res. 123, 52–57 (2013).
Taylor, D. et al. Recent developments in selenium research. Br. J. Biomed. Sci. 66, 107–116 (2009).
Jin, L. et al. Placental concentrations of mercury, lead, cadmium, and arsenic and the risk of neural tube defects in a Chinese population. Reprod. Toxicol. 35, 25–31 (2013).
Ou, Y. et al. Associations between toxic and essential trace elements in maternal blood and fetal congenital heart defects. Environ. Int. 106, 127–134 (2017).
Langley, R. L. et al. Adverse neurodevelopmental effects and hearing loss in children associated with manganese in well water, North Carolina. J. Environ. Occup. Sci. 4, 62–69 (2015).
Liu, J. et al. Placental concentrations of manganese and the risk of fetal neural tube defects. J. Trace Elem. Med. Biol. 27, 322–325 (2013).
Pi, X. et al. Concentrations of selected heavy metals in placental tissues and risk for neonatal orofacial clefts. Environ. Pollut. 242, 1652–1658 (2018).
Sharma, T. et al. Heavy metal levels in adolescent and maternal blood: association with risk of hypospadias. ISRN Pediatr. 2014, 714234 (2014).
Yalçin, S. S., Dönmez, Y., Aypar, E. & Yalçin, S. Element profiles in blood and teeth samples of children with congenital heart diseases in comparison with healthy ones. J. Trace Elem. Med. Biol. 63, 126662 (2021).
Guo, Y. et al. High maternal selenium levels are associated with increased risk of congenital heart defects in the offspring. Prenat. Diagn. 39, 1107–1114 (2019).
Ni, W. et al. Association between selected essential trace element concentrations in umbilical cord and risk for cleft lip with or without cleft palate: A case-control study. Sci. Total Environ. 661, 196–202 (2019).
Pi, X. et al. Higher concentration of selenium in placental tissues is associated with reduced risk for orofacial clefts. Clin. Nutr. 38, 2442–2448 (2019).
Takatani, T. et al. Individual and mixed metal maternal blood concentrations in relation to birth size: an analysis of the Japan Environment and Children’s Study (JECS). Environ. Int. 165, 107318 (2022).
Cedergreen, N. Quantifying synergy: a systematic review of mixture toxicity studies within environmental toxicology. PLoS One 9, e96580 (2014).
Kawamoto, T. et al. Rationale and study design of the Japan environment and children’s study (JECS). BMC Public Health 14, 25 (2014).
Mezawa, H. et al. Prevalence of congenital anomalies in the Japan environment and children’s study. J. Epidemiol. 29, 247–256 (2019).
Nakayama, S. F. et al. Blood mercury, lead, cadmium, manganese and selenium levels in pregnant women and their determinants: the Japan Environment and Children’s Study (JECS). J. Expo. Sci. Environ. Epidemiol. 29, 633–647 (2019).
Currie, L. A. Detection and quantification limits: origins and historical overview. Anal. Chim. Acta 391, 127–134 (1999).
Kobayashi, S. et al. Association of blood mercury levels during pregnancy with infant birth size by blood selenium levels in the Japan Environment and Children’s Study: a prospective birth cohort. Environ. Int. 125, 418–429 (2019).
Keil, A. P. et al. A quantile-based g-computation approach to addressing the effects of exposure mixtures. Environ. Health Perspect. 128, 47004 (2020).
Tsuji, M. et al. The association between whole blood concentrations of heavy metals in pregnant women and premature births: the Japan Environment and Children’s Study (JECS). Environ. Res. 166, 562–569 (2018).
Inadera, H. et al. Association of blood cadmium levels in pregnant women with infant birth size and small for gestational age infants: the Japan Environment and Children’s study. Environ. Res. 191, 110007 (2020).
Goto, Y. et al. Association of prenatal maternal blood lead levels with birth outcomes in the Japan Environment and Children’s Study (JECS): a nationwide birth cohort study. Int. J. Epidemiol. 50, 156–164 (2021).
Yamamoto, M. et al. Association between blood manganese level during pregnancy and birth size: the Japan environment and children’s study (JECS). Environ. Res. 172, 117–126 (2019).
Miyashita, C. et al. Association between the concentrations of metallic elements in maternal blood during pregnancy and prevalence of abdominal congenital malformations: the Japan environment and children’s study. Int. J. Environ. Res. Public Health 18, 10103 (2021).
Sanders, A. P. et al. Association between arsenic, cadmium, manganese, and lead levels in private wells and birth defects prevalence in North Carolina: a semi-ecologic study. BMC Public Health 14, 955 (2014).
Chung, S. E. et al. Maternal blood manganese and early neurodevelopment: the mothers and children’s environmental health (MOCEH) study. Environ. Health Perspect. 123, 717–722 (2015).
Chen, L. et al. Manganese concentrations in maternal-infant blood and birth weight. Environ. Sci. Pollut. Res. Int. 21, 6170–6175 (2014).
Pinsino, A., Roccheri, M. C., Costa, C. & Matranga, V. Manganese interferes with calcium, perturbs ERK signaling, and produces embryos with no skeleton. Toxicol. Sci. 123, 217–230 (2011).
Guilarte, T. R. et al. Evidence for cortical dysfunction and widespread manganese accumulation in the nonhuman primate brain following chronic manganese exposure: a 1H-MRS and MRI study. Toxicol. Sci. 94, 351–358 (2006).
Ding, D., Roth, J. & Salvi, R. Manganese is toxic to spiral ganglion neurons and hair cells in vitro. Neurotoxicology 32, 233–241 (2011).
Oikawa, S. et al. Mechanism for manganese enhancement of dopamine-induced oxidative DNA damage and neuronal cell death. Free Radic. Biol. Med. 41, 748–756 (2006).
Fitzgerald, P. & Dinan, T. G. Prolactin and dopamine: what is the connection? A review article. J. Psychopharmacol. 22, 12–19 (2008).
Dos Santos, N. R. et al. Manganese exposure and association with hormone imbalance in children living near a ferro-manganese alloy plant. Environ. Res. 172, 166–174 (2019).
Memon, N. S. et al. Correlation of manganese with thyroid function in females having hypo- and hyperthyroid disorders. Biol. Trace Elem. Res. 167, 165–171 (2015).
Mijal, R. S. & Holzman, C. B. Blood cadmium levels in women of childbearing age vary by race/ethnicity. Environ. Res. 110, 505–512 (2010).
Järup, L. et al. Health effects of cadmium exposure—a review of the literature and a risk estimate. Scand. J. Work Environ. Health 24, 1–51 (1998).
Zheng, S. et al. The antagonistic effect of selenium on lead-induced inflammatory factors and heat shock protein mRNA level in chicken cartilage tissue. Biol. Trace Elem. Res. 173, 177–184 (2016).
Zwolak, I. & Zaporowska, H. Selenium interactions and toxicity: a review. Selenium interactions and toxicity. Cell Biol. Toxicol. 28, 31–46 (2012).
Bernard, A. M. Biokinetics and stability aspects of biomarkers: recommendations for application in population studies. Toxicology 101, 65–71 (1995).
Spencer, A. Whole blood manganese levels in pregnancy and the neonate. Nutrition 15, 731–734 (1999).
Bower, C. et al. Age at diagnosis of birth defects. Birth Defects Res. A Clin. Mol. Teratol. 88, 251–255 (2010).
Acknowledgements
We thank all the mothers and their children who participated in the JECS. We wish to express our sincere appreciation to the collaborating hospitals and clinics. We also express our gratitude to all the JECS staff members in Hokkaido, Miyagi, Fukushima, Chiba, Kanagawa, Koshin, Toyama, Aichi, Kyoto, Osaka, Hyogo, Tottori, Kochi, Fukuoka, and South Kyushu/Okinawa Regional Centers, the National Center for JECS (program office), and the Medical Support Center.
Funding
The JECS was funded by the Ministry of the Environment, Japan. The findings and conclusions of this article are solely the responsibility of the authors and do not represent the official views of the Japanese Government.
Author information
Authors and Affiliations
Consortia
Contributions
Y.N., S.K., K.C., S.I., C.M., T.Y. and R.K. conceived and designed the study. Y.N., S.K., K.C., S.I., C.M., T.Y., Y.S., Y.I. and R.K. performed the data collection. Y.N., S.K., K.C., H.I. and N.T. performed the statistical analysis and contributed to the manuscript preparation and literature search. Y.N., S.K., K.C., S.I., C.M. and T.Y. interpreted the data. Y.N., S.K., K.C., S.I., C.M., T.Y., H.I., N.T., Y.S., Y.I. and R.K. contributed to the critical revision of the manuscript for important intellectual content. S.K., K.C., A.M. and R.K. contributed to the funds collection and were supervisors of the study. All authors approved the version of the manuscript to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Informed consent
Informed consent was obtained from all participants (patients).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: the authors identified a siginificant modification related to the results of the regression analysis. The primary focus of the analysis, manganese, remains almost unchanged. However, there are slight alterations in the 95% confidence intervals and p-values for cadmium, lead, mercury, and selenium in Table 3, Supplementary Table 2 and Supplementary Table 3.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nakamura, Y., Kobayashi, S., Cho, K. et al. Prenatal metal concentrations and physical abnormalities in the Japan Environment and Children’s Study. Pediatr Res (2023). https://doi.org/10.1038/s41390-023-02851-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-023-02851-4
- Springer Nature America, Inc.
This article is cited by
-
Environmental causes of birth defects: challenges and opportunities
Pediatric Research (2024)