Abstract
Backgroud
Urinary tissue inhibitor of metalloproteinase-2 (TIMP-2), insulin-like growth factor binding protein-7 (IGFBP-7) and the combination of TIMP-2 and IGFBP-7 ([TIMP-2]•[IGFBP7]) are proposed to be predictive biomarkers for acute kidney injury (AKI). The intention of our study was to determine whether there is any significant predictive value of these biomarkers for the occurrence of AKI and severe AKI in critically ill neonates.
Methods
Urinary samples were serially collected in 237 neonates during neonatal intensive care unit (NICU) stay for measurements of TIMP-2 and IGFBP-7 in this prospective study. AKI diagnosis was based on KDIGO classification without urine output or serum creatinine >1.2 mg/dL.
Results
Twenty neonates developed AKI, including 11 with KDIGO stage 1, defined as mild AKI, and 9 with stages 2 and 3, defined as severe AKI. Urinary IGFBP-7 and [TIMP-2]•[IGFBP7] remained associated with AKI after adjustment for gestational age, gender and illness severity. Urinary [TIMP-2]•[IGFBP7] achieved an AUC of 0.71 (P = 0.034) and displayed a sensitivity of 88.9% and a specificity of 50.9% for discriminating severe AKI at the optimal cut-off value of 0.045.
Conclusion
The combination of TIMP-2 and IGFBP-7 had independent discriminative value for severe AKI in critically ill neonates.
Similar content being viewed by others
Introduction
Acute kidney injury (AKI), which is one of the most common organ dysfunctions, is associated with high mortality and morbidity in critically ill patients.1,2,3 In recent years, many studies have focused on identifying early biomarkers for predicting AKI, which is critical to the improvement of clinical outcomes.4,5,6 The combination of urinary tissue inhibitor of metalloproteinase-2 (TIMP-2) with insulin-like growth factor binding protein-7 (IGFBP-7) ([TIMP-2]•[IGFBP7]) is suggested to be useful for identifying patients who are at risk for AKI or severe AKI, based on studies in adults and children.7,8,9,10
In heterogeneous critically ill adult patients, urinary [TIMP-2]•[IGFBP7] is associated with the severity of AKI across all three stages,8 and predicts the development of AKI well ahead of the clinical manifestations such as azotaemia and oliguria.7 Urinary TIMP-2 or IGFBP-7 alone also can be used to detect severe AKI in critically ill adult11 or children.12 However, although some studies have speculated on why urinary TIMP-2 and IGFBP-7 can act as a biomarker for AKI,7,13 the exact biological role in AKI beyond their utility as biomarkers is not fully understood since both proteins are capable of inducing a wide variety of cellular responses,14 and the utility of these proteins as biomarkers of AKI is still controversial.10,15 A previous study demonstrates that the urine levels of [TIMP-2]•[IGFBP7] are influenced by factors other than AKI, such as systemic inflammation and diabetes in critically ill adult patients; thus, the performance of these biomarkers in predicting AKI can be poor.15 Evidence is also lacked to confirm the association between these biomarkers and AKI in the heterogeneous critically ill neonatal or children populations.
Critically ill neonates are more likely to develop AKI due to factors such as perinatal hypoxic asphyxia, severe infection and the use of mechanical ventilation (MV) or nephrotoxic drugs,3,16 and the mortality rate is significantly higher in neonates with AKI than that in children.17 Compared with standard clinical criteria used in pediatric and adult patients, the diagnosis of neonatal AKI remains challenging. Because of inter-individual kidney maturational differences and maternal level, serum creatinine (SCr) and urine output are unreliable in the newborn period.3 Accordingly, the predictive value of these urinary biomarkers for AKI in critically ill neonates needs further assessment.
The objectives of the study were to investigate the influence of other factors on the urinary levels of TIMP-2, IGFBP-7 and the combination of TIMP-2 and IGFBP-7 ([TIMP-2]•[IGFBP7]), such as the gestational age, postnatal age, gender, birth weight, Apgar scores, and the illness severity assessed by the score for neonatal acute physiology (SNAP) in critically ill neonates, to determine the association of these urinary biomarkers with AKI or severe AKI, and to evaluate the diagnostic value of these biomarkers as independent AKI or severe AKI predictors in critically ill neonates.
Methods
Study population
Neonates admitted to the neonatal intensive care unit (NICU) from July to October 2016 were eligible for study inclusion. The exclusion criteria were death or discharge within 24 h of NICU admission. Neonates were prospectively recruited on admission and followed until NICU discharge or death. This study was approved by the Institutional Review Board at the Children’s Hospital of Soochow University. The study was conducted with the parental consent of each neonate when they were admitted.
Clinical data collection
Neonatal clinical information, including the gestational age, birth weight, gender and Apgar scores, was collected. Maternal information during pregnancy was also recorded, such as pregnancy complications, the mode of delivery and medication used. Clinical laboratory information, including SCr and blood urea nitrogen, was recorded for each neonate. The clinical diagnosis, the use of MV, antibiotics, steroids, caffeine, vasoactive drugs and surfactants during NICU stay and the NICU mortality rate were recorded. The clinical information were recorded daily until NICU discharge or death.
Assessment of illness severity
The SNAP, based on the physiological parameters collected on the first day of NICU admission, was calculated to determine the severity of the illness for the critically ill neonates, according to the methods described by Richardson,18 and in accordance with our previous studies.19
Diagnosis of AKI
The diagnosis and severity of AKI was made according to (1) the Neonatal AKI Improving Global Outcomes (KDIGO) classification without urine output: SCr rise ≥ 0.3 mg/dL within 48 h or SCr rise ≥ 1.5 × reference SCr within 7 days, and the reference sCr was defined as the lowest previous sCr value3 or (2) the neonatal SCr > 1.2 mg/dL.20 KDIGO stage 1 was defined as mild AKI, and KDIGO stages 2 and 3 were defined as severe AKI. All neonates had SCr on the first day of NICU admission and the SCr was routinely measured every 3–7 days during the NICU stay.
Urine sample collection and biochemical analyses
The urine samples of the neonates were first collected within the first 24 h after NICU admission and followed by serial collection every 48–72 h during NICU stay using a plastic bag and immediately stored at −80 °C. Urine samples were first centrifuged at 1500 × g at 4 °C for 15 min, and the supernatants were used for the measurement. Urine TIMP-2 and IGFBP-7 concentrations were detected by ELISA (Human TIMP-2 Duo Set ELISA DY971 and Human IGFBP-rp1/IGFBP-7 Duo Set ELISA DY1334-05, R&D Systems, USA). The protocol was carried out strictly according to the instructions. Urine samples were diluted 20–100 times to ensure that the enzymatic reactions were maintained within a linear range. The coefficients of variation within and between ELISA plates were less than 10%. We used urine TIMP-2/urine Cr (ng/mg) and urine IGFBP-7/urine Cr (ng/mg) to eliminate the influence of different urine flow rates on the results. For urine [TIMP-2]•[IGFBP7], the concentrations of TIMP-2 and IGFBP-7 in the urine were multiplied and then divided by 1000 to convert them into international general units, (ng/mL)2/1000, according to a previous study.7 The levels of Cr were measured by the sarcosine oxidase method on an automatic biochemical analyser (Siemens, Germany).
Data interpretation
Using the urine samples collected prior to the clinical diagnosis of AKI and the random urine samples collected during NICU stay from neonates who did not develop AKI, we determined the association of these biomarkers with AKI or severe AKI and calculated the diagnostic characteristics. In addition, the initial values of urinary TIMP-2, IGFBP-7 and [TIMP-2]•[IGFBP7] obtained from the first urine sample collected within the first 24 h after NICU admission were used for association analysis to investigate the influence of other factors on the levels of these urinary biomarkers.
Statistical analysis
Data analysis was performed using SPSS statistical software Version 20. The assumption of normality and homogeneity of variance were confirmed. For continuous variables, as all data were non-normally distributed, the median (interquartile range) is used to describe the characteristics of the patients. The Mann–Whitney U test was used to analyze the differences between two groups, and the Kruskal–Wallis H test was used to analyze the differences among three groups. For categorical variables, numbers (percentage) were used to describe the characteristics of the patients. The differences between groups were compared by using the chi-square test or Fisher’s exact test. The clinical and laboratory factors that could influence TIMP-2, IGFBP-7 and [TIMP-2]•[IGFBP7] were assessed by using univariate and multivariate linear regression analyses (enter method). The data for continuous variables were log-transformed to meet the assumption of the homogeneity of variance. The multicollinearity was assessed using variance inflation factor (VIF) and tolerance values, and VIF < 2 indicated the absence of multicollinearity. To identify independent variables associated with AKI and severe AKI, univariate and multivariate logistic regression analyses were performed and the odds ratio (OR) and adjusted OR (AOR) with a 95% confidence interval (CI) were calculated. Model fit was assessed by the Hosmer–Lemeshow goodness-of-fit test. A P-value > 0.05 suggested the absence of a biased fit. Discriminative performance was assessed with a receiver operating characteristic (ROC) curve and the area under the ROC curve (AUC). Using Sigma Plot 14.0 software, the non-parametric method of Delong was used to compare differences between AUCs, and the sensitivity and specificity were calculated. In addition, the logistic regression models was performed to estimate combinations of urinary biomarkers with clinical factors and the discriminatory ability of the combinations was evaluated by the AUC. A P-value < 0.05 was regarded as statistically significant.
Results
Patient characteristics
During the study period, 246 neonates who were directly admitted to the NICU with the parental consent were eligible. Among these neonates, 9 were excluded; 4 had a failure in collecting urine samples during the first 24 h after NICU admission and 5 died or were discharged from the NICU within 24 h. The remaining 237 cases were included in this study.
Of the 237 neonates, 20 (8.4%) developed AKI. According to the KDIGO classification, 11 neonates were classified as KDIGO stage 1, which was defined as mild AKI: 8 on the first day of NICU admission, 1 on the third, 1 on the tenth, and 1 on the 15th day of NICU admission. Nine neonates met the requirements of KDIGO stages 2 and 3, which were defined as severe AKI: 1 on the first day, 1 on the third, 1 on the forth, 1 on the fifth, 1 on the seventh, 2 on the ninth, 1 on the tenth, and 1 on the 12th day of NICU admission.
A comparison of the demographic and clinical characteristics between the neonates in the non-AKI and AKI groups was shown in Table 1. Although there was no significant difference in the overall gestational age between the two groups, the proportion of low gestational age neonates (26–28 weeks) was significantly higher in the AKI group than in the non-AKI group. The proportion of boys in the AKI group was 26% higher than that in the non-AKI group.
Urinary biomarkers
Since all AKI neonates developed AKI within the first 15 days after NICU admission, urine samples collected during the first 2 weeks were used for data analysis. Of the 237 neonates, 51 (21.5%) had one sample, 73 (30.8%) had two samples, 34 (14.3%) had three, 24 (10.0%) had four, and 55 (23.2%) had five or more than five samples available during the first 2 weeks after NICU admission. The missing samples were because of a failure in collecting urine or neonates being discharged from the NICU or death.
To identify whether urinary TIMP-2, IGFBP-7 and [TIMP-2]•[IGFBP7] would predict the development of subsequent AKI in critically ill neonates, the values of these biomarkers from the urine samples collected 0–5 days prior to the diagnosis of AKI and the values from the random urine samples collected during the first 2 weeks after NICU admission in neonates who did not develop AKI were analyzed. The comparisons of the urinary TIMP-2, IGFBP-7 and [TIMP-2]•[IGFBP7] levels among the neonates in non-AKI, mild AKI and severe AKI groups are shown in Fig. 1(a–c). The value of the urinary IGFBP-7 was significantly higher in neonates with mild AKI than that in those without AKI (P = 0.019) (Fig. 1b). Although there was no significant difference among the three groups for urinary [TIMP-2]•[IGFBP7] (P = 0.057), the value of urinary [TIMP-2]•[IGFBP7] in the severe AKI group was significantly higher than that in the non-AKI group (P = 0.028) (Fig. 1c).
Neonatal AKI KIGDO stage 1 was defined as mild AKI, and stages 2 and 3 were defined as severe AKI. a Urinary TIMP-2, b urinary IGFBP-7, c urinary [TIMP-2]•[IGFBP7]. Non-AKI: the values of these biomarkers were from the random urine samples collected during the first 2 weeks after admission. Mild and severe AKI: the values were from the urine samples collected 0–5 days prior to the diagnosis of AKI. Error bars represent median and interquartile range. Probability values: Kruskal–Wallis H test. *P < 0.05 vs. non-AKI by Mann–Whitney U test.
Correlation of the urinary biomarkers with the clinical factors
Univariate and multivariate linear regression analyses were used to analyze the association of initial urinary biomarkers with the clinical factors. All variables in Table 1 were analyzed in the univariate linear regression analysis, and variables with P < 0.1 in univariate analysis were listed in Table 2 and entered into the multivariate analysis after checking the multicollinearity by VIF and tolerance values. In the multivariate linear regression analyses, no variables remained associated with initial urinary IGFBP-7 levels. Being born small for gestational age (SGA) remained significantly associated with increased initial urinary TIMP-2 levels, and being male remained associated with higher levels of initial urinary [TIMP-2]•[IGFBP7] in multivariate analyses. The initial urinary [TIMP-2]•[IGFBP7] levels were significantly higher in boys than in girls (0.063 [0.026–0.135] vs. 0.025 [0.008–0.077], P < 0.001).
Analysis of variables that were potentially associated with AKI and severe AKI
To identify whether urinary biomarkers were independently associated with AKI and severe AKI in critically ill neonates, all variables in Table 1, which were considered confounding factors, were analyzed in the univariate logistic regression analysis and variables with P < 0.05 were listed in Table 3. In the multivariate logistic analysis, the urinary levels of IGFBP-7 and [TIMP-2]•[IGFBP7] remained associated with AKI after adjustment for gestational age, gender and SNAP. The association of urinary [TIMP-2]•[IGFBP7] with severe AKI also remained significant after adjustment for SNAP or after adjustment for gestational age, gender and SNAP in critically ill neonates, as shown in Table 3.
Ability of the urinary levels of TIMP-2 and IGFBP-7 and [TIMP-2]•[IGFBP7] to discriminate AKI and severe AKI
The discrimination performance of the urinary biomarkers for AKI and severe AKI was assessed with the AUC and ROC curves. None of these urinary biomarkers achieved an AUC of 0.70 for discriminating AKI, as shown in Table 4. Combining urinary [TIMP-2]•[IGFBP7] with urinary IGFBP-7, SNAP, gender and gestational age improved discrimination performance, which was superior to urinary [TIMP-2]•[IGFBP7] (P = 0.009), but not to urinary IGFBP-7 (P = 0.153), alone in discriminating AKI.
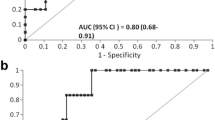
Urinary [TIMP-2]•[IGFBP7] displayed an AUC of 0.71 for discriminating severe AKI. The sensitivity and specificity were 88.9% and 50.9%, respectively, at the optimal cut-off value of 0.045 (ng/mL)2/1000, for discriminating severe AKI in critically ill neonates. The combination of urinary [TIMP-2]•[IGFBP7] with SNAP (AUC = 0.73) was similar to urinary [TIMP-2]•[IGFBP7] alone in discriminating severe AKI. The combination of urinary [TIMP-2]•[IGFBP7] with SNAP, gender and gestational age improved discrimination performance (AUC = 0.80) as shown in Table 4; however, the difference between the two AUCs (AUC = 0.80 vs. AUC = 0.71) did not reach statistically significant in discriminating severe AKI (P = 0.111). The ROC curves for the ability of urinary IGFBP-7, urinary TIMP-2 and urinary [TIMP-2]•[IGFBP7] to discriminate AKI and severe AKI in critically ill neonates are shown in Fig. 2(a, b).
Discussion
In this study, we analyzed the role of urinary TIMP-2, IGFBP-7 and [TIMP-2]•[IGFBP7] in predicting AKI and severe AKI in critically ill neonates. Our study showed that the combination of urinary TIMP-2 and IGFBP-7 had an independent discriminative value for severe AKI in heterogeneous critically ill neonates.
As far as we know, our study was the first to explore the correlation of urinary TIMP-2, IGFBP-7, [TIMP-2]•[IGFBP7] and AKI in critically ill neonates. The results showed that the incidence of AKI in NICU patients was 8.4%, which was lower than that in previous studies, including our own.3,16,21,22 As there are no fixed standard diagnostic criteria, the incidence and mortality of neonatal AKI vary greatly.3,16 The incidence of AKI also varied by gestational age and illness severity.21 The possible explanation might be that our study included a neonatal population with a wide range of gestational age; however, only 8 (3.4%) neonates were born at 26 weeks to <29 weeks, but 87 (36.7%) born in term. Nevertheless, this study encompassed the entire NICU population and evaluated the predictive value of these biomarkers for the occurrence of AKI, independent of gestational age and the severity of illness. The definition of AKI in our study was based on SCr. The values of SCr in neonates, especially in premature infants, are affected by many factors, such as maternal SCr levels, kidney development and renal tubular reabsorption function.16,23 As SCr does not change until 25–50% of kidney function decreases, depending only on SCr often leads to the underestimation and delayed diagnosis of AKI.24 Therefore, the concept of subclinical AKI based on early biomarkers has been proposed, emphasizing that even if patients do not meet the diagnostic criteria for AKI, there is still a great possibility that these patients may develop AKI.25
Previous studies have shown that most AKI biomarkers are susceptible to confounding factors, such as age, gender and the severity of the disease, in the prediction of AKI.3,23 In this study, urinary [TIMP-2]•[IGFBP7] was not correlated with the gestational age, SNAP, sepsis or other factors, except for gender. Therefore, when applied in the clinic, there are fewer factors that may interfere with the use of the combination of urinary TIMP-2 and IGFBP-7 in neonates. The gender difference in urinary [TIMP-2]•[IGFBP7] has never been reported before. A number of studies have demonstrated the presence of early gender differences in physiological responses to delivery and stress, in osmotic regulation of vasopressin and renal sodium handling and in urinary aquaporin-2 excretion in the early postnatal period.26,27 The exact mechanism underlying the gender difference in urinary [TIMP-2]•[IGFBP7] is unknown. In addition, the proportion of AKI was higher in male neonates than in female neonates in the study. The commentary to KDIGO Clinical Practice Guidelines for AKI suggested that females are at a higher risk for hospital-acquired AKI in adults.28 However, recent studies on the influence of gender on the occurrence of AKI are conflicting. The effect of gender on the occurrence of AKI is different in different research groups.1,29 Previous studies suggested that the main reason for the influence of gender on AKI comes from sex hormones. The haemodynamic response of the kidney to angiotensin, the degradation of noradrenaline by monoamine oxidase and the expression of renal prepro-endothelin are also different between males and females.30,31,32
So far, one interesting clinical study has been performed recently in neonates and suggests that urinary [TIMP-2]•[IGFBP7] proves valuable for the early diagnosis of AKI in indomethacin-treated very-low-birth-weight infants.4 Unlike the previous study, the population of NICU is heterogeneous and AKI etiology and timing are largely unknown. Our study demonstrates that urinary [TIMP-2]•[IGFBP7] had an independent predictive value for severe AKI, even after adjusting for gestational age, gender and illness severity, in the heterogeneous NICU population. The cut-off point of urinary [TIMP-2]•[IGFBP7] in the study was 0.045, with a sensitivity of 88.9% and a specificity of 50.9% for severe AKI. The cut-off point of urinary [TIMP-2]•[IGFBP7] in our study was much lower than that in previous studies conducted in infants (≤1 year of age) undergoing cardiac surgery,33,34 in which the cut-off point of urinary [TIMP-2]•[IGFBP7] for the occurrence of AKI was 0.78, with the sensitivity of 69% and the specifity of 69%.34 It is thought that the differences are related to different age cohorts. However, the influence of the study method cannot be completely ruled out. Notably, despite adding urinary [TIMP-2]•[IGFBP7] to the clinical model was better than individual biomarker alone, the combination of urinary [TIMP-2]•[IGFBP7] with SNAP, gender and gestational age did not substantially improve the prediction of severe AKI in critically ill neonates.
Moreover, although neither urinary IGFBP-7 nor urinary TIMP-2 alone achieved an AUC of 0.70 for discriminating AKI or severe AKI, urinary IGFBP-7, but not TIMP-2, was independently associated with the occurrence of AKI in NICU neonates after adjusting for gestational age, gender and illness severity. Both IGFBP-7 and TIMP-2 are involved in G1 cell cycle arrest during the very early phases of AKI; however, their mechanisms are somewhat different.7,35 The expression and secretion of IGFBP-7 and TIMP-2 in human kidney epithelial cells are different.35,36 The previous study conducted in critically ill patients at risk for AKI suggests that IGFBP-7 has a high predictive value for AKI in surgical patients, while TIMP-2 has a high predictive value for sepsis-induced AKI;7 however, there is much that remains poorly understood regarding the biological role of these biomarkers.37
This study has some limitations. First, the incidence and grade of AKI may be underestimated because SCr, which was not measured daily as part of the study, was used to define AKI but lacked the index of the urine output. The urine output is of great value in improving the diagnostic rate of AKI; however, the criteria of urine output for neonatal AKI were still ambiguous, and the accuracy of urine output in the diagnosis of neonatal AKI was not good. Previous studies suggested that neonatal AKI could be diagnosed when the urine output was < 0.5 mL/kg/h.3 Some other studies suggest that urine output <1.5 mL/kg/h was better for the diagnosis of neonatal AKI because the water content in neonates was more than that in adults and children.38,39 Second, we did not collect daily urine samples from the neonates. The values of urinary TIMP-2, IGFBP-7 and [TIMP-2]•[IGFBP7] from the urine samples collected 0–5 days prior to the diagnosis of AKI and the values from the random urine samples collected during the first 2 weeks after NICU admission in neonates who did not develop AKI were used for statistical analysis, which may cause discrepancy in postnatal age at sampling. We chose the values in this way to clarify the predictive value of these biomarkers for AKI and severe AKI. Third, this study included patients from a single center, 87 (36.7%) neonates were born in term and the illness severity of the neonates assessed by the SNAP was less severe, even as compared to our previous study.22 This may also explain why the number of AKI cases was relatively small in the study and may have a significant impact on the levels of these urinary biomarkers. Our findings may not be generalizable to critically ill neonates with extremely low gestational age or with more severe conditions. A multi-center study is needed; thus, a larger quantity of samples can be collected and investigated to further demonstrate the predictive value of urinary TIMP-2, IGFBP-7 and [TIMP-2]•[IGFBP7] for neonatal AKI. In future research, we will continue to explore this topic and to provide effective evidence for the early diagnosis and treatment of AKI in neonates.
Conclusions
The combination of urinary TIMP-2 and IGFBP-7 has an independent discriminative value for severe AKI in critically ill neonates.
References
Kaddourah, A., Basu, R. K., Bagshaw, S. M., Goldstein, S. L. & Investigators, A. Epidemiology of acute kidney injury in critically ill children and young adults. N. Engl. J. Med. 376, 11–20 (2017).
Peters, E. et al. A worldwide multicentre evaluation of the influence of deterioration or improvement of acute kidney injury on clinical outcome in critically ill patients with and without sepsis at ICU admission: results from The Intensive Care Over Nations audit. Crit. Care 22, 188 (2018).
Selewski, D. T. et al. Neonatal acute kidney injury. Pediatrics 136, e463–e473 (2015).
Waldherr, S. et al. Urinary acute kidney injury biomarkers in very low-birth-weight infants on indomethacin for patent ductus arteriosus. Pediatr. Res. 85, 678–686 (2019).
Hanna, M. et al. Early urinary biomarkers of acute kidney injury in preterm infants. Pediatr. Res. 80, 218–223 (2016).
Ostermann, M. et al. Kinetics of urinary cell cycle arrest markers for acute kidney injury following exposure to potential renal insults. Crit. Care Med. 46, 375–383 (2018).
Kashani, K. et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit. Care 17, R25 (2013).
Hoste, E. A. et al. Derivation and validation of cutoffs for clinical use of cell cycle arrest biomarkers. Nephrol. Dial. Transplant. 29, 2054–2061 (2014).
Wetz, A. J. et al. Quantification of urinary TIMP-2 and IGFBP-7: an adequate diagnostic test to predict acute kidney injury after cardiac surgery? Crit. Care 19, 3 (2015).
Vijayan, A. et al. Clinical use of the urine biomarker [TIMP-2] x [IGFBP7] for Acute Kidney Injury Risk Assessment. Am. J. Kidney Dis. 68, 19–28 (2016).
Yamashita, T. et al. Evaluation of urinary tissue inhibitor of metalloproteinase-2 in acute kidney injury: a prospective observational study. Crit. Care 18, 716 (2014).
Bai, Z. et al. Serum and urine FGF23 and IGFBP-7 for the prediction of acute kidney injury in critically ill children. BMC Pediatr. 18, 192 (2018).
Bihorac, A. et al. Validation of cell-cycle arrest biomarkers for acute kidney injury using clinical adjudication. Am. J. Respir. Crit. Care Med. 189, 932–939 (2014).
Lameire, N., Vanmassenhove, J., Van Biesen, W. & Vanholder, R. The cell cycle biomarkers: promising research, but do not oversell them. Clin. Kidney J. 9, 353–358 (2016).
Bell, M., Larsson, A., Venge, P., Bellomo, R. & Martensson, J. Assessment of cell-cycle arrest biomarkers to predict early and delayed acute kidney injury. Dis. Markers 2015, 158658 (2015).
Perico, N., Askenazi, D., Cortinovis, M. & Remuzzi, G. Maternal and environmental risk factors for neonatal AKI and its long-term consequences. Nat. Rev. Nephrol. 14, 688–703 (2018).
Sutherland, S. M. et al. AKI in hospitalized children: epidemiology and clinical associations in a national cohort. Clin. J. Am. Soc. Nephrol. 8, 1661–1669 (2013).
Richardson, D. K., Gray, J. E., McCormick, M. C., Workman, K. & Goldmann, D. A. Score for Neonatal Acute Physiology: a physiologic severity index for neonatal intensive care. Pediatrics 91, 617–623 (1993).
Li, Y. et al. Addition of SNAP to perinatal risk factors improves the prediction of bronchopulmonary dysplasia or death in critically ill preterm infants. BMC Pediatr. 13, 138 (2013).
Hoffman, S. B., Massaro, A. N., Soler-Garcia, A. A., Perazzo, S. & Ray, P. E. A novel urinary biomarker profile to identify acute kidney injury (AKI) in critically ill neonates: a pilot study. Pediatr. Nephrol. 28, 2179–2188 (2013).
Jetton, J. G. et al. Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc. Health 1, 184–194 (2017).
Li, Y. et al. Urine interleukin-18 and cystatin-C as biomarkers of acute kidney injury in critically ill neonates. Pediatr. Nephrol. 27, 851–860 (2012).
Prowle, J. R. Acute kidney injury: creatinine and AKI–through a glass, darkly. Nat. Rev. Nephrol. 9, 193–195 (2013).
Askenazi, D. J., Ambalavanan, N. & Goldstein, S. L. Acute kidney injury in critically ill newborns: what do we know? What do we need to learn? Pediatr. Nephrol. 24, 265–274 (2009).
Haase, M., Kellum, J. A. & Ronco, C. Subclinical AKI–an emerging syndrome with important consequences. Nat. Rev. Nephrol. 8, 735–739 (2012).
Stachenfeld, N. S., Splenser, A. E., Calzone, W. L., Taylor, M. P. & Keefe, D. L. Sex differences in osmotic regulation of AVP and renal sodium handling. J. Appl Physiol. (1985) 91, 1893–1901 (2001).
Zelenina, M. et al. Urinary aquaporin-2 excretion during early human development. Pediatr. Nephrol. 21, 947–952 (2006).
Bagshaw, S. M., George, C., Bellomo, R. & Committee, A. D. M. Early acute kidney injury and sepsis: a multicentre evaluation. Crit. Care 12, R47 (2008).
Neugarten, J. & Golestaneh, L. Female sex reduces the risk of hospital-associated acute kidney injury: a meta-analysis. BMC Nephrol. 19, 314 (2018).
Satake, A. et al. Protective effect of 17beta-estradiol on ischemic acute renal failure through the PI3K/Akt/eNOS pathway. Kidney Int. 73, 308–317 (2008).
Tanaka, R. et al. Sex differences in ischaemia/reperfusion-induced acute kidney injury depends on the degradation of noradrenaline by monoamine oxidase. Clin. Exp. Pharm. Physiol. 44, 371–377 (2017).
Muller, V. et al. Sexual dimorphism in renal ischemia-reperfusion injury in rats: possible role of endothelin. Kidney Int. 62, 1364–1371 (2002).
Gist, K. M. et al. Kinetics of the cell cycle arrest biomarkers (TIMP-2*IGFBP-7) for prediction of acute kidney injury in infants after cardiac surgery. Pediatr. Nephrol. 32, 1611–1619 (2017).
Gist, K. M. et al. Acute kidney injury biomarkers predict an increase in serum milrinone concentration earlier than serum creatinine-defined acute kidney injury in infants after cardiac surgery. Ther. Drug Monit. 40, 186–194 (2018).
Emlet, D. R. et al. Insulin-like growth factor binding protein 7 and tissue inhibitor of metalloproteinases-2: differential expression and secretion in human kidney tubule cells. Am. J. Physiol. Ren. Physiol. 312, F284–F296 (2017).
Emlet, D. R., Wen, X. & Kellum, J. A. Comments on the Review ‘Biomarkers in acute kidney injury - pathophysiological basis and clinical performance' Acta Physiol. 2017, 219, 556-574: an update on kidney localization of IGFBP7 and TIMP2. Acta Physiol. (Oxf.) 222, e12934 (2018).
Schrezenmeier, E. V., Barasch, J., Budde, K., Westhoff, T. & Schmidt-Ott, K. M. Biomarkers in acute kidney injury - pathophysiological basis and clinical performance. Acta Physiol. (Oxf.) 219, 554–572 (2017).
Bezerra, C. T., Vaz Cunha, L. C. & Liborio, A. B. Defining reduced urine output in neonatal ICU: importance for mortality and acute kidney injury classification. Nephrol. Dial. Transplant. 28, 901–909 (2013).
Liborio, A. B., Branco, K. M. & Torres de Melo Bezerra, C. Acute kidney injury in neonates: from urine output to new biomarkers. BioMed. Res. Int. 2014, 601568 (2014).
Acknowledgements
We thank the staff in biochemistry laboratory for technical assistance. This work was supported by grants from the National Natural Science Foundation of China (81370773, 81741054, 81971432), JiangSu province science and technology support Program (Social Development BE2016675), Natural Science Foundation of Jiangsu province (BK20171217), Key talent of women’s and children’s health of JiangSu province (FRC201738), SuZhou clinical key disease diagnosis and treatment technology foundation (LCZX201611), SuZhou science and technology development project (SYS201760). The funders had no role in the study design, data collection, preparation of the manuscript, and decision to publish.
Author information
Authors and Affiliations
Contributions
J.C. participated in data analysis and drafted the manuscript. Y.S. performed the experiments. S.W., X.D. and H.H. participated in collecting the data and samples. X.D. participated in data analysis. Z.B. and X.L. participated in data analysis and interpretation. J.W. participated in the design of the study and coordination. Y.L. had primary responsibility for study design, performing the experiments, data analysis, interpretation of data, and writing of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, J., Sun, Y., Wang, S. et al. The effectiveness of urinary TIMP-2 and IGFBP-7 in predicting acute kidney injury in critically ill neonates. Pediatr Res 87, 1052–1059 (2020). https://doi.org/10.1038/s41390-019-0698-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0698-8
- Springer Nature America, Inc.
This article is cited by
-
Urinary TIMP-2*IGFBP-7 to diagnose acute kidney injury in children receiving cisplatin
Pediatric Nephrology (2024)
-
Predictive value of urinary cell cycle arrest biomarkers for all cause-acute kidney injury: a meta-analysis
Scientific Reports (2023)
-
Gut microbiota and neonatal acute kidney injury biomarkers
Pediatric Nephrology (2023)
-
Artificial intelligence in early detection and prediction of pediatric/neonatal acute kidney injury: current status and future directions
Pediatric Nephrology (2023)
-
Derivation and validation of urinary TIMP-1 for the prediction of acute kidney injury and mortality in critically ill children
Journal of Translational Medicine (2022)