Abstract
Neonatal respiratory failure is a common and serious clinical problem which in a considerable proportion of infants requires invasive mechanical ventilation. The basic goal of mechanical ventilation is to restore lung function while limiting ventilator-induced lung injury, which is considered an important risk factor in the development of bronchopulmonary dysplasia (BPD). Over the last decades, new conventional mechanical ventilation (CMV) modalities have been introduced in clinical practice, aiming to assist clinicians in providing lung protective ventilation strategies. These modalities use more sophisticated techniques to improve patient-ventilator interaction and transfer control of ventilation from the operator to the patient. Knowledge on how these new modalities work and how they interact with lung physiology is essential for optimal and safe use. In this review, we will discuss some important basic lung physiological aspects for applying CMV, the basic principles of the old and new CMV modalities, and the evidence to support their use in daily clinical practice.
Similar content being viewed by others
Introduction
Neonatal respiratory failure is a common and serious clinical problem associated with high morbidity and mortality.1 Despite the increasing use of non-invasive respiratory support modalities, a considerable proportion of preterm infants still require invasive mechanical ventilation at some point during their admission.2,3 The primary goal of invasive mechanical ventilation is to correct the compromised lung function, to restore adequate gas exchange, and reduce the work of breathing.
Unfortunately, invasive mechanical ventilation also has adverse effects, with ventilator-induced lung injury (VILI) being the most important one.4 Animal studies have identified the most important risk factors for VILI:1 volutrauma: end-inspiratory lung overdistension mostly caused by using high tidal volumes (VT);2 atelectotrauma: repetitive opening and collapse of unstable lung units and regional overdistension due to redistribution of the applied VT;3 oxygen toxicity: oxidative stress in an environment of reduced anti-oxidant capacity. It has been shown that VILI is enhanced by surfactant dysfunction and lung inflammation.5,6
VILI is considered one of the major risk factors for development of bronchopulmonary dysplasia (BPD).7 For this reason, applying a lung protective ventilation strategy during invasive mechanical ventilation has been one of the important challenges for neonatologists. Ideally, such a strategy would avoid the use of high VT, reverse atelectasis, stabilize lung units during both in- and expiration, and avoid the use of high concentrations of oxygen.8 Over the last decades, several new ventilation modes have been developed and introduced in clinical practice to assist the clinician in applying a lung protective ventilation strategy. These modes differ in their characteristics and complexity, and how they interact with basic lung physiology. In this review, we will discuss some basic lung physiology important for conventional mechanical ventilation (CMV), the basic principles of the currently available CMV modalities, and the evidence to support their use in daily clinical practice.
Physiological principles to consider during mechanical ventilation
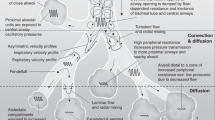
Understanding basic lung physiology and the (compromising) effect of the underlying lung disease is essential when selecting the optimal ventilation mode and setting.
Compliance of the respiratory system in newborn infants is defined by the change in lung volume per change in transpulmonary pressure.9 In case of restrictive lung disease, compliance is reduced, which means that a higher transpulmonary pressure is needed to deliver the desired VT. During CMV, this requires a higher driving pressure, defined as the difference between peak inflation pressure (PIP) and positive end-expiratory pressure (PEEP). Ideally compliance is measured during a passive state, i.e. with no contribution of the patients’ breathing effort. However, in clinical practice most infants breathe spontaneously during CMV, and under these conditions, the measured volume change per pressure change is referred to as dynamic compliance.
The chest wall compliance in newborn infants is relatively high, and as such is less able to counteract the elastic recoil forces of the lung.10 As a result, the end-expiratory lung volume (EELV) tends to decrease, especially if compliance is low. The newborn infant tries to preserve EELV by closing or narrowing the glottis and the upper airways during spontaneous breathing. Following intubation, this control of EELV will be lost and needs to be replaced by sufficient PEEP. Failing to do so will result in a low EELV due to alveolar collapse, with serious physiological consequences, such as an increase in intrapulmonary right-to-left shunt leading to hypoxemia, a decrease in compliance, an increase in pulmonary vascular resistance, and an increase in airway resistance.9
Airway resistance is defined as pressure gradient needed to move gas at a constant flow through the airways and is often increased in neonatal lung disease due to secretion in the airways or the presence of an endotracheal tube (ETT).9 It is important that the inspiratory time (TI) during CMV is long enough to yield gas equilibration at the alveolar level, and the expiratory time (TE) is adequately long to enable complete exhalation. To assist the clinician in selecting the optimal TI and TE, many ventilators now provide the so-called time constant (τ), which is defined by the product of compliance and resistance. τ describes the exponential rise and decay in VT over time during, respectively, inspiration and expiration. It has been determined that it takes four time constants to deflate 98% of the delivered VT. So, setting a TE longer than four times the expiratory τ will ensure adequate exhalation of the delivered VT.
Aerated parts of the respiratory system not involved in gas exchange are called dead space.9 Anatomical dead space consists of the (upper) airways, which means that adding an ETT and flow sensor will significantly increase dead space. This needs to be taken into account when determining the optimal VT during CMV. The alveolar dead space consists of aerated but not perfused alveoli and is mainly caused by overdistension of the lungs.
Pressure targeted ventilation
The basic principle of pressure targeted ventilation (PTV) is creating a positive pressure at the airway opening which exceeds the alveolar pressure and will result in an influx of air. Most frequently used is time-cycled, pressure-limited (TCPL) ventilation, during which a constant gas flow is provided through the patient circuit which is connected to the patient via the ETT at the Y-piece. The ventilator delivers a mechanical inflation by closing the expiration valve, thereby building up the airway pressure to the targeted PIP, which is maintained during the preset TI. The set flow rate determines the rate of pressure built up (rise time) during the inspiration phase.
At the start of expiration, the expiration valve opens and the airway pressure drops passively to the targeted PEEP. Although the flow is continuous in the patient circuit, at the Y-piece it usually has a decelerating pattern (Fig. 1).
The actual VT that is delivered during a pressure targeted inflation is variable and depends on the set driving pressure, the pulmonary mechanics, and the spontaneous effort of the infant (Fig. 2a). Low compliance and high resistance of the respiratory system will result in a lower delivered VT for a given driving pressure. Multiplying the VT with the set frequency will provide the delivered minute volume, which is the most important determinant for carbon dioxide wash-out. It is important in neonatal ventilation that the delivered VT is measured by a flow sensor positioned at the Y-piece. Measuring volume changes within the ventilator provides inaccurate VT because of the volume lost in the patient circuit. In the presence of ETT leak, the expiratory VT is more accurate than the inspiratory VT.
Pressure (upper panel) and tidal volume (lower panel) over time during time-cycled pressure-limited ventilation without volume guarantee (a) and with volume guarantee (b). Note the automated adjustments of the peak inflation pressure during volume guarantee resulting in three out of five pressure inflation with a tidal volume on target as compared to only one inflation when volume guarantee is not activated.
Oxygenation is mainly controlled by the fraction of inspired oxygen in the delivered gas and the level of PEEP.
A second mode of PTV is pressure control ventilation (PCV), which uses a variable flow instead of a constant flow rate. The clinicians can set a rise time for the PIP and the ventilator will adjust the peak flow accordingly.
There are no magic numbers when setting the ventilator during PTV. In fact, setting the ventilator needs a patient tailored dynamic approach as the lung condition and patients’ own breathing effort change over time. The driving pressure should be set to deliver an adequate VT at the given compliance and resistance of the respiratory system. This is usually a VT varying between 4 and 7 ml/kg, depending on the anatomical and alveolar dead space.11 TI and TE (and thus frequency) will depend on the τ of the respiratory system. PEEP should be set at an appropriate level to optimize EELV and reduce intrapulmonary right-to-left shunt.
A theoretical advantage of PTV is the limitation of PIP, thereby reducing the risk of “barotrauma”. This benefit has been questioned as experimental studies have shown that VILI is caused by high VT and not so much high PIP.12 Another advantage of PTV is that, compared to volume targeted modes, it is less influenced by ETT leak. Finally, the continuous gas flow used during TCPL allows unrestricted spontaneous breathing of the infant.
A disadvantage of PTV is the variability of the delivered VT at a given driving pressure, which may increase the risk of both hypo- and hypercapnia, and lung injury due to (too) low and high VT.13,14
It is unclear if and to what extent the possible advantages and disadvantages during PTV translate to patient outcome. This is also true for the optimal settings during PTV. Most of the available research is on comparing PTV to volume-targeted modes, which will be discussed in the next paragraph.
Volume targeted ventilation
During volume targeted ventilation (VTV), the primary target is not the PIP but the delivered VT. Thereby, the risk of delivering (too) low or high VT is reduced, and this will potentially also reduce the risk of hypo- and hypercapnia, and VILI with subsequent BPD.
There are basically two modes of VTV. The first is volume controlled ventilation (VCV), which has been the long-term standard in adult intensive care. During VCV, the ventilator targets a fixed VT set by the clinician using a block wave inspiratory flow pattern (Fig. 1). In contrast to PTV, the delivered PIP is no longer fixed, but varies depending on lung mechanics and the patients’ contribution to the work of breathing. To enable delivery of a preset VT, most ventilators target the inspiratory VT measured within the ventilator. This will result in an overestimation of the volume delivered to the patient, as part of the VT will be lost in the patient circuits and—if present—through leak around the ETT.
The number of studies investigating the efficacy and safety of VCV in newborns is limited to two randomized trials. The largest of these two, comparing VCV to TCPL in infants less than 32 weeks’ gestation, showed that VCV was feasible and safe, but did not result in differences in important short-term neonatal outcomes.15 At 2-year follow-up, infants in the VCV group showed less pulmonary morbidity.16
The second more widely used mode of VTV is volume guarantee (VG) ventilation, although the nomenclature may differ depending on the ventilator. During VG ventilation, the ventilator delivers a pressure targeted inflation and measures the expired VT. If this delivered VT deviates from the set VT, the PIP is automatically adjusted on a breath-to-breath basis, in an attempt to match the set VT as much as possible (Fig. 2b). The maximum level to which the ventilator can escalate the PIP is set by the clinician. Some ventilators differentiate between triggered and non-triggered mechanical inflations when calculating the necessary adjustment of the PIP. To avoid large pressure swings and the subsequent risk of (too) low or high VT delivery, the magnitude of the incremental and decremental pressure steps is limited by the algorithm. As a result, it may take several inflations before the PIP is reached that targets the set VT.
Based on the above, it is clear that VG ventilation is a misnomer, as the delivered VT is not guaranteed but stabilized. This was also shown by a small randomized controlled trial reporting less frequent high VT inflations and hypocapnia when VG was added to PTV.13 Other short-term crossover trials showed that adding VG to PTV resulted in adequate gas exchange with lower ventilator pressures.17 Randomized trials comparing VG ventilation to pressure targeted modes have been captured in a systematic review.18 It is important to realize that this meta-analysis also contains a limited number of studies that used VCV. The results of this meta-analysis show that compared with modes of PTV, VTV reduces the combined outcome death or BPD at 36 weeks postmenstrual age, pneumothorax, and the combined outcome intraventricular hemorrhage grade 3/4 and periventricular leukomalacia.
There are a few limitations that need to be considered when using VTV in daily clinical practice. First, a large leak (>50%) around the ETT expiratory will compromise VT measurement and thus VG ventilation. Second, if the PIP limit is set too low, the ventilator may not be able to increase the PIP to match the set VT.19 This will result in low VT ventilation with possible adverse effects on gas exchange. Finally, it is important to acknowledge that during VTV, ventilator support is inversely proportional to the breathing effort of the infant. This may result in a situation characterized by significant respiratory distress and effort by the patient while the supporting PIP is very low. These circumstances are usually caused by a VT set lower than required.11
The optimal VT during VTV is not a fixed number, but should be individualized, depending on the underlying lung disease, the patient characteristics, and the outcome of interest. Higher anatomical (flow sensor, distension of larger airways) or alveolar (BPD) dead space may justify the use of higher VT.11 From a lung injury perspective, limiting VT might be more appropriate although it is important to emphasize that using too low VT may also increase the risk of VILI, especially when combined with low levels of PEEP.14
Proportional assist modalities
The basic aim of proportional assist modalities is to compensate disease-related changes in compliance and resistance and the resulting increased work of breathing. This means that in contrast to VTV, the ventilatory support is proportional to the breathing effort of the infant. In order to provide this proportional assistance, the ventilator needs a reliable signal to assess the patients’ the breathing effort. The following signals can be used for this purpose:1 flow changes detected by a flow sensor, and2 diaphragmatic electrical activity (EAdi).
The modality proportional assist ventilation (PAV) uses volume and flow changes created by the patient to unload the supra-physiological breathing effort.20 The measured changes in (spontaneous) inspired volume are used to unload elastic work of breathing during inspiration and changes in airway flow to unload inspiratory and expiratory resistive work of breathing. This way the driving pressure is applied proportional to the spontaneous breathing effort allowing the patient to have full control of amplitude (i.e driving pressure) and timing (i.e. TI and TE) of each breath. The so-called gain set by the clinician determines how much pressure (cmH2O) is provided per unit change in volume or flow. Most ventilators allow for separate control of elastic and resistive unloading. By calculating the (disease related) decrease in compliance of the respiratory system and the added resistance of the ETT, the required gain for complete elastic and resistive unloading can be determined. It is clear that sensor malfunction due to rain out or secretions, but also ETT leak, may hamper PAV. Furthermore, PAV requires adequate respiratory drive and in its absence the patient will not be ventilated. For this reason, a more controlled back-up mode should be set in case of insufficient respiratory drive.
Although extensively studied in different animal models of lung injury, the clinical application of PAV has mainly been evaluated in (cross-over) observational studies. These studies have shown short-term benefits of PAV such as, maintaining adequate gas exchange and a lowering oxygen index at lower mean airway pressures, and improving thoraco-abdominal synchrony compared to other modes of ventilations.21,22 Randomized trials are needed to determine if these short-term benefits translate to improved patient outcome.
The modality neurally adjusted ventilatory assist (NAVA) works very similar to PAV but uses the electrical activity of the diaphragm (EAdi) as a measure of breathing effort. The EAdi signal is registered by a nasogastric tube (EAdi catheter) mounted with four electrodes placed at the level of the diaphragm (Fig. 3). It provides the diaphragmatic electrical activity needed to inhale the VT and lowest tonic electrical activity needed to maintain an adequate EELV.23 Based on the generated electrical activity of the diaphragm, the ventilator determines the driving pressure to unload work of breathing. The clinician needs to set the so-called NAVA-level, which determines how much driving pressure (cmH2O) is generated per µV of EAdi increase above the tonic activity.24 Initially a NAVA level is set that generates a similar PIP as the controlled mode used before switching to NAVA. Next the gain is adjusted in steps of 0.1–0.2 cmH2O/µV to avoid a peak EAdi < 5 µV (too much unloading) and > 15 µV (too little unloading). The EAdi signal is also used for the onset (i.e. trigger) and cycling-off of inspiration. Compared to other triggering devices, EAdi is a relatively stable signal that is less affected by movement or airflow artefacts.25 Similar to PAV, during NAVA the patient is in control of both the amplitude and the timing of ventilation. Spontaneous breathing effort is essential for successful NAVA ventilation and in its absence a controlled back-up mode should take over.
The clinical application of NAVA has mainly been assessed in cross-over observational studies in both preterm and term newborn infants. Compared with more controlled modes of ventilation, NAVA reduces the PIP, the fraction of inspiratory oxygen, and the work of breathing in infants with respiratory distress syndrome (RDS) or evolving BPD.26,27 To date, only one randomized trial compared NAVA to TCPL ventilation in a relatively small and mature group of preterm infants with RDS.28 In addition to feasibility and safety, this study confirmed the need for lower PIP levels during NAVA, but other outcomes did not differ between the groups.
Synchronization
Ideally the support delivered by the ventilator should be fully synchronized with the spontaneous breathing efforts of the patient. Asynchrony may result in patient discomfort, less effective oxygenation and ventilation, high airway pressure, pneumothorax, and an increased risk of intraventricular hemorrhage.29,30 Randomized trials have shown that synchronization reduces duration of ventilation compared with non-synchronized ventilation.31 However, synchronized ventilation does not appear to reduce mortality or BPD.
To achieve synchronization, the onset of a spontaneous breath is detected by the trigger sensor connected to the ventilator. Ideally, the time between the onset of the spontaneous breath and the delivery of the mechanical support should be as short as possible, and the end of the mechanical inflation should be in sync with the end of spontaneous inspiration.
Different signals can be used to detect onset and/or termination of spontaneous breaths. The most widely used trigger signals are airway flow and airway pressure. The change in airway flow or the resulting volume at the start of a spontaneous breath triggers the start of a mechanical inflation. The clinician determines the sensitivity of the trigger by setting the threshold of flow or volume change that qualifies as an onset of inspiration. Airway flow can also be used to synchronize the end of inspiration in ventilation modes that use a decelerating inspiratory flow pattern. At the start of inflation, there will be a steep increase in inspiratory flow to build up the PIP and the ventilator sets the maximal required flow at 100%. As the airway pressure approached PIP, the flow decelerates to zero, indicating the end of inspiration. As the flow reaches a preset percentage (often 10–15%) of the maximal flow, the inspiration phase is terminated.
By positioning the flow sensor at the Y-piece of the patient circuit, flow and volume triggering becomes very sensitive even at relatively small flows and volumes in preterm infants. However, flow triggering also has disadvantages. First, the flow sensor will increase anatomical dead space. Second, condensation in the flow sensor or ETT leak may impair accurate flow measurement. Finally, accumulation of condensation in the patient circuits may cause auto-triggering.
Triggering based on airway pressure is based on a fall in PEEP below a set threshold during inspiration. This technique does not require a flow sensor at the Y-piece and is therefore not influenced by ETT leak nor does it increase anatomical dead space. However, the patient needs to generate a higher work-of-breathing to trigger the ventilator. Furthermore, synchronization of breath termination is not possible with airway pressure. Studies have shown that flow triggering is superior to pressure triggering with respect to trigger delay, sensitivity, and asynchrony.32
Abdominal wall motion can also be used for detecting the onset (but not the termination) of inspiration. For this, a pneumatic capsule is placed on the abdomen just below the ribcage. As the abdomen expands at the start of inspiration, the pressure in the capsule will increase and this will trigger the ventilator. This technique is not affected by leak and the response time is relative short. However, studies have shown that the detection of spontaneous breaths is not always sensitive enough.33
Relatively new is triggering based on the electrical activity of the diaphragm using a NAVA catheter. Both the onset and the termination of inflation can be synchronized. This technique does not increase dead space, is independent of ETT leak, and has a very short delay time. Disadvantages are that this technique is only available on a specific ventilator and requires an invasive and costly catheter. Studies have shown that diaphragmatic trigger is superior to flow triggering, although the impact on important clinical outcome parameters is unknown.25,28
Most pressure and volume targeted modes of ventilation require a so-called synchronization modality. This modality determines how often a trigger is followed by a mechanical inflation. During synchronized intermittent mandatory ventilation (SIMV), the number of triggered spontaneous breathing efforts are limited to the set SIMV frequency. Additional spontaneous breaths are not mechanically supported. During Assist Control (A/C) or synchronized intermittent positive pressure ventilation (SIPPV), every detected spontaneous breath is supported by the ventilator. The set frequency serves as a “backup” rate in case breathing is insufficient. Pressure support ventilation (PSV) is very similar to the A/C mode in that every spontaneous breath is supported with positive pressure. In addition, the end of inspiration is flow-cycled and the frequency is fully controlled by the patient. The latter requires a backup ventilation mode in case of insufficient respiratory drive.
Several studies have compared different modes of synchronization but failed to show clear (clinical outcome) differences.31
New modes under investigation
Developing new modes of CMV is an ongoing process and some modalities currently under investigation may enter clinical practice in the near future.
Airway Pressure Release Ventilation (APRV) is a TCPL ventilation modality that applies a high constant distending pressure (Phigh) for a prolonged time (Thigh) combined with time-cycled releases to a lower pressure (Plow) for a short period of time (Tlow or release time). These parameters are set in a physiology-driven manner, taking into consideration the flow tracing and the inflection points of the pressure-volume curve.34 Spontaneous breathing occurs independent of the respiratory cycle. The application of Phigh for a long time should facilitate alveolar recruitment and maintain an adequate EELV. The short releases in airway pressure combined with spontaneous breathing should facilitate carbon dioxide clearance. Adult and pediatric studies of APRV have shown variably results, with some reporting improved hemodynamics, decreased sedative use, improved gas exchange while applying lower mean airway pressures, and better patient comfort.34,35,36 Animal studies in models of neonatal lung injury reported better oxygenation, lung mechanics and hemodynamics, during APRV compared to commonly used conventional modalities.37,38,39 Case series in newborns have shown similar results.40
Mandatory minute ventilation (MMV) is a modality that targets a minimum preset minute volume. The patient is allowed to breathe spontaneously and all breaths are pressure supported. At regular intervals (for example every 10 s), the ventilator estimates the minute volume based on the spontaneous rate and VT in that short period. If the estimated minute volume is below the set target, extra mechanical inflations are started to guarantee the preset minute volume. These mechanical inflations can be pressure or volume targeted. The actual rate of mechanical inflations depends on the spontaneous effort of the patient. If the spontaneous minute volume is above the target, no mechanical inflations are added. This is fundamentally different from SIMV, as during this synchronization mode the number of SIMV breaths is fixed and independent of the spontaneous effort of the patient. To date, only two observational studies have evaluated MMV in newborns, demonstrating that MMV maintains adequate gas exchange with less mechanical support than SIMV.41,42
Variable ventilation (VV) tries to mimic the variability of VT and respiratory rate observed in normal physiological breathing, which is especially present in newborn infants. This means that VT applied during VV vary on a breath-to-breath basis, with a generated sequence according to the distribution of spontaneous tidal breathing within certain boundaries set by the physician. The ventilatory rate is usually changed in an opposite way to ensure a relatively stable minute volume. To date, VV has only been assessed in animal studies which suggest that VV might boost endogenous surfactant production and attenuate lung injury.43,44
Summary
Modalities of CMV in newborn infants have evolved considerable over the last decades. Improved hardware and software have allowed the introduction of new modes, such as VTV and more sensitive trigger modalities. Furthermore, control of mechanical support is shifting more and more from the operator to the patient. Clinicians should be aware of the fact that selecting and setting the optimal ventilatory mode for each individual patient requires sufficient knowledge on lung (patho)physiology and the characteristics of the modality. Although small observational studies have reported short-term benefits for some of these newer modes, it is important to acknowledge that most modes have been introduced in clinical practice without rigorous evaluation in randomized controlled trials. With the exception of VTV, it therefore remains unclear if these new modalities actually impact important clinical outcomes, such as BPD. This should be the focus of future studies.
References
Angus, D. C. et al. Epidemiology of neonatal respiratory failure in the United States: projections from California and New York. Am. J. Respir. Crit. Care Med. 164, 1154–1160 (2001).
Soll, R. F. et al. Obstetric and neonatal care practices for infants 501 to 1500 g from 2000 to 2009. Pediatrics 132, 222–228 (2013).
Baud, O. et al. Effect of early low-dose hydrocortisone on survival without bronchopulmonary dysplasia in extremely preterm infants (PREMILOC): a double-blind, placebo-controlled, multicentre, randomised trial. Lancet 387, 1827–1836 (2016).
Dreyfuss, D. & Saumon, G. Ventilator-induced lung injury: lessons from experimental studies. Am. J. Respir. Crit. Care Med. 157, 294–323 (1998).
Coker, P. J. et al. Increased sensitivity to mechanical ventilation after surfactant inactivation in young rabbit lungs. Crit. Care Med. 20, 635–640 (1992).
Ikegami, M., Kallapur, S. G. & Jobe, A. H. Initial responses to ventilation of premature lambs exposed to intra-amniotic endotoxin 4 days before delivery. Am. J. Physiol. Lung Cell. Mol. Physiol. 286, L573–L579 (2004).
Gagliardi, L. et al. Do differences in delivery room intubation explain different rates of bronchopulmonary dysplasia between hospitals? Arch. Dis. Child Fetal Neonatal Ed. 96, F30–F35 (2011).
van Kaam, A. Lung-protective ventilation in neonatology. Neonatology 99, 338–341 (2011).
Harris, T. R. & Wood, B. R. Physiological principles. in Assisted Ventilation of the Newborn 3rd edn (eds Goldsmith, J. P. & Karotkin, E. H.) 21–68 (Saunders, Philadelphia, PA, 1996).
Papastamelos, C. et al. Developmental changes in chest wall compliance in infancy and early childhood. J. Appl. Physiol. 78, 179–184 (1995).
Keszler, M. Volume-targeted ventilation: one size does not fit all. Evidence-based recommendations for successful use. Arch. Dis. Child Fetal Neonatal Ed. 104 (2018).
Dreyfuss, D. et al. High inflation pressure pulmonary edema. Respective effects of high airway pressure, high tidal volume, and positive end-expiratory pressure. Am. Rev. Respir. Dis. 137, 1159–1164 (1988).
Keszler, M. & Abubakar, K. Volume guarantee: stability of tidal volume and incidence of hypocarbia. Pediatr. Pulmonol. 38, 240–245 (2004).
Lista, G. et al. Lung inflammation in preterm infants with respiratory distress syndrome: effects of ventilation with different tidal volumes. Pediatr. Pulmonol. 41, 357–363 (2006).
Singh, J., Byrne, S. & Donn, S. M. Mechanical ventilation of very low birth weight infants: is volume or pressure a better target variable? J. Pediatr. 149, 308–313 (2006).
Singh, J. et al. Long term follow-up of very low birthweight infants from a neonatal volume versus pressure mechanical ventilation trial. Arch. Dis. Child Fetal Neonatal Ed. 94 (2009).
Cheema, I. U. & Ahluwalia, J. S. Feasibility of tidal volume-guided ventilation in newborn infants: a randomized, crossover trial using the volume guarantee modality. Pediatrics 107, 1323–1328 (2001).
Klingenberg, C. et al. Volume-targeted versus pressure-limited ventilation in neonates. Cochrane Database Syst. Rev. 10, CD003666 (2017).
Belteki, G. & Morley, C. J. Frequency, duration and cause of ventilator alarms on a neonatal intensive care unit. Arch. Dis. Child Fetal Neonatal Ed. 103, F307–F311 (2018).
Schulze, A. & Bancalari, E. Proportional assist ventilation in infants. Clin. Perinatol. 28, 561–578 (2001).
Schulze, A. et al. Proportional assist ventilation in low birth weight infants with acute respiratory disease: a comparison to assist/control and conventional mechanical ventilation. J. Pediatr. 135, 339–344 (1999).
Musante, G. et al. Proportional assist ventilation decreases thoracoabdominal asynchrony and chest wall distortion in preterm infants. Pediatr. Res. 49, 175–180 (2001).
Stein, H., Firestone, K. & Rimensberger, P. C. Synchronized mechanical ventilation using electrical activity of the diaphragm in neonates. Clin. Perinatol. 39, 525–542 (2012).
Firestone, K. S. et al. Effect of changing NAVA levels on peak inspiratory pressures and electrical activity of the diaphragm in premature neonates. J. Perinatol. 35, 612–616 (2015).
Alander, M. et al. Comparison of pressure-, flow-, and NAVA-triggering in pediatric and neonatal ventilatory care. Pediatr. Pulmonol. 47, 76–83 (2012).
Stein, H. D. Neurally adjusted ventilatory assist in neonates weighing. J. Pediatr. 160, 786–9.e1 (2012).
Rosterman, J. L. et al. The impact of neurally adjusted ventilatory assist mode on respiratory severity score and energy expenditure in infants: a randomized crossover trial. J. Perinatol. 38, 1–5 (2017).
Kallio, M. et al. Neurally adjusted ventilatory assist (NAVA) in preterm newborn infants with respiratory distress syndrome-a randomized controlled trial. Eur. J. Pediatr. 175, 1175–1183 (2016).
Perlman, J. M. et al. Reduction in intraventricular hemorrhage by elimination of fluctuating cerebral blood-flow velocity in preterm infants with respiratory distress syndrome. N. Engl. J. Med. 312, 1353–1357 (1985).
Greenough, A. et al. Pancuronium prevents pneumothoraces in ventilated premature babies who actively expire against positive pressure inflation. Lancet 1, 1–3 (1984).
Greenough, A. et al. Synchronized mechanical ventilation for respiratory support in newborn infants. Cochrane Database Syst. Rev. 9, CD000456 (2016).
Dimitriou, G., Greenough, A. & Cherian, S. Comparison of airway pressure and airflow triggering systems using a single type of neonatal ventilator. Acta Paediatr. 90, 445–447 (2001).
John, J. et al. Airway and body surface sensors for triggering in neonatal ventilation. Acta Paediatr. 83, 903–909 (1994).
Daoud, E. G. Airway pressure release ventilation. Ann. Thorac. Med. 2, 176–179 (2007).
Lalgudi Ganesan, S. et al. Airway pressure release ventilation in pediatric acute respiratory distress syndrome. a randomized controlled trial. Am. J. Respir. Crit. Care Med. 198, 1199–1207 (2018).
Schultz, T. R. et al. Airway pressure release ventilation in pediatrics. Pediatr. Crit. Care Med. 2, 243–246 (2001).
Martin, L. D., Wetzel, R. C. & Bilenki, A. L. Airway pressure release ventilation in a neonatal lamb model of acute lung injury. Crit. Care Med. 19, 373–378 (1991).
Arrindell, E. L. et al. Lung volume recruitment in a preterm pig model of lung immaturity. Am. J. Physiol. Lung Cell. Mol. Physiol. 309, L1088–L1092 (2015).
Kollisch-Singule, M. et al. Limiting ventilator-associated lung injury in a preterm porcine neonatal model. J. Pediatr. Surg. 52, 50–55 (2017).
Gupta, S. et al. Airway pressure release ventilation: a neonatal case series and review of current practice. Can. Respir. J. 20, e86–e91 (2013).
Claure, N. et al. Computer-controlled minute ventilation in preterm infants undergoing mechanical ventilation. J. Pediatr. 131, 910–913 (1997).
Guthrie, S. O. et al. A crossover analysis of mandatory minute ventilation compared to synchronized intermittent mandatory ventilation in neonates. J. Perinatol. 25, 643–646 (2005).
Berry, C. A. et al. Variable ventilation enhances ventilation without exacerbating injury in preterm lambs with respiratory distress syndrome. Pediatr. Res. 72, 384–392 (2012).
Bartolák-Suki, E. et al. Optimization of variable ventilation for physiology, immune response and surfactant enhancement in preterm lambs. Front. Physiol. 8, 425 (2017).
Author information
Authors and Affiliations
Contributions
All authors contributed to the drafting and revising of the article and provided final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
van Kaam, A.H., De Luca, D., Hentschel, R. et al. Modes and strategies for providing conventional mechanical ventilation in neonates. Pediatr Res 90, 957–962 (2021). https://doi.org/10.1038/s41390-019-0704-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0704-1
- Springer Nature America, Inc.
This article is cited by
-
Diaphragmatic electromyography in infants: an overview of possible clinical applications
Pediatric Research (2024)
-
Detection and quantitative analysis of patient-ventilator interactions in ventilated infants by deep learning networks
Pediatric Research (2024)
-
Predictors of extubation failure in newborns: a systematic review and meta-analysis
Italian Journal of Pediatrics (2023)
-
Pilot study of an interprofessional pediatric mechanical ventilation educational initiative in two intensive care units
BMC Medical Education (2023)
-
Low inflating pressures during neonatal tidal volume targeted ventilation: occurrence and significance
Journal of Perinatology (2023)