Abstract
Background
Echocardiographic determination of the right ventricular end-systolic remodeling index (RVES RI) has clinical value for the assessment of pulmonary hypertension (PH) in adults. We aim to determine RVES RI values in pediatric PH and to correlate RVES RI data with echocardiographic variables and NYHA functional class (FC).
Methods
Prospective echocardiography study in 49 children with PH. The 49 matched control subjects were chosen from 123 healthy children used to construct pediatric normal reference values. The associations with invasive hemodynamic variables were also investigated in a validation cohort of 12 PH children and matched controls.
Results
RVES RI was increased in children with PH vs. healthy controls (1.45 ± 0.16 vs. 1.16 ± 0.06; p < 0.01; confirmed in the validation cohort). RVES RI was associated with invasive hemodynamic variables, i.e. the mean pulmonary artery pressure. RVES RI values increased with worsening NYHA-FC. The highest RVES RI values were observed in PH children with NYHA FC 3 (1.60 ± 0.12).
Conclusions
RVES RI is a useful indicator of RV remodeling and dilation in the setting of increased RV pressure load, especially when the degree of regurgitation of the tricuspid and pulmonary valves is insufficient to numerically estimate RV systolic pressure and mPAP, due to incomplete Doppler envelopes.
Similar content being viewed by others
Introduction
Right ventricular (RV) volume and/or pressure overload causes not only cardiac remodeling but also systolic and diastolic ventricular dysfunction of the RV.1,2 An accurate assessment of RV size and function is critically important for guiding treatment and follow-up in a number of pediatric cardiovascular conditions, e.g., pulmonary hypertension (PH).3,4,5,6,7 Survival of patients with PH has been shown to be closely related to RV function.4,8,9 Several echocardiographic parameters, including the tricuspid annular plane systolic excursion (TAPSE), the tricuspid annular peak systolic velocity (S′), and RV end-systolic dimensions, have been shown to be associated with prognosis in children with PH.10,11 Echocardiographically measured parameters such as RV end-diastolic basal diameter or the RV end-diastolic area can easily be obtained and therefore may be used as non-invasive measures of RV size and performance in children.12 A simple index that would incorporate both the longitudinal component of RV adaptation and the end-systolic dimension would add significant information of RV behavior in pediatric PH. The novel RV end-systolic remodeling index (RVES RI), defined as the ratio of end-systolic RV free wall longitudinal/septal length, has been demonstrated to be a simple reproducible prognostic marker in adults with PH.13 With increasing pressure afterload, the RV remodeling progresses from physiological RV hypertrophy to maladaptation/RV dilation. During this process, echocardiographic and hemodynamic RV parameters, including RVES RI as a surrogate of RV remodeling, are expected to worsen, but pediatric experience is sparse.
The aim of this study was first to determine reference values for RVES RI for children and to assess correlations between this new index and conventionally used echocardiographic parameters in children with PH.14,15 We hypothesize that RVES RI provides easily obtainable, comprehensive information on RV volume and shortening in children with PH and is associated with invasive and echocardiographic variables altered in RV pressure overload.
Materials and methods
Healthy control study group
Our healthy control study group consisted of 123 children without any significant health condition (67 males; 56 females, age range: 1 day to 18 years (median: 5.7 years), body weight (BW): 2.6 kg to 76.0 kg; body surface area (BSA): 0.19–1.99 m2, neonates (n = 11), infants (n = 19), children (1–10 years; n = 47), and adolescents (11–18 years; n = 45), Table 1). For the comparison with the PH study group, 49 healthy subjects were matched according to sex and age (0–<1, 1–<4, 4–18 years) to patients with PH. These 49 healthy subjects had comparable BW and BSA when compared to the 49 children with PH. The control subjects were recruited prospectively from healthy children referred to our cardiology service for evaluation of a heart murmur or a family history of heart disease. All patients with congenital heart disease (CHD) such as pulmonary stenosis, acquired heart diseases, or chromosomal syndromes were excluded. Patients with patent ductus arteriosus were also excluded because ductal left-to-right shunt flow causes turbulence within the pulmonary artery (PA) and may therefore influence volume-dependent measurements. The normal pulsed wave (PW) PA Doppler profile is smooth, without “notching” of the Doppler envelope. A PA Doppler notch can be associated with increased pulmonary vascular resistance (PVR) in PH patients,16 and therefore patients with a “notched” Doppler envelope were excluded from analysis of the healthy cohort. In line with our clinical experience, adequate RVES RI measurements were possible in 98% of the study echocardiograms. Patients were examined in a resting state. In our healthy cohort, we confirmed that all children had a normal left ventricular ejection fraction (LVEF), a normal age-related mitral annular plane systolic excursion,17 normal pulmonary artery acceleration time (PAAT),18 a normal RV size,19 and normative TAPSE and RV tricuspid annular peak systolic velocity (S′).20,21
PH study group
The PH study group from Graz consisted of 49 children with PH as defined by a mPAP >20 mm Hg, i.e., 22 children with pulmonary arterial hypertension (PAH) associated with congenital heart disease (PAH-CHD),22 15 patients with PH associated with bronchopulmonary dysplasia (PH-BPD),23 and 12 patients with idiopathic PAH (IPAH), (median age: 5.3 years; age range 1.5 months to 18.0 years; 30 males, 19 females; BSA: 0.20–1.92 m2, Table 2. At the time of enrollment, all patients had been clinically stable without change of medications within the preceding 4 months. For the PAH-CHD subgroup, inclusion criteria were status post repair of a post-tricuspid left-to-right shunt such as ventricular septal defects (VSDs) and atrio-ventricular septal defects; patients with significant residual cardiovascular shunt (Qp/Qs >1.5) or isolated atrial septal defect were excluded. All patients in the IPAH and PAH-CHD subgroups underwent cardiac catheterization prior to enrollment, thereby excluding any significant intracardiac shunt. Patients with acquired heart diseases or chromosomal syndromes were excluded from this study. Patients with persistent patent ductus arteriosus and with significant postoperative (residual) intracardiac shunt were also excluded because the left-to-right shunt flow may influence the RV morphology.
The respective CHDs were surgically repaired in all patients at a mean age of 5.7 months (range: 0.3–14.2 months). All of these patients were status post complete repair patients without significant shunts who have persistent PH. PH was defined as a mean pulmonary artery pressure (mPAP) ≥ 20 mm Hg at rest (definition by the WSPH 2018), a pulmonary capillary wedge pressure ≤15 mm Hg, and a PVR >3 mm WU·m2 [iWU].24 By directly measuring pressures and indirectly measuring flow, we determined the cardiac output (Qs) (using Fick’s principle), mixed venous oxygen saturation, and mPAP (Table 2).
All PH-BPD patients had at least measurable mild-to-moderate tricuspid regurgitation (TR) so that TR jets could be well interrogated with continuous wave (CW)-Doppler. The RV systolic pressure was estimated by applying the modified Bernoulli equation.25 A TR velocity >2.8 m/s was considered a reasonable cut-off to define elevated pulmonary pressure in the absence of pulmonary stenosis.26
Validation cohort
In order to validate our findings, including the cut-off value to distinguish PH from non-PH condition, we conducted a blinded image analysis of echocardiograms prospectively obtained at the tertiary University Children’s Hospital at Hannover Medical School. The validation cohort consisted of 12 pediatric PH patients (5 IPAH, 4 PAH-CHD, 3 PH-associated lung disease) and 9 age-matched, non-PH controls. All subjects underwent echocardiography at the day of admission at the Children’s Hospital, Hannover Medical School and cardiac catheterization the next day (Table 3). The corresponding DICOM echocardiographic image clips of full studies were sent to the study center in Graz where the images were (re-)analyzed in a blinded fashion, in order to limit selection bias and interobserver variability.
Image acquisition
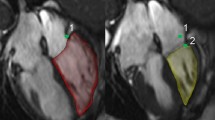
To minimize variability, a strict institutional protocol for image acquisition was used for this prospective study. Age, BW, BL, and BSA were measured at the time of echocardiography, and the BSA was calculated using the Mosteller formula.27 The echocardiographic measurement of the RVES RI in children is shown in Fig. 1.
a Nine-year-old child with PH related to congenital heart disease (CHD). The right ventricular end-systolic remodeling index (RVES RI) represents a simple ratio of end-systolic lateral height:septal height (representing RV longitudinal length measured straight from the septal tricuspid annulus point to the RV insertion on the interventricular septum). The green line marks the septal height, the red line the lateral height. b Healthy nine-year-old child with normal RV size and function. RA right atrium, RV right ventricle. The RVES RI was measured at end systole, which was defined as the time frame preceding tricuspid valve opening, showing the minimal RV area (in most instances, the time of the end of the T wave).
Echocardiographic protocol
Echocardiograms were performed using a commercially available echocardiographic system (Sonos iE33, Philips, Andover, MA, USA) using transducers of 5-1, 8-3, and 12-4 MHz depending on patient age, size, and weight. Images were recorded digitally and analyzed using the off-line software (Xcelera Echo; Philips Medical Systems, The Netherlands). All measures were averaged over three cycles and analyzed according to the latest guidelines by two blinded certified readers.28 Indices of RV size and function were measured on the focused RV apical four-chamber view. In seven cases, echocardiography was performed the day before the cardiac catheterization; in one case, the echocardiography was conducted 7 days prior to the invasive catheterization study; and in all other cases, transthoracic echocardiography was performed on the same day as the cardiac catheterization study.
RVES remodeling index
RVES RI is defined as the ratio of end-systolic RV free wall longitudinal length:septal height (RVES RI = end-systolic lateral free wall length/septal height).13 Internal scaling free wall length to septal length rather was shown to have the advantage of scaling using the same dimension (dimensional consistency) and being less influenced by edematous state or obesity.13 The lateral wall length was measured from the lateral tricuspid annulus to the insertion point of the RV on the interventricular septum. The septal length (representing the RV length at the level of the septum) was measured as a straight line from the septal tricuspid annulus to the RV insertion on the interventricular septum. The RVES RI was measured at end systole, which was defined as the time frame preceding tricuspid valve opening, showing the minimal RV area (in most instances, the time of the end of the T wave). While in the original adult study protocol13 an exact endocardial tracking approach was used (more curvy line), we preferred a more straight approach to measure the lateral RV wall, thereby ignoring certain trabeculations of the RV.
We measured the PAAT as the interval between the onset of ejection and the peak PA flow velocity in accordance with previous studies, acknowledging existing pediatric reference values.18
The TAPSE reflects the longitudinal excursion of the tricuspid annulus toward the apex and is measured with M mode in the apical four-chamber view.20
PW tissue Doppler imaging was performed using transducer frequencies of 2.5–3.5 MHz with spectral Doppler filters adjusted until a Nyquist limit of 15–20 cm/s. The tricuspid annular peak systolic velocity (S′) was recorded and analyzed off-line.21
The ratio of RV diameter to LV diameter is measured from a two-dimensional parasternal short axis image at the LV papillary muscle level as the ratio of the anterior-posterior dimension to the RV and LV diameters at end systole. The RV/LV ratio was derived to combine a measure of RV size with septal shift secondary to elevated end-systolic RV pressure.29
The left ventricular eccentricity index (LVEI) is the ratio of the minor axis of the LV parallel to the septum divided by the minor axis perpendicular to the septum.29
The estimation of systolic PAP (PASP) is based on the peak velocity of the jet of tricuspid regurgitation (TRV). The simplified Bernoulli equation and CW-Doppler is used to assess velocity within the TRV jet: right ventricular systolic pressure [(RVSP) = PASP = 4 × (TRV)2 + right atrial (RA) v wave = approx. 4 × (TRV)2 + mean right atrial pressure (mRAP)]. Thus the CW-Doppler-derived TRV is a surrogate of RVSP and PASP, in the absence of an RV outflow tract obstruction or pulmonary artery stenosis. The time intervals between cardiac catheterization and echocardiography was <12 h in most cases (n = XX), 12–24 h in X cases, and 7 days in 1 case. All measurements were taken by 3 well-trained observers, reviewing 3–5 consecutive beats, and data were averaged as previously recommended.12
Invasive hemodynamic assessment
Cardiac catheterization was performed through the internal jugular or right femoral vein. RAP, systolic pulmonary arterial pressure, mPAP, PVR/SVR ratio, and pulmonary vascular resistance index (PVRi) were determined at both centers.
Statistics
Reliability of both the interobserver and intraobserver measurements (measurements of 15 children and 30 observations were included) were analyzed by a random effects model analysis of variance (intraclass correlation coefficient (ICC). Data are presented as mean and SD for normally distributed continuous variables and median (interquartile range (IQR)) when the distribution was skewed. Categorical variables are presented as absolute and relative frequencies. Differences between children with PH and age- and sex-matched healthy children were analyzed using independent t Test. The ability of RVES RI values to identify children with PH was analyzed. Therefore, sensitivity, specificity, negative predictive value, and positive predictive value for a cut-off score of mean + 2 SDs was calculated.
Receiver-operating characteristic curves (ROCs) were used to calculate the best cut-off score. Area under the curve (AUC) and corresponding 95% confidence intervals (CIs) were calculated. Therefore, Youden Index was calculated, which maximize sensitivity and specificity. A validation cohort was used to evaluate the ability of this “best cut-off” to distinguish between PH patients and controls (here: non-PH patients) in a different cohort. ROC analysis was also performed for the ability of RVES RI to identify patients with a PVRi >6 WU·m2. Correlations were performed using Spearman’s rank correlation coefficient or Pearson’s correlation when appropriate. Correlation coefficients were calculated for the whole group and PH subgroups separately. A p value <0.05 was considered to be statistically significant. The statistical analyses were performed using IBM SPSS Statistics 24.0.0 (IBM Corporation; Armonk, USA).
Results
Control subjects data
In the 123 healthy children and adolescents studied (Table 1), the mean RVES RI was found to be 1.16 ± 0.06. The RVES RI did not correlate with increasing age (r = −0.082, p = 0.369), BL (r = −0.048, p = 0.599), or BSA (r = −0.077, p = 0.396) of our healthy subjects. The lower bound of pediatric normal reference values (−2 SD) was found to be 1.04, the upper bound 1.28.
PH subject data
In our PH pediatric patients studied, the mean RVES RI (1.45 ± 0.16) values were significantly higher compared to 49 age- and sex-matched healthy controls (p < 0.001) chosen from 123 healthy normal subjects (Table 2). PH-BPD patients showed significant lower RVES RI values (1.37 ± 0.10) compared to iPAH patients (1.52 ± 0.15; p = 0.001) and PH-CHD patients (1.46 ± 0.17; p = 0.018). Intraobserver and interobserver reliability for RVES RI was high (ICCintra: 0.83, 95% CI: 0.65–0.92; ICCinter: 0.93, 95% CI: 0.87–0.97).
Validation cohort
In order to validate our findings, including the cut-off value to distinguish PH from non-PH condition, we conducted a blinded image analysis of echocardiograms prospectively obtained at the tertiary University Children’s Hospital at Hannover Medical School. The validation cohort consisted of 12 pediatric PH patients (5 IPAH, 4 PAH-CHD, 3 PH-associated lung disease) and 9 age-matched, non-PH controls. All subjects underwent echocardiography at the day of admission at the Children’s Hospital, Hannover Medical School, and cardiac catheterization the next day (Table 3). The corresponding DICOM echocardiographic image clips of full studies were sent to the study center in Graz where the images were (re-)analyzed in a blinded fashion, in order to limit selection bias and interobserver variability.
Correlations with RV systolic function variables
RVES RI values in the PH group negatively correlated with the RV systolic function parameters TAPSE (r = −0.487, p < 0.001; subgroup analysis: IPAH, r = −0.765; PH-BPD, r = −0.366; PAH-CHD: r = −0.705), with the S′ (r = −0.471, p = 0.001; subgroup analysis: IPAH, r = −0.752; PH-BPD, r = −0.355; PAH-CHD: r = −0.507) and with the PAAT (r = −0.343, p < 0.016; subgroup analysis: IPAH, r = −0.841; PH-BPD, r = −0.239; PAH-CHD: r = −0.240). We used as cut-off point an RVES RI of >+2 SD to investigate whether >+2 RVES RI values correctly indicates PH in children. Using this cut-off score (>+2.0), 44 of the 49 PH children were identified as having PH (sensitivity: 89.8%, specificity: 95.9%, positive predictive value: 95.7%, negative predictive value: 90.4%; Fig. 2).
ROC analysis
ROC analysis for detecting PH patients showed an excellent performance of the RVES RI (p < 0.001, AUC: 0.980, 95% CI: 0.958–1.000) with a best cut-off score—according to Youden Index—of 1.28 (false negative: IPAH: n = 1, PH-BPD: n = 2, PAH-CHD: n = 2; Fig. 3). Applying ROC analysis to the validation cohort resulted in a slightly lower AUC (p = 0.002, AUC: 0.898, 95% CI: 0.767–1.000). For the detection of PH patients with PVRi >6 WU, the AUC was smaller (p < 0.001, AUC: 0.832, 95% CI: 0.706–0.958) with the best cut-off score—according to Youden Index—of 1.39 (sensitivity: 0.91, specificity: 0.63; Fig. 4). To detect PH patients with PVRi >3–<6, the AUC is 0.777 (95% CI: 0.625–0.929), the best cut-off score is: >1.39; sensitivity: 73.5%; and the number of patients with PVRi ≤3 is so small (n = 3) that no specificity is calculated.
Correlations with RV size and variables
RVES RI positively correlated with the RV/LV end-systolic diameter ratio (r = 0.586; p < 0.001; subgroup analysis: IPAH, r = 0.445; PH-BPD, r = 0.595; PAH-CHD: r = 0.593) as a surrogate of RV dilation. The RVES RI also correlated positively with the LVEI (r = 0.633; p < 0.001; subgroup analysis: IPAH, r = 0.446; PH-BPD, r = 0.598; PAH-CHD: r = 0.669) as a surrogate of LV underfilling and end-systolic compression (“D-sign”).
Correlations with NYHA functional class (FC)
In the 49 pediatric PH patients the RVES RI values increased with worsening NYHA FC (p = 0.002). The highest RVES RI values were observed in children and adolescents with PH with NYHA FC 3 (n = 7; 1.60 ± 0.12), followed by NYHA FC 2 (n = 18, 1.48 ± 0.12) and NYHA FC 1 (n = 17; 1.37 ± 0.12). An NYHA category analysis revealed that children with NYHA FC 1 had significant lower RVES RI values compared to children with NYHA FC 2 (p = 0.026) and lower RVES RI versus children with NYHA FC 3 (p = 0.001). For NYHA FC 4, no subgroup analysis was performed owing to the small sample sizes.
Correlations with hemodynamic variables
The RVES RI values correlated with the ratio of systemic pulmonary artery pressure (sPAP)/systolic systemic arterial pressure (sSAP; r = 0.724; p < 0.001; subgroup analysis: IPAH, r = 0.898; PH-BPD, r = 0.249; PAH-CHD: r = 0.681) and fairly well with mPAP (r = 0.464, p < 0.004; subgroup analysis: IPAH, r = 0.695; PH-BPD, r = 0.438; PAH-CHD: r = 0.404) and the PVRi (r = 0.579, p < 0.001; subgroup analysis: IPAH, r = 0.535; PH-BPD, r = 0.021; PAH-CHD: r = 0.806), determined by cardiac catheterization.
Discussion
To the best of our knowledge, this is the first study demonstrating that the RVES RI is increased in children with PH. With this study, we introduce the RVES RI in clinical care of children with PH and demonstrate an association of this index with RV size and function parameters, with LV eccentricity index, with NYHA FC, and—importantly—with invasive hemodynamic variables.
We provide for the first time pediatric normative values for this parameter. Normative values are essential for a comparison of normal or potentially abnormal values in children with cardiovascular diseases, such as PH.
This new RVES RI has the advantage of simplicity and reproducibility. Compared with other remodeling indices such as the RV sphericity, the RVES RI has been shown to be more predictive of outcome in adult PAH.13 It is likely that the RVES RI is dependent not only on RV dimensions but also on longitudinal systolic RV function. RV end-systolic dimensions provide information on both size and function. In the children with PH studied here, RVES RI values were significantly increased compared to healthy control subjects. ROC analysis for detecting PH patients showed an excellent performance of the RVES RI. The RVES RI correlated with echocardiographic variables of systolic RV function (e.g., TAPSE), and invasive hemodynamics (e.g., PVRi) in the 49 pediatric PH patients.
Echocardiography is non-invasive and easily obtainable also in small children, therefore allowing for repetitive management and longitudinal follow-up. In pediatric PH associated with CHD, the RV longitudinal function parameters TAPSE and tricuspid annular peak systolic velocity (S′) have been shown to be significantly impaired, with a constant decrease of the S′ value with disease progression.7 In our pediatric PH cohort, TAPSE and S′ in TDI did inversely correlate with RVES RI, a finding that could be confirmed in our external validation cohort. Of note, children with PH were found to maintain normal TAPSE values early in the disease course, except when PH occurred or persisted after repair of CHD.30
The RV/LV end-systolic dimension ratio was derived to combine a measure of RV size with septal shift secondary to elevated RV pressure, in the parasternal short axis view.7 We previously showed that children with more severe PH had increased RV/LV and right-to-left atrial dimension ratios.31 When stratified by using NYHA FC, sicker PH children had greater RV and right atrial dimensions with lower exercise capacity, while the TAPSE decreased.31 This enhances the need for multi-parametric approaches such as including information of longitudinal RV behavior and end-systolic size. In the pediatric PH cohort under study, the RV/LV ratio positively correlated with RVES RI. The RVES RI was associated with lower FC and worse hemodynamics, demonstrating that this index adds information on ventricular–ventricular interaction. Similar results were found in our validation cohort. Therefore, the new RVES RI can add information on RV deformation that may proof to be superior to the conventionally used echocardiographic variables in future larger studies.
The abnormal pressure elevation in the RV with respective flattening of the interventricular septum leads to the D-shaped appearance of the LV with reduced volumes but preserved global systolic function.7 An LV eccentricity index >1 has been shown to be prognostic in adult IPAH patients who also had a decreased RV systolic function.32 We found the LVEI to positively correlate with the RVES RI, indicating that RV deformation acts in combination with RV/LV interaction.
RVES RI positively correlated to NYHA FC of our children with PH and in the validation cohort, suggesting a clinical relevance of this index in follow-ups of children with PH.
RVES RI values in our children with PH positively correlated with hemodynamic variables such as the ratio of sPAP/sSAP (r = 0.724; r < 0.001) and with PVRi (r = 0.579, p > 0.001). We found only a moderate correlation of the index to the mean PAP (r = 0.464, p < 0.004), which might be explained mainly due to the fact that the RVES RI is a RV remodeling/size parameter and a sufficient high correlation to the mean pressure (measured in the pulmonary artery) would obviously be behind the possibilities of this simply echocardiographic index. The RVES RI also showed a good performance in differentiating patients with a PVRi >6 mm WU and patients with a PVRi <6 mm WU (AUC: 0.832, 95% CI: 0.706–0.958), a finding that could be confirmed in the Hannover validation cohort. This demonstrates that the RVES RI increases with increasing afterload in pediatric PH and, as such, makes the RVES RI potentially interesting as a follow-up parameter. As RVES RI integrates ventricular enlargement, it can be questioned whether this index would be validated in other conditions, such as in children with tetralogy of Fallot, in which the range of RV dilation might be lower.
Limitations in this study
The etiological heterogeneity of the pediatric PH group, containing 22 patients with associated, repaired CHD, IPAH, and PH-BPD, drives probably the variance of the data. In patients with PAH-CHD s/p surgical repair, the structure and shape of the RV and the interventricular septum are affected by, for example, VSDs, patches, and/or an abnormal RV outflow tract. These morphological aspects may limit the use of such index in patients with PAH-CDH. Moreover, the PH-BPD group is different from the two other PH groups, as PH was diagnosed occurring earlier in life and may improve with growth and lung maturation. Of note, longitudinal data of the RVES RI, concerning medium- and long-term outcome of our pediatric PH patients, are missing in our study design. Echocardiographic determination of the RVES RI has clinical value for the assessment of PH in adults, but when we started this study only few data were available for the pediatric patients. Therefore, at this time point we could not hypothesize how large a difference between healthy children and children with PH would be. Since the group of pediatric patients with PH is a small group, we used all available data of our center and compared it to a matched group of healthy children. Therefore, we asked our ethic committee to get the allowance to include all available patients and got the permission to include up to 55 patients. Owing to the mentioned reasons, no formal sample size calculation was performed.
Conclusion
The RVES RI, defined as the ratio of end-systolic RV free wall longitudinal/septal length, is a valuable addition to the non-invasive surrogate markers of PAH and associated RV dysfunction and particularly important in children with PH who often have only mildly dilated RVs with high mPAP and thus often only mild TR with incomplete TRV envelopes, making estimation of RVSP unreliable. We suggest including the RVES RI in echocardiographic protocols when evaluating children with suspected PH.
References
Grapsa, J. et al. Right ventricular remodeling in pulmonary arterial hypertension with three-dimensional echocardiography: comparison with cardiac magnetic resonance imaging. Eur. J. Echocardiogr. 11, 64–73 (2010).
Vonk Noordegraaf, A. & Galiè, N. The role of the right ventricle in pulmonary arterial hypertension. Eur. Respir. Rev. 20, 243–253 (2011).
Hansmann, G. Pulmonary hypertension in infants, children, and young adults. J. Am. Coll. Cardiol. 69, 2551–2569 (2017).
Vonk Noordegraaf, A., Haddad, F., Bogaard, H. J. & Hassoun, P. M. Noninvasive imaging in the assessment of the cardiopulmonary vascular unit. Circulation 131, 899–913 (2015).
Hansmann, G. et al. Executive summary. Expert consensus statement on the diagnosis and treatment of paediatric pulmonary hypertension. The European Paediatric Pulmonary Vascular Disease Network, endorsed by ISHLT and DGPK. Heart 102(Suppl 2), ii86–ii100 (2016).
Lammers, A. et al. Diagnostics, monitoring and outpatient care in children with suspected pulmonary hypertension/pediatric pulmonary hypertensive vascular disease. Expert Consensus Statement on the Diagnosis and Treatment of Paediatric Pulmonary Hypertension—The European Paediatric Pulmonary Vascular Disease Network, endorsed by ISHLT and DGPK. Heart 102, 1–13 (2016).
Koestenberger, M., Apitz, C., Abdul-Khaliq, H. & Hansmann, G. Transthoracic echocardiography for the evaluation of children and adolescents with suspected or confirmed pulmonary hypertension. Expert consensus statement on the diagnosis and treatment of paediatric pulmonary hypertension. The European Paediatric Pulmonary Vascular Disease Network, endorsed by ISHLT and DGPK. Heart 102(Suppl 2), ii14–ii22 (2016).
McLaughlin, V. V. et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation 119, 2250–2294 (2009).
Galiè, N. et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Heart J. 37, 67–119 (2016).
Fine, N. M. et al. Outcome prediction by quantitative right ventricular function assessment in 575 subjects evaluated for pulmonary hypertension. Circ. Cardiovasc. Imaging 6, 711–721 (2013).
Ryo, K. et al. Characterization of right ventricular remodeling in pulmonary hypertension associated with patient outcomes by 3-dimensional wall motion tracking echocardiography. Circ. Cardiovasc. Imaging 8, e003176 (2015).
Rudski, L. G. et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 23, 685e713 (2010).
Amsallem, M. et al. Right heart end-systolic remodeling index strongly predicts outcomes in pulmonary arterial hypertension: comparison with validated models. Circ. Cardiovasc. Imaging https://doi.org/10.1161/CIRCIMAGING.116.005771 (2017).
Koestenberger, M. et al. Diagnostics in children and adolescents with suspected or confirmed pulmonary hypertension. Paediatr. Respir. Rev. 23, 3–15 (2017).
Koestenberger, M. et al. Transthoracic echocardiography in the evaluation of pediatric pulmonary hypertension and ventricular dysfunction. Pulm. Circ. 6, 15–29 (2016).
Arkles, J. S. et al. Shape of the right ventricular Doppler envelope predicts hemodynamics and right heart function in pulmonary hypertension. Am. J. Respir. Crit. Care Med. 183, 268–276 (2011).
Koestenberger, M. et al. Left ventricular long-axis function: reference values of the mitral annular plane systolic excursion in 558 healthy children and calculation of z-score values. Am. Heart J. 164, 125–131 (2012).
Koestenberger, M. et al. Normal reference values and z scores of the pulmonary artery acceleration time in children and its importance for the assessment of pulmonary hypertension. Circ. Cardiovasc. Imaging https://doi.org/10.1161/CIRCIMAGING.116.005336 (2017).
Koestenberger, M. et al. Reference values and calculation of z-scores of echocardiographic measurements of the normal pediatric right ventricle. Am. J. Cardiol. 114, 1590–1598 (2014).
Koestenberger, M. et al. Right ventricular function in infants, children and adolescents: reference values of the tricuspid annular plane systolic excursion (TAPSE) in 640 healthy patients and calculation of z score values. J. Am. Soc. Echocardiogr. 22, 715–719 (2009).
Koestenberger, M. et al. Reference values of tricuspid annular peak systolic velocity in healthy pediatric patients, calculation of Z score, and comparison to tricuspid annular plane systolic excursion. Am. J. Cardiol. 109, 116–121 (2012).
Kozlik-Feldmann, R. et al. Pulmonary hypertension in children and adolescents with congenital heart disease (PAH-CHD, PPHVD-CHD). Expert Consensus Statement on the Diagnosis and Treatment of Pediatric Pulmonary Hypertension—The European Paediatric Pulmonary Vascular Disease Network, endorsed by ISHLT and DGPK. Heart 102, 42–48 (2016).
Hilgendorff, A., Bonnet, D., Apitz, C. & Hansmann, G. Pulmonary hypertension associated with acute or chronic lung diseases in the preterm and term neonate. Expert Consensus Statement on the Diagnosis and Treatment of Pediatric Pulmonary Hypertension—The European Paediatric Pulmonary Vascular Disease Network, endorsed by ISHLT and DGPK. Heart 102, 49–56 (2016).
Rosenzweig, E. B. et al. Paediatric pulmonary arterial hypertension: updates on definition, classification, diagnostics and management. Eur. Respir. J. 53(1), 1801916 (2019).
Yock, P. G. & Popp, R. L. Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation 70, 657–662 (1984).
McQuillan, B. M., Picard, M. H., Leavitt, M. & Weyman, A. E. Clinical correlates and reference intervals for pulmonary artery systolic pressure among echocardiographically normal subjects. Circulation 104, 2797–2802 (2001).
Mosteller, R. Simplified calculation of body-surface area. N. Engl. J. Med. 317, 1098 (1987).
Lang, R. M. et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 28, 1.e14–39.e14 (2015).
Friedberg, M. K. Imaging right-left ventricular interactions. JACC Cardiovasc. Imaging 11, 755–771 (2018).
Hauck, A., Guo, R., Ivy, D. D. & Younoszai, A. Tricuspid annular plane systolic excursion is preserved in young patients with pulmonary hypertension except when associated with repaired congenital heart disease. Eur. Heart J. Cardiovasc. Imaging 18, 459–466 (2017).
Koestenberger, M. et al. Ventricular-ventricular interaction variables correlate with surrogate variables of clinical outcome in children with pulmonary hypertension. Pulm. Circ. 9, 2045894019854074 (2019).
Ghio, S. et al. Prognostic relevance of the echocardiographic assessment of right ventricular function in patients with idiopathic pulmonary arterial hypertension. Int. J. Cardiol. 140, 272–278 (2010).
Acknowledgements
G.H. currently receives grant support from the German Research Foundation (DFG; KFO 311 HA 4348/6-1).
Author information
Authors and Affiliations
Contributions
Each author listed below has seen this Research Article and approved the submission of this manuscript and takes full responsibility for the manuscript. M.K. is the lead author of this study, made substantial contributions to the design and content of this study, and wrote the manuscript. A.A. made substantial contributions to the acquisition and analysis of the data of this article. P.C. delivered the data analysis of the validation cohort study and revised the manuscript for important intellectual content. A.G., S.S., and S.K.-K. made substantial contribution to the conception and of the drafting of this manuscript. A.A. and M.C. made substantial contributions to the analysis of the data. G.C. revised this manuscript for important intellectual content. D.H. made substantial contributions to the acquisition of the data. G.H. made a critical revision of this article and gave final approval of the version of this article.
Corresponding authors
Ethics declarations
Competing interests
The authors of this manuscript declare no relationship with any companies whose products or services may be related to the topic of the article. The authors declare no conflict of interest. This article resulted from a collaborative project of the European Pediatric Pulmonary Vascular Disease Network, Berlin (www.pvdnetwork.org).
Ethics
Subjects or their parents have given their written informed consent. This study complies with all institutional guidelines related to patient confidentiality and research ethics including institutional review board approval of the local Graz Ethics Board (IRB #29-588). The validation cohort study was approved by the ethics committee of Hannover Medical School (IRB #2200). All cardiac catheterizations and echocardiograms were clinically indicated. Informed consent for study participation was obtained from the legal caregivers.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Koestenberger, M., Avian, A., Chouvarine, P. et al. Right ventricular end-systolic remodeling index in the assessment of pediatric pulmonary arterial hypertension. The European Pediatric Pulmonary Vascular Disease Network (EPPVDN). Pediatr Res 88, 285–292 (2020). https://doi.org/10.1038/s41390-020-0748-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-0748-2
- Springer Nature America, Inc.
This article is cited by
-
Pulmonary hypertension in bronchopulmonary dysplasia
Pediatric Research (2021)