Abstract
Nephrotic syndrome (NS) is a common kidney disease of childhood, affecting 2–7 children per 100,000. A potentially life-threatening complication affecting children with NS is thromboembolism (TE). However, there remains a paucity of information regarding the burden of TE and its associated risk factors in this population. A systematic review was performed on observational studies examining TE events in children with NS, published in Medline, Embase, CINAHL, and CENTRAL, until May 2021. Meta-analyses were separately conducted on the prevalence of TE within articles exclusively studying children with congenital NS and among articles including all forms of NS. Out of 13,626 articles, 22 were included (14,290 children). The pooled prevalence of symptomatic TE among articles including patients with all forms of NS was 3.60% (95% CI 1.95–5.63), which increased to 8.70% (95% CI 5.11–12.96) in articles with exclusively congenital NS patients. Children with steroid-resistant NS were at a higher risk of TE compared to steroid-sensitive children (OR 4.40, 95% CI 1.34–15.59, p = 0.013). Focal segmental glomerulosclerosis was the most common histology present in patients with TE (51.2%). Children diagnosed with NS have a significant risk of TE, particularly in patients with congenital NS and steroid resistance.
Impact
-
The prevalence of symptomatic thromboembolic (TE) events in children with nephrotic syndrome (NS) was 3.60% (95% CI 1.95–5.63), which increased more than two-fold in children with congenital NS to 8.70% (95% CI 5.11–12.96).
-
Potential risk factors for TE events in this population include congenital forms of NS and steroid resistance.
-
This review provides a better estimate of the prevalence of TE in children with NS, while identifying potentially higher-risk populations who may benefit from TE screening and thromboprophylaxis.
Similar content being viewed by others
Introduction
Childhood nephrotic syndrome (NS) is one of the most common childhood kidney diseases, affecting 2–7 children per 100,000.1 NS may present in children in a primary form, including idiopathic NS, or secondary forms such as IgA nephropathy or due to underlying systemic disease (e.g., systemic lupus erythematosus), infections (e.g., hepatitis B and C), or medication use (e.g., non-steroidal anti-inflammatory drugs).2 In addition, NS presenting within the first 3 months and first year of age are considered congenital NS and infantile NS, respectively, which are typically due to genetic mutations.3 Patients with childhood NS are at risk for serious infections, acute kidney injury, and treatment-related adverse events, such as decreased bone mineral density and immunosuppression from long-term corticosteroid use.4 Among the non-infectious complications, thromboembolism (TE) is one of the most serious and potentially life-threatening when it occurs in the pulmonary circulation, where it results in pulmonary embolism (PE).
The pathophysiology of TE is secondary to multiple concurrent processes. First, the urinary loss of anticoagulant proteins such as antithrombin (AT) and protein S, coupled with a compensatory upregulated production of procoagulant proteins, including fibrinogen, Factor V, and Factor VIII, place patients in a procoagulant state.5 These processes combined with the dysregulation of platelet function and aggregability and decreased fibrinolytic activity place children at a higher risk of TE, particularly deep vein thrombosis (DVT).6
The available literature on TE in children with NS is limited in sample size and estimates an incidence of approximately 2.9%, although significant variation in the incidence of TE exists between studies, ranging from 1.8 to 28.1%.1 Several cohort studies have reported the prevalence of TE events in children with NS; however, differences in disease stage and chronicity, genetic predispositions, as well as patient characteristics contribute to the heterogeneity of reported TE prevalence.1 In addition, inter-study differences such as study design, sample size, disease definition, and detection methods of TE limit the accuracy of such estimates. Although research continues to shed light on this process, it is unclear why some children present with serious TE complications that lead to increased mortality. This makes management decisions challenging, particularly regarding screening protocols for children at risk for TE and thromboprophylaxis treatment. Thus, the purpose of this review was to systematically describe the prevalence and presentation of TE in pediatric NS patients. Moreover, specific patient and disease characteristics were also identified to better inform clinical decision-making, specifically regarding higher-risk populations who may benefit from screening and thromboprophylaxis.
Methods
This systematic review was reported based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Supplementary Table S1).7
Eligibility criteria
English-language studies (cohort studies, case–control studies, and case series with more than five patients) on children and young adults (age 0–21 years) with a diagnosis of primary, secondary, or congenital/infantile NS due to any cause, which reported on the presence of symptomatic events of TE were included. Case reports, conference proceedings, and review articles were excluded. The references of prominent review articles were also screened.
Information sources and searches
Databases included Medline, Embase, CINAHL, and CENTRAL. Search strategies were developed in collaboration with an expert librarian (S.S.) and included keywords and MeSH terms (Supplementary Data S1). Databases were searched for relevant articles published from inception until May 2021. Citations of review articles were searched for additional relevant published data. Articles were screened via Covidence and Refworks software.
Study selection
Two content experts (K.D. and H.G.) independently selected studies for inclusion using our selection criteria. Titles and abstracts were first reviewed, followed by a full-text review for selected studies. Study selection was completed by consensus, with disagreements adjudicated by a third author (R.C.). If the same cohort of subjects was included in more than one article, the study with the larger sample size was included; in duplicate cohorts with the same sample size, the newer publication was included.
Assessment of methodological quality
Two independent reviewers (K.D. and H.G.) assessed the quality of included studies using the Newcastle-Ottawa scale (NOS) for observational studies. The respective NOS for case–control and cohort studies were used as applicable.8 Quality was assessed based on patient selection, comparability, and outcomes. Scores for each category were then added, and based on the total score, a study was determined to be either high or low methodological quality. Disagreements in scoring were resolved by a third reviewer (R.C.).
Data extraction
Data were extracted independently by two reviewers using standardized data collection forms. Extracted data included demographic characteristics, prevalence, timing, and location of TE, histology of NS, protein C, protein S, and AT levels, and whether screening for TE was performed. An attempt was made to contact the authors where data were missing.
Outcome measures
The primary outcome was to determine the prevalence of TE in children with all forms of NS and among those with congenital NS. Secondary outcomes included the location of TE, the timing of TE events relative to the diagnosis of NS, as well as the incidence rate of TE if follow-up data were reported. Subgroup analyses were also performed to better understand differences in the risk of TE by age, sex, steroid sensitivity status, and histology (i.e., minimal change disease (MCD), focal segmental glomerulosclerosis (FSGS), and secondary forms of NS). In addition, the prevalence of TE was performed separately for studies that described only congenital NS, as the clinical features of this cohort of patients are different from other forms of NS, which may place them at a higher risk of TE.9
Statistical analysis
Data synthesis was completed using Covidence software. Prevalence was defined as the number of individuals with NS who developed TE over any time during the study period divided by the total number of patients with NS. The prevalence meta-analyses were conducted with a random-effects model using the metafor package from RStudio version 1.4.1717, R version 4.1.0 (R Project for Statistical Computing; Boston, MA).10 Prevalence estimates were first transformed using the Freeman–Tukey double-arcsine method, followed by conversion back to prevalence estimates.11,12,13 Heterogeneity was quantified in included studies using the inconsistency index (I2) and χ2 test p values. Odds ratios of TE prevalence were reported based on specified subgroups: age (0–1, 1–10 years, and >10 years), sex, and type of NS according to steroid sensitivity status and histology, respectively. Odds ratios and 95% confidence intervals were calculated using the epitools package from RStudio. Dichotomous data were presented as frequencies and percentages. Funnel plots were generated using the metafor package to explore for publication bias, which was assessed by plot asymmetry through visual inspection and the Egger test. Separate meta-analyses and funnel plots were generated for studies that exclusively examined congenital NS patients and for the remaining studies that were not exclusive to congenital NS patients. Within articles including all forms of NS, any subsamples of congenital NS patients were analyzed separately with articles exclusively including congenital NS patients if data specific to the congenital NS group was provided and included more than five congenital NS patients, similar to the study inclusion criteria mentioned above.
Results
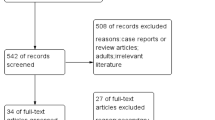
Out of 13,626 articles returned by the database search, 2388 duplicates were removed. Following title and abstract screening, and subsequent full-text review of 218 articles for eligibility, 22 articles were included (Fig. 1), which represented a total of 14,290 patients (Table 1). There were 53 articles that were not accessible despite attempts by the authors and a librarian. Of the 22 articles included in our review, 16 were retrospective, 4 were prospective studies, and 2 were cross-sectional. Of the included studies, 4 studies (18%) were of good quality, 1 study (5%) of fair quality, and 17 studies (77%) of poor quality (Supplementary Table S2). Most of the poor-quality studies were due to an absence of a control cohort for the outcome of interest.
Studies included children with primary NS, including idiopathic NS, congenital NS, and other secondary forms of NS. Of these studies, four (18%) included the screening of each child for TE beyond physical exam with some form of imaging (Supplementary Table S3). Of the 14,290 patients included, 502 patients (3.5%) developed at least one thromboembolic complication. Among studies reporting all forms of NS (n = 16), representing a total of 14,039 patients, the pooled prevalence of TE was 3.60% (95% CI 1.95–5.63), as shown in Fig. 2. Seven of these studies included a subset of congenital NS patients; however, these samples were not analyzed separately as the number of congenital NS patients was either not provided, did not meet the minimum criteria of six patients, or did not provide TE data specific to this group. The remaining studies (n = 6) exclusively evaluated patients with congenital NS. Of the 251 children with congenital NS, the prevalence of TE was 8.70% (95% CI 5.11–12.96), as shown in Fig. 3.
Of the 22 studies that were included in the final analysis, 12 studies were longitudinal and provided follow-up data. Among 475 cases of TE, there were a total of 30,487.3 patient-years of follow-up. The overall incidence of patients with TE was 15.58 per 1000 patient-years of follow-up. The patient-year data of individual studies are shown in Table 2. All 12 studies were conducted on patients with NS of all forms, apart from the study conducted by Mahan et al., which exclusively investigated patients with congenital NS.14
Location
Among 16 studies that reported the location of TE, there were 146 events that were classified into 7 categories. DVT was the most common location for TE accounting for 72 events (49.3%), followed by cerebral venous sinus thrombosis or cerebral infarct (13.7%), PE (12.3%), renal vein thrombosis (11.0%), other arterial thrombosis (6.8%), intra-abdominal splanchnic vein thrombosis (4.8%), and cardiac thrombosis (2.1%). Locations of all TE events can be visualized in Fig. 4.
Timing
The majority of studies assessed patients for TE during periods of relapse requiring hospitalization or outpatient follow-up. TE during periods of remission was rarely reported. The limited data on the timing of TE made it not possible to determine the number of patients diagnosed with TE at the onset of diagnosis, or any association between the time following the initial diagnosis of NS and developing TE.
Protein C, protein S, and antithrombin levels
There were five studies that examined protein C and S and two studies that compared AT levels in NS patients who developed TE. Three of these studies found that patients who developed TE exhibited lower levels of protein C and protein S than healthy controls or normal reference ranges.15,16,17 Anand et al. reported all patients who experienced TE had reduced levels of protein C and protein S; however, reduced AT levels were not predictive of events of TE.15 Tavil et al. identified deficiencies of AT, protein C, and protein S to be potential risk factors for TE events, reporting 29%, 18%, and 12% of patients experiencing TE exhibiting deficiencies of each protein, respectively.17 Conversely, two studies found protein C to be significantly elevated in children with NS when compared to healthy control, although no correlation was made to TE in either study.18,19
Subgroup analysis of studies including all forms of NS
Age
Only five studies reported the ages of each child diagnosed with TE (39 children total) (Supplementary Table S3). This did not include studies that only examined congenital NS patients. This included one patient less than 1 year of age (with congenital NS), 24 patients from 1 to 10 years of age, and 14 patients over 10 years old. Given that the ages of NS patients without TE were not consistently reported in these studies, specific age groups that may be at higher risk of TE were unable to be determined.
Sex
Six studies reported the sex of all participants in the study, including both children who did and did not develop TE (Supplementary Table S3). Two of these studies looked exclusively at congenital NS patients. These six studies included a total of 525 males and 380 females, of which 26 (5.0%) and 13 (3.4%) patients developed TE respectively. The odds ratio for males developing TE was 1.46 (95% CI 0.75–2.99, p = 0.32).
Histology
The histology of children experiencing TE was reported in six studies (Supplementary Table S3). Given the limited number of patients presenting with each histologic subtype, further analysis on the risk of TE was not performed. Among the six studies, there were 43 patients who developed TE, of which 22 patients (51.2%) had FSGS. Six patients (14.0%) had MCD, 1 patient (2.3%) had membranoproliferative glomerulonephritis, 3 patients had idiopathic membranous nephropathy (7.0%), and 11 (25.6%) were unknown.
Steroid sensitivity
Two studies included in this review reported the prevalence of TE for both steroid-sensitive and steroid-resistant children with TE (Supplementary Table S3).20,21 Cumulatively, these studies included 336 patients with steroid-sensitive NS, 5 of whom developed TE (1.5%). These studies also included 111 steroid-resistant NS patients, 7 of whom developed TE (6.3%). The odds of developing TE with steroid resistance compared to steroid sensitivity was 4.40 (95% CI 1.34–15.59, p = 0.013).
Publication bias
Publication bias was not present for the prevalence of TE in studies including only patients with congenital NS based on Egger’s test (p = 0.32). Similarly, publication bias was also not present in studies including all forms of NS (p = 0.06). The respective funnel plots are shown in Supplementary Figs. S1 and S2.
Discussion
Estimates of TE prevalence in childhood NS have been subject to methodological limitations including retrospective study designs, small sample sizes, varying TE definitions, NS histopathology distribution, and imaging methods used to detect TE. Given the heterogeneous literature on TE in pediatric NS patients, we aimed to systematically review the prevalence and clinical presentation of symptomatic TE in this population. We found that TE had a prevalence of 3.60% among children with all forms of NS, and within articles with exclusively congenital NS patients, the prevalence was 8.70%. Moreover, we studied several risk factors associated with TE including age, sex, histology, steroid sensitivity status, and protein C, protein S, and AT levels. Unfortunately, there were insufficient data in the studies to accurately assess the association between many of these risk factors and the prevalence of TE, apart from the impact of sex and steroid sensitivity, which demonstrated a non-significant difference between males and females and 4.4 times greater odds in steroid-resistant children.
The two-fold increase in TE prevalence seen in studies exclusively on congenital NS patients compared to all forms of NS has been similarly demonstrated in literature. A higher incidence of TE events in congenital NS children (10%) has been reported, as well as in children with secondary causes of NS (17.1%) and membranous nephropathy (25%).22 The increased susceptibility to TE in infants with congenital NS may be partially explained by the massive proteinuria that they experience soon after birth that results in the loss of anticoagulant proteins, including AT and plasminogen.3 In addition, the use of central venous access devices (CVAD) for nutrition and albumin infusion, low intravascular volume status due to low oncotic pressure, use of diuretics, and immobilization are also important factors that lead to a higher risk of TE in children with congenital NS.23 CVADs are a known risk factor for TE in NS patients as they are associated with vessel wall irritation, disrupted laminar blood flow, catheter-induced infection and local inflammation; all possibly contributing to activation of the coagulation system.1 However, in our review, there were insufficient data to explore the risk of TE with CVADs.
Among studies that indicated the anatomical location of TE, the most common type were DVTs, occurring in 49.3% of all TE events. Previous work conducted by Kayali et al. using data from the National Hospital Discharge Survey found the relative risk of DVT in patients with NS was 6.81 times higher compared to those without NS; however, as this cohort was largely composed of adults, the results may not be as applicable to children.24 Consistent with our data, Kayali et al. found that DVT was more common than renal vein thrombosis.24 In addition, CVAD use was associated with 45% of those events, further indicating it as a risk factor for TE in these patients.24
Steroid-resistant children had higher odds of TE than steroid-sensitive children; however, this finding was limited by the small number of studies reporting steroid responsiveness in patients who experienced TE. These results may be partially explained by the increased production of pro-thrombotic proteins by the liver, as well as urinary losses of AT that are likely exacerbated by the increased disease progression often seen with steroid-resistant children.22,25 In addition, inherent differences in the disease chronicity of various forms of NS may contribute to the increased prevalence of TE in steroid-resistant children, who usually have a prolonged duration of proteinuria.26 Studies have found the risk of venous TE to be directly proportional to the severity of proteinuria and inversely proportional to the albumin level.27
In regard to sex, males were found to have 1.46 times higher odds of TE prevalence compared to females, although this was not statistically significant. It is unclear why male patients with NS appeared to have a higher prevalence of TE; however, this has been previously reported by Stabouli et al. (70.1% males) in patients with NS who developed cerebral TE.28 Similarly, Zhu et al. found that the prevalence of PE in children and younger adults with NS was higher in male patients than female patients (29% vs. 14.9%, p = 0.002).29
This review also found that a majority of patients who developed TE presented with FSGS on histology (51.2%) compared to other presentations. Other pediatric studies have similarly noted that FSGS and membranous histology appear to increase the risk of NS-associated TE, when compared to other histopathological subtypes or steroid-sensitive NS.9 Similar studies in adult populations found patients with membranous nephropathy (7.85%) to have a greater prevalence of venous TE when compared with FSGS (2.97%) or IgA nephropathy (0.36%).27 Differences in disease mechanisms are believed to be responsible for histological diagnosis being an independent risk factor for venous thrombosis. A greater prevalence of steroid resistance and thus longer disease chronicity in patients with FSGS may contribute to an increased prevalence of TE seen in this population.30
TE events in NS children can be potentially life- or limb-threatening, especially in cases of PE or extreme limb ischemia. For NS patients at high risk for TE, prophylactic anticoagulation may be considered; however, there are limited data on the clinical efficacy or risks associated with prophylactic anticoagulation. The use of prophylactic anticoagulation is known to be effective for preventing clinically significant TE, but not without an increased risk of bleeding episodes.31 The American College of Chest Physicians (ACCP) guidelines from 2012 suggest consideration of prolonged anticoagulation treatment (>3 months) or secondary prophylaxis in NS patients who have been diagnosed with a thrombus.32 Similarly, the KDIGO 2021 Clinical Practice Guidelines recommend prophylactic full-dose anticoagulation in adult NS patients if serum albumin < 20–25 g/L and if any of the following criteria is met: proteinuria >10 g/day, body mass index >35 kg/m2, a genetic predisposition for TE, heart failure, recent surgery or prolonged immobilization.33 However, there are no recommendations on primary prophylaxis for pediatric NS in either the ACCP guidelines or the recent American Society of Hematology venous TE guidelines.34 Larger prospective studies are needed to help identify children at high risk, who can be closely monitored or considered for primary mechanical and/or anticoagulation prophylaxis.
The results of this study were limited by the lack of consistent reporting on patients’ demographic data, location of TE, timing of TE with respect to disease progression, histology presentation, steroid sensitivity, and protein C, protein S, and AT levels, which made it difficult to perform subgroup analyses and draw strong conclusions. Second, the reported TE prevalence may have been affected by the use of TE screening in four studies as mentioned above (Supplementary Table S3), which may detect asymptomatic TE in addition to symptomatic cases. Third, the use of anticoagulation prophylaxis in some of the congenital NS studies may contribute to the underestimation of the true prevalence of TE in this population.35,36 It is also important to note that TE events may go undiagnosed as they can present asymptomatically both in clinical and research settings. Fourth, there was a lack of data to determine the risk of TE in NS patients stratified by histology. For that reason, despite a higher pooled prevalence of TE in FSGS patients, we cannot conclusively state that FSGS patients were at a greater risk of TE. Fifth, some studies which reported data on TE in idiopathic NS also had a few patients with CNS; thus, likely inflating the reported prevalence when compared to a cohort excluding CNS patients.9,15,17,18,19,20,21,37,38,39,40,41 Lastly, our study was unable to differentiate the prevalence of TE in patients with primary NS compared to others with secondary causes of NS, as secondary causes of NS were not described in most studies.
Despite these limitations, this is the first systematic review and meta-analysis exploring the prevalence and clinical presentation of TE in pediatric NS patients. The true prevalence of TE in children with NS may in fact be higher than the three percent that is commonly cited in the literature, particularly among those with congenital forms of NS. Notably, NS patients with FSGS and steroid-resistant status appear to have a higher risk of experiencing TE. Further research is required to inform potential prophylactic measures for these higher-risk groups and to identify additional contributing factors for TE events.
Data availability
All data generated or analyzed during this study are included in this published article and its Supplementary information files.
References
Kerlin, B. A., Haworth, K. & Smoyer, W. E. Venous thromboembolism in pediatric nephrotic syndrome. Pediatr. Nephrol. 29, 989–997 (2014).
Noone, D. G., Iijima, K. & Parekh, R. Idiopathic nephrotic syndrome in children. Lancet 392, 61–74 (2018).
Jalanko, H. Congenital nephrotic syndrome. Pediatr. Nephrol. 24, 2121–2128 (2009).
Park, S. J. & Shin, J. I. Complications of nephrotic syndrome. Korean J. Pediatr. 54, 322–328 (2011).
Kendall, A. G., Lohmann, R. C. & Dossetor, J. B. Nephrotic syndrome. Arch. Intern. Med. 127, 1021–1027 (1971).
Pannicucci, F. et al. Comprehensive study of hemostasis in congenital nephrotic syndrome. Nephron 33, 9–13 (1983).
Moher, D. et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med. 6, e1000097 (2009).
Wells, G. A. et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomized studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/nosgen.pdf (2015).
Kerlin, B. A. et al. Epidemiology and risk factors for thromboembolic complications of childhood nephrotic syndrome: A Midwest Pediatric Nephrology Consortium (MWPNC) study. J. Pediatr. 155, 105–110 (2009).
Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 36, 1–48 (2010).
Freeman, M. F. & Tukey, J. W. Transformations related to the angular and the square root. Ann. Math. Stat. 21, 607–611 (1950).
Barendregt, J. J., Doi, S. A., Lee, Y. Y., Norman, R. E. & Vos, T. Meta-analysis of prevalence. J. Epidemiol. Community Health 67, 974–978 (2013).
Miller, J. J. The inverse of the Freeman–Tukey double arcsine transformation. Am. Stat. 32, 138 (1978).
Mahan, J. D., Mauer, S. M., Sibley, R. K. & Vernier, R. L. Congenital nephrotic syndrome: evolution of medical management and results of renal transplantation. J. Pediatr. 105, 549–557 (1984).
Anand, N. K., Chand, G., Talib, V. H., Chellani, H. & Pande, J. Hemostatic profile in nephrotic syndrome. Indian Pediatr. 33, 1005–1012 (1996).
Mittal, A., Aggarwal, K. C., Saluja, S., Aggarwal, A. & Sureka, B. Platelet functions and coagulation changes in Indian children with nephrotic syndrome. J. Clin. Diagn. Res. 7, 1647–1650 (2013).
Tavil, B. et al. Case series of thromboembolic complications in childhood nephrotic syndrome: Hacettepe experience. Clin. Exp. Nephrol. 19, 506–513 (2015).
Mehls, O., Andrassy, K., Koderisch, J., Herzog, U. & Ritz, E. Hemostasis and thromboembolism in children with nephrotic syndrome: differences from adults. J. Pediatr. 110, 862–867 (1987).
Özkayın, N., Sevgi, M. & Kaan, K. Hypercoagulability risk factors in children with minimal change disease and the protective role of protein-C activity. Int Urol. Nephrol. 36, 599–603 (2004).
Lee, B. H. et al. Idiopathic membranous nephropathy in children. Fetal Pediatr. Pathol. 21, 1707–1715 (2006).
Lilova, M. I., Velkovski, I. G. & Topalov, I. B. Thromboembolic complications in children with nephrotic syndrome in Bulgaria (1974–1996). Pediatr. Nephrol. 15, 74–78 (2000).
Kerlin, B. A., Ayoob, R. & Smoyer, W. E. Epidemiology and pathophysiology of nephrotic syndrome – associated thromboembolic disease. Clin. J. Am. Soc. Nephrol. 7, 513–520 (2012).
Holmberg, C., Antikainen, M., Ronnholm, K., Ala Houhala, M. & Jalanko, H. Management of congenital nephrotic syndrome of the Finnish type. Pediatr. Nephrol. 9, 87–93 (1995).
Kayali, F., Najjar, R., Aswad, F., Matta, F. & Stein, P. D. Venous thromboembolism in patients hospitalized with nephrotic syndrome. Am. J. Med. 121, 226–230 (2008).
Beins, N. T. & Dell, K. M. Long-term outcomes in children with steroid-resistant nephrotic syndrome treated with calcineurin inhibitors. Front Pediatr. 3, 104 (2015).
Tullus, K., Webb, H. & Bagga, A. Management of steroid-resistant nephrotic syndrome in children and adolescents. Lancet Child Adolesc. Health 2, 880–890 (2018).
Barbour, S. J. et al. Disease-specific risk of venous thromboembolic events is increased in idiopathic glomerulonephritis. Kidney Int. 81, 190–195 (2012).
Stabouli, S., Chrysaidou, K., Kupferman, J. C. & Zafeiriou, D. I. Neurological complications in childhood nephrotic syndrome: a systematic review. Eur. J. Paediatr. Neurol. 23, 384–391 (2019).
Zhu, H. et al. Prevalence and associated risk factors of pulmonary embolism in children and young adults with nephrotic syndrome: a Chinese large cohort study. J. Thorac. Imaging 36, 326–332 (2021).
Li, S. J., Tu, Y. M., Zhou, C. S., Zhang, L. H. & Liu, Z. H. Risk factors of venous thromboembolism in focal segmental glomerulosclerosis with nephrotic syndrome. Clin. Exp. Nephrol. 20, 212–217 (2016).
Kelddal, S., Nykjær, K. M., Gregersen, J. W. & Birn, H. Prophylactic anticoagulation in nephrotic syndrome prevents thromboembolic complications. BMC Nephrol. 20, 1–6 (2019).
Monagle, P. et al. Antithrombotic therapy in neonates and children: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 141, e737–e801 (2012).
Rovin, B. H. et al. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 100, S1–S276 (2021).
Monagle, P. et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: treatment of pediatric venous thromboembolism. Blood Adv. 2, 3292–3316 (2018).
Dufek, S. et al. Management of children with congenital nephrotic syndrome: challenging treatment paradigms. Nephrol. Dial. Transpl. 34, 1369–1377 (2019).
Dobbie, L. J., Lamb, A., Eskell, L., Ramage, I. J. & Reynolds, B. C. Thromboprophylaxis in congenital nephrotic syndrome: 15-year experience from a national cohort. Pediatr. Nephrol. 36, 1183–1194 (2021).
Carpenter, S. et al. Association of infections and venous thromboembolism in hospitalized children with nephrotic syndrome. Pediatr. Nephrol. 34, 261–267 (2019).
Gipson, D. et al. Inpatient health care utilization in the United States among children, adolescents, and young adults with nephrotic syndrome. Am. J. Kidney Dis. 61, 910–917 (2013).
Chang, J. & Lin, C. Long-term outcome of heavy proteinuria in patients under 2 years of age. Pediatr. Nephrol. 18, 1044–1048 (2003).
Citak, A., Emre, S., Sâirin, A., Bilge, I. & Nayir, A. Hemostatic problems and thromboembolic complications in nephrotic children. Pediatr. Nephrol. 14, 138–142 (2000).
Fabri, D., Belangero, V. M., Annichino-Bizzacchi, J. M. & Arruda, V. R. Inherited risk factors for thrombophilia in children with nephrotic syndrome. Eur. J. Pediatr. 157, 939–942 (1998).
Funding
The preparation of this manuscript was supported by funding from the McMaster Medical Student Research Excellence Award.
Author information
Authors and Affiliations
Contributions
K.D. and H.G. were involved with all aspects of data collection, screening, analysis, and writing of the manuscript. Y.X. and J.C. were involved with the data collection and analyses and the writing and revision of the manuscript. S.S. assisted with the development of the search strategy and data collection. M.B. and A.K.C.C. provided their input on the data analysis and manuscript revision. R.C. supervised all aspects of this project from inception to manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dadgar, K., Xue, Y., Chung, J. et al. Childhood nephrotic syndrome and the clinical profile of thromboembolism: a systematic review and meta-analysis. Pediatr Res 93, 1463–1469 (2023). https://doi.org/10.1038/s41390-022-02302-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02302-6
- Springer Nature America, Inc.