Abstract
Background
Neonates with congenital heart disease (CHD) undergoing cardiopulmonary bypass (CPB) surgery have increased risk of impaired neurodevelopmental outcomes secondary to brain injury. This study aims to characterize pre- and post-operative continuous EEG (cEEG) patterns to detect abnormal cerebral activity in infants with CHD and investigate whether an association exists between the degree of encephalopathy in pre- and post-operative cEEG.
Methods
This retrospective cohort study conducted between 2010 and 2018 at a tertiary hospital in Cleveland, OH included infants with CHD with cEEG monitoring, who underwent CPB surgery within first 6 months of life.
Results
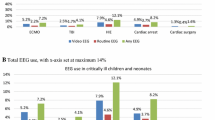
Study included 77 patients, of which 61% were males who were operated at median age 6 days. Pre-operatively, 69% and 87% had normal cEEG and sleep–wake cycles, respectively. Post-operatively, 80% had abnormal cEEG. Longer circulatory arrest time and CPB were associated with lack of continuity (p 0.011), excessive discontinuity (p 0.007) and prolonged inter-burst interval (IBI) duration (p value < 0.001). A significant association existed between severity of encephalopathy in immediate and 24-h post-operative period (p value < 0.001).
Conclusions
More than 80% of neonates with CHD have abnormal post-operative EEG. Longer circulatory arrest time and CPB were associated with lack of continuity, excessive discontinuity, and prolonged IBI duration on post-operative EEG.
Impact
-
This study shows that majority of neonates with congenital heart disease (CHD) have normal pre-operative EEG with a continuous background and normal sleep–wake cycles. Also, 80% of neonates had abnormal post-operative EEG.
-
Longer duration of arrest time and bypass time was associated with lack of continuity, excessive discontinuity, and prolonged IBI duration during post-operative EEG monitoring.
-
These findings will help clinicians when counseling parents in the intensive care unit, risk stratification, and long-term neurodevelopmental monitoring in these high-risk patients.
Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Hoffman, J. I. E. & Kaplan, S. The incidence of congenital heart disease. J. Am. Coll. Cardiol. 39, 1890–1900 (2002).
Massey, S. L. et al. Electroencephalographic patterns preceding cardiac arrest in neonates following cardiac surgery. Resuscitation 144, 67–74 (2019).
Reller, M. D., Strickland, M. J., Riehle-Colarusso, T., Mahle, W. T. & Correa, A. Prevalence of congenital heart defects in Metropolitan Atlanta, 1998-2005. J. Pediatr. 153, 807–813 (2008).
Gaynor, J. W. et al. Is cardiac diagnosis a predictor of neurodevelopmental outcome after cardiac surgery in infancy? J. Thorac. Cardiovasc. Surg. 140, 1230–1237 (2010).
Mahle, W. T. et al. An MRI study of neurological injury before and after congenital heart surgery. Circulation 106, 1085 (2002).
McQuillen, P. S. et al. Temporal and anatomic risk profile of brain injury with neonatal repair of congenital heart defects. Stroke 38, 736–741 (2007).
Mulkey, S. B. et al. Multi-tiered analysis of brain injury in neonates with congenital heart disease. Pediatr. Cardiol. 34, 1772–1784 (2013).
Mulkey, S. B. et al. Amplitude-integrated EEG in newborns with critical congenital heart disease predicts preoperative brain magnetic resonance imaging findings. Pediatr. Neurol. 52, 599–605 (2015).
Shellhaas, R. A. et al. The American clinical neurophysiology society’s guideline on continuous electroencephalography monitoring in neonates. J. Clin. Neurophysiol. 28, 611–617 (2011).
Limperopoulous, C. et al. Association between electroencephalographic findings and neurologic status in infants with congenital heart defects. J. Child Neurol. 16, 471–476 (2001).
Mebius, M. J. et al. Amplitude-integrated electroencephalography during the first 72 h after birth in neonates diagnosed prenatally with congenital heart disease. Pediatr. Res. 83, 798–803 (2018).
Ter Horst, H. J., Mud, M., Roofthooft, M. T. R. & Bos, A. F. Amplitude integrated electroencephalographic activity in infants with congenital heart disease before surgery. Early Hum. Dev. 86, 759–764 (2010).
Latal, B. et al. Postoperative amplitude-integrated electroencephalography predicts four-year neurodevelopmental outcome in children with complex congenital heart disease. J. Pediatr. 178, 55–60 (2016).
Claessens, N. H. P. et al. Amplitude-integrated electroencephalography for early recognition of brain injury in neonates with critical congenital heart disease. J. Pediatr. 202, 199–205 (2018).
Gunn, J. K., Beca, J., Hunt, R. W., Olischar, M. & Shekerdemian, L. S. Perioperative amplitude-integrated EEG and neurodevelopment in infants with congenital heart disease. Intensive Care Med. 38, 1539–1547 (2012).
Gunn, J. K. et al. Amplitude-integrated electroencephalography and brain injury in infants undergoing norwood-type operations. Ann. Thorac. Surg. 93, 170–176 (2012).
Clancy, R. R. et al. Electrographic neonatal seizures after infant heart surgery. Epilepsia 46, 84–90 (2005). Published online.
Gaynor, J. W. et al. Increasing duration of deep hypothermic circulatory arrest is associated with an increased incidence of postoperative electroencephalographic seizures. J. Thorac. Cardiovasc Surg. 130, 1278–1286 (2005).
Shellhaas, R. A., Gallagher, P. R. & Clancy, R. R. Assessment of neonatal electroencephalography (EEG) background by conventional and two amplitude-integrated EEG classification systems. J. Pediatr. 153, 369–374 (2008).
Clancy, R. R. et al. Preoperative risk-of-death prediction model in heart surgery with deep hypothermic circulatory arrest in the neonate. J. Thorac. Cardiovasc. Surg. 119, 347–357 (2000).
Bellinger, D. C. et al. Developmental and neurologic status of children after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. N. Engl. J. Med. 332, 549–555 (1995).
Limperopoulos, C. et al. Predictors of developmental disabilities after open heart surgery in young children with congenital heart defects. J. Pediatr. 141, 51–58 (2002).
Hovels-Gurich, H. H., Seghaye, M. C., Dabritz, S., Messmer, B. J. & Von Bernuth, G. Cognitive and motor development in preschool and school-aged children after neonatal arterial switch operation. J. Thorac. Cardiovasc. Surg. 114, 578–585 (1997).
Hövels-Gürich, H. H. et al. Long term behavioural outcome after neonatal arterial switch operation for transposition of the great arteries. Arch. Dis. Child. 87, 506–510 (2002).
Miller, G., Tesman, J. R., Ramer, J. C., Baylen, B. G. & Myers, J. L. Outcome after open-heart surgery in infants and children. J. Child Neurol. 11, 49–53 (1996).
Rogers, B. T. et al. Neurodevelopmental outcome of infants with hypoplastic left heart syndrome. J. Pediatr. 126, 496–498 (1995).
Gaynor, J. W. et al. The relationship of postoperative electrographic seizures to neurodevelopmental outcome at 1 year of age after neonatal and infant cardiac surgery. J. Thorac. Cardiovasc. Surg. 131, 181–189 (2006).
Limperopoulos, C. et al. Neurologic status of newborns with congenital heart defects before open heart surgery. Pediatrics 103, 402–408 (1999).
Dittrich, H. et al. Neurodevelopment at 1 year of age in infants with congenital heart disease. Heart 89, 436–441 (2003).
Majnemer, A. et al. Long-term neuromotor outcome at school entry of infants with congenital heart defects requiring open-heart surgery. J. Pediatr. 148, 72–77 (2006).
Khalil, A. et al. Brain abnormalities and neurodevelopmental delay in congenital heart disease: systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 43, 14–24 (2014).
Latal, B. et al. Can preoperative cranial ultrasound predict early neurodevelopmental outcome in infants with congenital heart disease? Dev. Med. Child Neurol. 57, 639–644 (2015).
Licht, D. J. et al. Preoperative cerebral blood flow is diminished in neonates with severe congenital heart defects. J. Thorac. Cardiovasc. Surg. 128, 841–849 (2004).
Mahle, W. T. et al. An MRI study of neurological injury before and after congenital heart surgery. Circulation 106, 1085 (2002).
Van Hauten, J. P., Rothman, A. & Bejar, R. High incidence of cranial ultrasound abnormalities in full-term infants with congenital heart disease. Am. J. Perinatol. 13, 47–53 (1996).
Te Pas, A., Van Wezel-Meijler, G., Bökenkamp-Gramann, R. & Walther, F. Preoperative cranial ultrasound findings in infants with major congenital heart disease. Acta Paediatr. Int. J. Paediatr. 94, 1597–1603 (2005).
Newburger, J. W. et al. A comparison of the perioperative neurologic effects of hypothermic circulatory arrest versus low-flow cardiopulmonary bypass in infant heart surgery. N. Engl. J. Med. 329, 1057–1064 (1993).
Brown, W. R., Moody, D. M., Challa, V. R., Stump, D. A. & Hammon, J. W. Longer duration of cardiopulmonary bypass is associated with greater numbers of cerebral microemboli. Stroke 31, 707–713 (2000).
Newman, M. F. et al. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N. Engl. J. Med. 344, 395–402 (2001).
Kurth, C. D., Steven, J. M. & Nicolson, S. C. Cerebral oxygenation during pediatric cardiac surgery using deep hypothermic circulatory arrest. Anesthesiology 82, 74–82 (1995).
Nomura, F. et al. Cerebral oxygenation measured by near infrared spectroscopy during cardiopulmonary bypass and deep hypothermic circulatory arrest in piglets. Pediatr. Res. 40, 790–796 (1996).
Lamblin, M. D. et al. Electroencephalography of the premature and term newborn. Maturational aspects and glossary. Neurophysiol. Clin. 29, 123–219 (1999).
Toet, M. C. et al. Cerebral oxygen saturation and electrical brain activity before, during, and up to 36 h after arterial switch procedure in neonates without pre-existing brain damage: its relationship to neurodevelopmental outcome. Exp. Brain Res. 165, 343–350 (2005).
Birca, A. et al. Interplay of brain structure and function in neonatal congenital heart disease. Ann. Clin. Transl. Neurol. 3, 708–722 (2016).
Bernet, V. et al. Effect of sedation and analgesia on postoperative amplitude-integrated EEG in newborn cardiac patients. Pediatr. Res. 67, 650–655 (2010).
Olischar, M., Davidson, A. J., Lee, K. J. & Hunt, R. W. Effects of morphine and midazolam on sleep-wake cycling in amplitude-integrated electroencephalography in post-surgical neonates ≥32 weeks of gestational age. Neonatology 101, 293–300 (2012).
Funding
No financial assistance was received in support of this study.
Author information
Authors and Affiliations
Contributions
S.P. helped design the study, designed data collection instruments, collected data, drafted the initial manuscript, and reviewed and revised the manuscript. N.F. conceptualized and designed the study and reviewed the manuscript. E.P.-K. and A.M.-N. helped design the study and reviewed and revised the manuscript for important intellectual content. L.F. helped design data collection instruments and collected data. S.W. helped with data stratification and statistical analysis. H.A. coordinated and supervised data collection and critically reviewed the manuscript for important intellectual content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
No patient consent was required for this retrospective cohort study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Padiyar, S., Friedman, N., Pestana-Knight, E. et al. Continuous electroencephalography (cEEG) in infants with congenital heart disease (CHD). Pediatr Res 94, 715–723 (2023). https://doi.org/10.1038/s41390-023-02520-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02520-6
- Springer Nature America, Inc.
This article is cited by
-
Perioperative Neuromonitoring in Children with Congenital Heart Disease
Neurocritical Care (2023)