Abstract
Background
Hypertonia is characterized by increased resting muscle tone. Previous studies have shown that adult patients with hypertonia displayed autonomic imbalance. However, cardiac sympatho-vagal control in infants with hypertonia have not been explored. The main aim was to estimate cardiac autonomic control in infants with hypertonia using heart rate variability (HRV).
Methods
Thirty infants (0–2 years old) were studied. Heart rate (HR) and R-R interval time series were obtained in 15 Control and 15 Hypertonia infants. HRV was analyzed in time and frequency domains. Additionally, non-linear analysis and entropy measurements were performed.
Results
Infants with hypertonia showed cardiac autonomic imbalance as evidenced by alterations in HRV, characterized by an increased power spectral density of low frequency (LF) over high frequency (HF) components of HRV. Indeed, a ∼7% increase in LF, and ∼30% reduction in HF, were found in infants with hypertonia vs. control infants. In addition, time domain and non-linear HRV analysis (Root-mean-square of successive normal sinus R-R interval difference, entropy, and R-R interval variability) were all significantly decreased in hypertonia vs. control subjects.
Conclusions
Our results showed that hypertonia infants displayed HRV disturbances, which suggest an alteration in overall autonomic cardiac modulation in infants with hypertonia compared with healthy condition.
Similar content being viewed by others
Introduction
According to an NIH-funded Task force on Childhood Motor Disorders, hypertonia is defined as velocity-dependent increase in muscle tone with exaggerated tendon jerks, resulting from hyperexcitability of the stretch reflex.1 Clinically, hypertonia is characterized by resistance to externally imposed movement that increases with the speed of stretch.1,2,3 Hypertonia is commonly observed in several pathologies, including hydrocephalus, stroke, multiple sclerosis, and traumatic brain or spinal injury.3 Although cerebral palsy is the most common cause of hypertonia in children,3 a significant number of infants display spontaneous hypertonia in the absence of pyramidal neuron lesions.4 The precise mechanisms underpinning development of spontaneous hypertonia in newborns is incompletely understood, however it has been proposed that this condition is related to poor maternal health (i.e., drug abuse, metabolic disorders, trauma) during pregnancy.4
In addition to abnormal control of movement, several studies suggest that hypertonia is associated with abnormal function of the autonomic nervous system.5,6 Indeed, abnormal autonomic function is observed in clinically distinct hypertonia populations.5,6 Treatment for hypertonia typically focuses on the recovery of skeletal muscle function, however it has been proposed that abnormal cardiac autonomic control, mostly associated to parasympathetic modulation, in adult hypertonic patients with concomitant cerebral palsy, is an important risk factor that contributed to the development of cardiovascular diseases and could contribute to the progression of the overall pathophysiological state.5 Indeed, parasympathetic withdrawal is associated to increase mortality events in patients with cardiovascular dysfunction.7 However, regarding to hypertonia, it is not clear whether abnormal autonomic function occurs soon after birth (spontaneous hypertonia) or develops later in adulthood (i.e., hypertonia with concomitant cerebral palsy). Considering this lack of evidence, addressing autonomic function and especially vagal component in infants with spontaneous hypertonia may be relevant for the development of therapeutic strategies intended to improve cardiovascular outcomes in these patients. A significant barrier to these studies is the difficulty associated with measuring autonomic function directly and non-invasively. Fortunately, several indirect and non-invasive methods have been developed to study autonomic function.
Assessment of heart rate variability (HRV) is one non-invasive method for estimating autonomic control to the heart based on the analysis of spontaneous fluctuations in heart rate (HR).8,9,10 Despite that HRV is an indirect estimator of overall cardiac autonomic function, the technique has been shown to be a robust and reliable prognostic tool for studying cardiovascular health in humans,8,11 and has been validated in many pathological and non-pathological conditions.9,12,13,14 Thus, we utilize HRV analysis to determine if cardiac autonomic balance was altered in infants with spontaneous hypertonia and delayed motor development relative to healthy age-matched controls.
Methods
Ethical statement
Protocols were approved by the Ethical Committee of the Corporación de Rehabilitación Club de Leones Cruz del Sur and were performed according to the standards set by the Declaration of Helsinki. Parents were informed by phone call that their sons met the inclusion criteria for a clinical study and were subsequently invited to an orientation session to learn more about the research protocol. Parents that were interested in participating in the study with their sons were asked to give informed consent. All parents of children included in the study gave written informed consent.
Experimental subjects
Study subjects (n = 30) were recruited from the rehabilitation center of the Club de Leones Cruz del Sur and from the rehabilitation center from the Ejército de Chile —Punta Arenas, according to the following inclusion criteria: (i) age between 0 and 2 years, (ii) diagnosed with hypertonia, and (iii) not have acute fever in the last 48 h. All infants were free of any medication including pain relief drugs and anti-pyretic drugs at the time of the study. Also, recruited infants needs to show no history of hospital re-admissions after delivery. Hypertonia was diagnosed by a physiatrist when five or more of the following criteria where met:3,15 (i) increased axial tone, (ii) increased upper limb tone, (iii) increased lower limb tone, (iv) increased osteotendinous reflex, (v) clonus, (vi) delayed psychomotor development, (vii) persistent plantar and/or palmar reflex, and (viii) signs of Babinski.3,15 The number/percent of subjects respect to each of the diagnostic criteria are shown in Table 1. Exclusion criteria were the following: (i) infants over 2 years old, (ii) under concomitant anticonvulsant drug therapy or any another drug that may influence the autonomic nervous system (i.e., Baclofen, valproic acid), and (iii) infants with metabolic disease, heart disease, or acute infection. Study groups consisted of 15 term infants with hypertonia (median: 9 months of age: 8 females and 7 males) and 15 healthy control infants (median: 11 months of age; 5 females and 10 males). The infants of both groups were born without complications at delivery and obtained an APGAR score of 10/10. Infants were exclusively breastfed for the first 6 months and then started on solid food. All the study subjects were more than 6 months old at the time of HRV analysis. The demographic characteristics of the study subjects including sex, age, gestational age, current age, birth weight, current weight, current height, and body mass index (BMI) are presented in Table 2.
Study design
A cross-sectional prospective study was performed to determine the influence of hypertonia on autonomic function by using non-invasive HRV analysis. All measurements were performed between 15:30 and 19:00 h. at a room temperature of 25 °C. HR and R-R intervals were recorded during 20 minutes at rest using a HR monitor. Using this data, time, and frequency domain analysis as well as non-linear analysis and entropic measurements were also performed. Variables included in the HRV analysis were: (i) the power spectral density of very low frequency component of HRV (VLF), (ii) the low frequency component of HRV (LF) associated to both cardiac sympathetic and parasympathetic modulation, and (iii) the high frequency component of HRV (HF) mostly associated with cardiac parasympathetic modulation, (iv) the LF/HF ratio as a cardiac autonomic balance indicator, (v) mean RR interval, (vi) mean HR, (vii) Root-mean-square of successive normal sinus R-R interval difference (RMSSD, an indirect index of parasympathetic modulation), (viii) standard deviation of NN intervals (SDNN, an indicator of overall autonomic modulation), (ix) number of pairs of successive NN (R-R) intervals that differ by more than 50 ms (NN50), (x) proportion of NN50 divided by the total number of NN (R-R) intervals (PNN50, indicator of parasympathetic modulation), (xi) standard deviation of points perpendicular to the axis of line of identity (SD1, an estimator of short-term variability in cardiac rhythm modulation), and standard deviation of points along the axis of line of identity (SD2, an estimator of short-term variability in cardiac rhythm modulation) of Poincaré plots from the R-R interval, (xii) approximate entropy (ApEn), and (xiii) sample entropy (SampleEn) as an indicators of overall autonomic modulation.5,8,9,11,12,13,14 Additionally, correlations between gestational and current weight, gestational and current height, and BMI with HRV parameters were performed. For HRV analysis in the frequency domain, data were expressed in absolute as well as in normalized units (n.u.).
Experimental procedures
Infants were undressed by one of the parents (thoracic and abdominal area) and a stretch band containing a bluetooth-based wireless hear rate sensor (H7 transmitter, Polar, Kempele, Finland) was gently secured to the abdomen at the level of the sternum (Fig. 1). The HR sensor was moistened with warm water to ensure good skin contact and conductivity. Once the sensor was installed the infant was dressed again and the parent was asked to hold the child to keep him/her calm. Once the child was calm, R-R intervals and the derived HR were recorded for 20 minutes using a wireless device (Polar V800, Kempele, Finland). The Polar V800 device has been previously validated for acquisition of HR signals for HRV analysis in adults and infants.16 All recordings were performed in awake subjects that were not nursing. All subjects tolerated the procedure well, and no clinical complications were observed during the procedure.
Position of Polar v800 for recording of R-R interval time series. The Polar v800 was positioned between the thoracic and abdominal area after which time infants were dressed to maintain stable body temperature. Written informed consent was obtained from the individual´s parent for the publication of this image
Data collection
Data was collected for a period of 20 minutes with a sampling rate of 1 kHz for ECG raw data. After detection of the QRS complex the device downsamples the signal (1–2 Hz) to interpolate R-R interval time series. The first 5 minutes of each recording was discarded, and the last 10 minutes of the recordings were used for HRV analysis. R-R time series were downloaded via Polar proprietary software (Polar FlowSync 2.6.2, Kempele, Finland) and exported into text files for later analysis. HRV was analyzed using Kubios software as previously described.17 Periods with excessive noise, usually due to movement, were visually identified and manually eliminated from the analysis by an operator blinded to group assignment. Accordingly, data with excessive noise were interpolated using a very low correction filter for R-R intervals greater or less than 0.25 ms or a low artifact correction filter when RR intervals were greater or less than 0.35 ms.18 The signal was then detrended using smoothness methods (Lambda = 500). Both the HR monitor device and the related software comply with the guidelines recommended by the Taskforce of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology standards for the measurement of HRV.11
Frequency domain analysis of HRV
HRV spectra was calculated by using an autoregressive algorithm (A.R.) to improve resolution in short-term measurements. Welch’s periodogram method (50% overlap window) was used to avoid spectral leakage. Frequency domain parameters were: total power, very-low frequency component of HRV (VLF; 0.00–0.04 Hz), low frequency component of HRV (LF; 0.04–0.3 Hz), high frequency component of HRV (HF; 0.3–1.2 Hz), and the LF/HF power ratio.19 We partially modified the cut-off frequencies proposed by Lasky et al. (2009) to avoid any confounding effect of different respiratory rates among patients. Mainly, the HF cut-off was expanded to cover up to the 99th percentile of respiratory rate distributions reported in infants from 0 to 2 years of age.20
Time domain and non-linear analysis of HRV
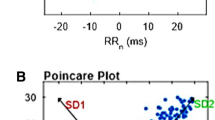
Autonomic control was also assessed by time domain and non-linear analysis of HRV. Time domain metrics were as follows: Mean RR, mean HR, Root-mean-square of successive normal sinus R-R interval difference (RMSSD), Standard deviation of all NN intervals (SDNN), number of pairs of successive NN (R-R) intervals that differ by more than 50 ms (NN50), and proportion of NN50 divided by the total number of NN (R-R) intervals pNN50. Non-linear analysis was performed by means of construction of Poincaré plots of R-R intervals and analyzed quantitatively by SD1 (transversal dispersion of Poincaré plot) and SD2 (longitudinal dispersion of Poincaré plot).
Data entropy
Entropy is a measure of the degree of disorder in dynamical systems that allow estimation of the complexity of biological data.21 Entropy measurements have previously been employed in the analysis of cardiovascular pathologies.22,23 Both, approximate entropy (ApEn) and sample entropy (SampleEn) were calculated in R-R time series using Kubios software (Finland, version 3.0.2).
Statistical analysis
Descriptive statistics included standard measures of central tendency and variability for continuous data. Data was expressed as mean ± standard deviation (SD), normality of the data was assessed using Shapiro Wilk test and the homoscedasticity of the variance was determined by Levene’s test. Differences between groups were assessed using unpaired T-tests for variables with normal distribution (LF n.u., HF n.u., SD2, RR, HR, SDNN, SampleEn, ApEn, SD1/SD2, HF%) and Mann–Whitney test for variables with non-normal distribution (TOTAL POWER, LF, HF, LF/HF, SD1, RMSSD, NN50, PNN50, LF%, VLF) (GraphPad Prism software Inc., version 7.0, La Jolla, CA). The statistical power (1-β) for each comparison was also calculated considering arithmetic mean, standard deviation, and number of subjects with an α < 0.05 (GPower, version 3.9.9.3, Dusseldorf, Germany). Partial correlation coefficient was calculated between current age and HRV indexes relative to current weight, current height and body mass index (BMI). All correlation plots were performed with residual data from partial correlation analysis (IBM SPSS statistics software Version 19; SPSS Inc., Chicago, IL). Correlation coefficient was categorized as strong correlation (r values > 0.7), good correlation (r values between 0.5 a 0.7), moderate correlation (r values between 0.3 to 0.5), and poor correlation (r values < 0.3).24 A priori, an α of p < 0.05 was considered statistically significant.
Results
HR and R-R intervals
HR and R-R interval time series are shown in Table 3 and Fig. 2, respectively. The hypertonia group had a significantly higher resting HR compared to the age-matched control group (129.93 ± 11.58 vs. 143.40 ± 9.93 bpm, Control vs. Hypertonia, respectively; p < 0.05; SP: 0.76) (Table 3). Accordingly, the R-R interval time series were significantly lower in hypertonic group compared to the control subjects (466.15 ± 43.47 vs. 421.13 ± 30.59 ms, Control vs. Hypertonia, respectively; p < 0.05; SP: 0.73) (Fig. 2c, d).
R-R interval time series in Control and Hypertonia groups. a Representative tachogram obtained from one Control infant and one infant with Hypertonia. b Summary data showing the differences in HR between groups. c Representative R-R interval time series obtained from one Control infant and one infant with Hypertonia. d Summary data showing the differences in R-R interval between groups. Note that the Hypertonia group had significantly shorter R-R intervals and a significant increase in resting HR. Values are mean ± standard deviation (S. D.). *p < 0.05, Unpaired Student T test. n = 15 per group
Frequency domain HRV analysis in Hypertonia
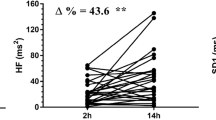
Spectral components of HRV in both hypertonia and control subjects are shown in Fig. 3. Compared to control group, the hypertonia group had an increase of the LF component (86.03 ± 6.17 vs. 80.31 ± 4.84 n.u., Hypertonia vs. Control group, respectively; SP: 0.63), a decrease of HF component (13.97 ± 6.17 vs. 20.06 ± 4.39 n.u., Hypertonia vs. Control group, respectively; p < 0.05; SP: 0.70) and an increase of the LF/HF ratio (7.54 ± 3.93 vs. 4.41 ± 1.78 n.u., Hypertonia vs. Control group, respectively; p < 0.05; SP: 0.63) (Fig. 3 and Table 3). No differences were observed in Total Power, VLF and LF non-normalized data in HRV parameters between hypertonia group and control subjects (Table 3).
Infants with hypertonia displayed autonomic imbalance. a Representative HRV spectrums obtained from one Control infant (white) and one infant with Hypertonia (black). Note that spectrums from the Hypertonia group illustrate reduction of the HF component of HRV. b Summary data showing the increase in the LF component of HRV in Hypertonia group compared to the Control group. c Summary data showing the decrease in the HF component of HRV in Hypertonia group compared to the Control group. d Summary data showing the differences in the LF/HF ratio. Note that the Hypertonia group displayed an increase in the LF/HF compared to the Control group. Values are mean ± standard deviation (S.D.). *p < 0.05, Mann–Whitney test (LF/HF ratio); Unpaired Student T test (LF and HF). n = 15 per group
Non-linear analysis of HRV
Poincaré plots from control and hypertonia groups are shown in Fig. 4. The hypertonia group showed a marked decrease in the short-term variability of HR compared to the control group. Compared to age-matched controls, the hypertonia group had a significant decrease of SD1 (6.02 ± 2.55 vs. 8.57 ± 2.88 ms, Hypertonia vs. Control group, respectively; p < 0.05; SP: 0.56) (Fig. 4b), without significant modifications in the long-term HR variability (SD2) (Fig. 4c). In addition, we calculate SD1/SD2 ratio as an indicator of the relationship between overall HRV and parasympathetic.8 Hypertonia group showed a significant decrease of SD1/SD2 ratio compared to the control group (0.30 ± 0.08 vs. 0.40 ± 0.05, Hypertonia vs. Control group, respectively; p < 0.05; SP: 0.97) (Table 3).
Hypertonia mainly affects short-term R-R variability without differences in long-term R-R variability. a Representative Poincaré plots of R-R intervals from one Control infant (white) and one infant with Hypertonia (black). b Summary data showing short-term (SD1) and c long-term R-R interval variability (SD2). Note that the Hypertonia group showed a decreased SD1 compared to the Control group. Values are mean ± standard deviation (S. D.). *p < 0.05, Mann–Whitney test (SD1); Unpaired Student T test (SD2). n = 15 per group
Time domain and entropy measures of HRV
Time domain and entropy measurements of HRV are shown in Fig. 5 and summarized in Table 3. Compared to control subjects, the hypertonia group (Hypertonia vs. Control group) displayed a significantly lower RMSSD (8.52 ± 3.63 vs. 12.12 ± 4.07 ms; p < 0.05: SP: 0.56), ApEn (1.26 ± 0.20 vs. 1.47 ± 0.07 ms; p < 0.05: SP: 0.83) and SampEn (1.27 ± 0.27 vs. 1.61 ± 0.14 ms; p < 0.05: SP: 0.89). No differences were found in SDNN and PNN50 between hypertonia and control group (Table 3).
Time domain and entropy measurements of HRV are altered in infants with hypertonia. a Summary data showing time domain HRV analysis. RMSSD was decreased in the Hypertonia group compared to the Control group. b Summary data showing no differences in the SDNN between Control and Hypertonia groups. c Summary data of ApEn and d SampEn obtained in control infants and infants with hypertonia. Note that the Hypertonia group displayed a decrease in HR entropy compared to the Control group. Values are mean ± standard deviation (S. D.). *p < 0.05, Mann–Whitney test (RMSSD); Unpaired Student T test (SDNN, ApEn, SampEn). n = 15 per group
Partial correlation analysis
A negative correlation was also observed between HR and age in the control group (r = −0.63; p < 0.05: Strong correlation) (Figure 6a, Table 4). Accordingly, a positive correlation between R-R interval time series and age were observed in the control group (r = 0.61; p < 0.05; Strong correlation) (Table 4). In contrast, there was no significant correlation between R-R and HR vs. current age in hypertonia subjects (R-R vs. Current age: r = −0.19; p > 0.05; Poor correlation; and HR vs. Current age: r = 0.19; p > 0.05: Poor correlation) (Fig. 6, Table 4). As illustrated in Fig. 3 and summarized in Table 3, the hypertonia group experienced lower values of HF component of HRV compared to control subjects. We found that the positive correlation between HF component of HRV and age observed in control group (HF vs. Current age; r = 0.47; p < 0.05; Moderate correlation), was absent in the hypertonia group (HF vs. Current age; r = 0.09; p > 0.05; Poor correlation) (Fig. 6, Table 4). In addition, we observed a negative correlation between the LF component of HRV and age in the control group (LF vs. Current age; r = −0.53; p < 0.05; Moderate correlation) but not in the hypertonia group (LF vs. Current age: r = −0.09; p > 0.05; Poor correlation) (Fig. 6, Table 4). LF/HF ratio, SD1, SD2, RMSSD, SDNN, NN50, PNN50, ApEn, SampleEn were not significant correlate with current age in control and hypertonia subjects (Table 4). All of these partial correlations were independent of current weight, height, and BMI.
Normal decrease of heart rate (HR) and increases in cardiac parasympathetic modulation with aging are absent in infants with hypertonia independently of the changes in body weight, height, and body mass index (BMI). Partial correlations between age of the subjects and a HR and b HF component of HRV adjust to body weight, height and BMI. Dashed lines (and white circles) represent linear partial regressions for the Control group and solid lines (and black triangles) represent partial linear regressions for the Hypertonia group. Control group showed a significant correlation between residuals from HR and HF component of HRV (physiological response) and current age that are absent in Hypertonia population
Discussion
The primary aim of this study was to estimate autonomic function in infants with hypertonia and delayed motor development. The main findings of our study were: (i) children diagnosed with hypertonia display increased resting HR compared to age-matched Control subjects; (ii) children with hypertonia showed an increase of LF component, decrease HF component and consequently increased LF/HF ratio, suggesting cardiac autonomic control impairment, which is mainly associated to parasympathetic withdrawal, and (iii) the normal physiological increase in parasympathetic modulation of the HR during early life was disrupted in infants with hypertonia.
HRV analysis in infants
HRV analysis has been used as an indirect surrogate of cardiac autonomic balance. Accordingly, it has been largely used to estimate autonomic dysfunction in children with a variety of pathological conditions, including congenital heart disease, Fontan circulation, attention deficit hyperactivity disorder, and type I diabetes.25,26,27,28 Our data suggest that children diagnosed with hypertonia and delayed motor development also have autonomic dysfunction as evidenced by alterations in HRV. Indeed, the HF component (mostly associated with respiratory sinus arrhythmia and parasympathetic control to the heart) in hypertonia was markedly decreased compared to control infants. Our observations of HRV differences in infants with hypertonia are similar to previous studies in children with cardiovascular diseases.27 Considering that autonomic dysfunction and especially vagal modulation, contribute to increase pathophysiological states in adult patients with hypertonia5 and importantly, that patients with parasympathetic withdrawal display an increase mortality events,7,28 it is plausible to hypothesize that infants with spontaneous hypertonia may be at risk and/or prone for the development of future cardiovascular diseases during the adulthood. Future research should address this point to fully determined the contribution of early life alterations in HRV in infants with hypertonia and its consequences in the adulthood.
In addition to spectral analysis, using non-linear, linear, and entropy analysis of HRV we found similar results, that suggest that infants with hypertonia may display cardiac autonomic dysfunction characterized by a significant decrease in cardiac vagal modulation. Indeed, we observed that SD1, RMSSD, and entropy are all significantly decreases in Hypertonia group. It has been proposed that alteration in RMSSD can be considered an independent risk factor for mortality in cardiorenal disease.28 Also, alterations in Poincaré plots (short and long-term R-R variation) have been shown to have clinically relevant prognostic value in patients with mild to moderate heart failure.29,30 Finally, entropy analysis of HR also has prognostic value in both animal models and human heart disease patients.6,31 All these indicators of HRV analysis, strongly suggest that infant with hypertonia displayed autonomic control impairment to the heart. However, something interesting to note is that the parasympathetic withdrawal in infants with hypertonia was a consistent observation, independent of the type of HRV analysis used to assess autonomic balance (i.e., linear vs. non-linear). The HF component (frequency domain), the RMSSD (linear domain), and SD1 (non-linear domain), that has been proposed as estimators of vagal modulation,11,32,33 were significantly lower in the Hypertonia group compared to Control group. Interestingly, this change in vagal modulation appears to be specific to the etiology of the hypertonia since it has also been described an increased HF component of the HRV in children with cerebral palsy-related hypertonia.5 Therefore, alterations in cardiac vagal modulation in the setting of different hypertonia etiologies deserves future investigations.
Schechtman and colleagues (1989)34 showed that cardiac vagal modulation estimated through HRV analysis increases with age. Present results confirm previous observations showing differences in cardiac vagal component with age in healthy subjects, but more importantly, our results emphasize the loss of this physiologically relevant regulatory mechanism of cardiac function in infants with hypertonia. The loss of vagal component of HRV could be associated to baroreflex control impairment, which may negatively impact cardiac performance in children with hypertonia.35 Future investigations should address the specific mechanisms underlying the decrease vagal modulation in infants with spontaneous hypertonia and its relation, if any, with alterations in baroreflex function.
Regarding physical development and autonomic nervous system, it has been proposed that delayed growth negatively influences autonomic function.36 In the present study, we found a slight but significant difference in the infant current height. Also, we found a significant correlation between the current height and current weight with PNN50 and NN50 (mainly associated to parasympathetic control)10,11 in Control infants and that this correlation was lost in infants with hypertonia. Thus, our data support and extend findings of previous studies suggesting that alterations in growth may negatively influence cardiac autonomic balance. However, we cannot rule out the possibility that this phenomenon may occur in the opposite direction, that is that altered autonomic function negatively influences growth. This is a novel hypothesis that deserves future investigation.
Limitations
A limitation of the present study is that our experimental sample size (n = 15 per group) was limited. Even though we can confirm that differences between groups in HRV indicators that suggest the presence of parasympathetic withdrawal (our major relevant finding) are not false positives since the statistical power was between 0.56 and 0.70 (medium to large)37, we acknowledge that a greater multi-center study is needed to support the efficacy of any intervention intended to improve autonomic function in infants with hypertonia. Other limitation of our study was that we only performed short-term recordings. However, previous studies have validated the use of short-term recordings (5–10 min.) for non-linear and frequency domain of HRV analysis.11 In addition, respiration was not monitored during this study. To our knowledge, there are no studies showing altered resting breathing patterns in infants with hypertonia. Nevertheless, we observed a slight but not significant increase in the VLF component of the HRV in the hypertonia group compared to controls. This may be relevant since alterations in the VLF component of the HRV has been shown to be present in pathological conditions whereas oscillatory breathing patterns are present. Then, future studies are needed to address the changes, if any, in ventilatory rhythm/pattern in infants with spontaneous hypertonia and how it may influence HRV.
Conclusions
Hypertonia is commonly observed in multiple pathologies including amyotrophic lateral sclerosis, cerebral palsy, ischemic, or hemorrhagic vascular lesions, traumatic brain injury, or hypoxic encephalopathy.15 Our results show for the first time that children diagnosed with spontaneous hypertonia (without any other concurrent neurological disorder) display alterations in HRV mostly characterized by decrease HF component of HRV, suggesting decrease cardiac vagal modulation. Considering that autonomic imbalance is recognized as an independent risk factor for the development of cardiovascular disease, results of the present study showing alterations in HRV in infants with spontaneous hypertonia may be useful in the clinical setting to assess cardiovascular risk in this population. Future studies should focus on identifying the pathophysiological mechanisms underlaying the alterations in HRV associated with hypertonia.
References
Sanger, T. D. et al. Task force on childhood motor disorders. classification and definition of disorders causing hypertonia in childhood. Pediatrics 111, e89–e97 (2003).
Delwaide, P. J. Pyramidal syndrome: its physiopathology and treatment. Recent. Prog. Med. 80, 681–685 (1989).
Evans, S. H., Cameron, M. W. & Burton, J. M. Hypertonia. Curr. Probl. Pediatr. Adolesc. Health Care 47, 161–166 (2017).
Hart, A. R. et al. Neonatal hypertonia—a diagnostic challenge. Dev. Med. Child Neurol. 57, 600–610 (2015).
Ferreira, M. C. et al. Autonomic nervous system in individuals with cerebral palsy: a controlled study. J. Oral. Pathol. Med. 40, 576–581 (2011).
Hung, C. Y. et al. Cardiac autonomic status is associated with spasticity in post-stroke patients. NeuroRehabilitation 34, 227–233 (2014).
Pradhapan, P. et al. Effect of heart rate correction on pre- and post-exercise heart rate variability to predict risk of mortality—an experimental study on the FINCAVAS cohort. Front. Physiol. 5, 208 (2014).
Vanderlei, L. C. et al. Basic notions of heart rate variability and its clinical applicability. Rev. Bras. Cir. Cardiovasc. 24, 205–217 (2009).
Billman, G. E. Heart rate variability—a historical perspective. Front. Physiol. 2, 86 (2011).
Cygankiewicz, I. & Zareba, W. Heart rate variability. Handb. Clin. Neurol. 117, 379–393 (2013).
Camm, J. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task force of the european society of cardiology and the north american society of pacing and electrophysiology. Circulation 93, 1043–1065 (1996).
Tsuji, H. et al. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation 94, 2850–2855 (1996).
Thayer, J. F., Yamamoto, S. S. & Brosschot, J. F. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int. J. Cardiol. 141, 122–131 (2010).
Huang, J. C. et al. Heart rate variability predicts major adverse cardiovascular events and hospitalization in maintenance hemodialysis patients. Kidney Blood. Press. Res. 42, 76–88 (2017).
Purves D., et al. Neuroscience 2nd edition. (Sunderland, Sinauer Associates, 2001).
Gamelin, F. X. et al. Validity of the polar S810 to measure R-R intervals in infants. Int. J. Sports Med. 29, 134–138 (2008).
Tarvainen, M. P. et al. Kubios HRV—heart rate variability analysis software. Comput. Methods Prog. Biomed. 113, 210–220 (2014).
Jarrin, D. C. et al. Measurement fidelity of heart rate variability signal processing: the devil is in the details. Int. J. Psychophysiol. 86, 88–97 (2012).
Lasky, R. E. et al. Changes in the PQRST intervals and heart rate variability associated with rewarming in two newborns undergoing hypothermia therapy. Neonatology 96, 93–95 (2009).
Fleming, S. et al. Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: a systematic review of observational studies. Lancet 377, 1011–1018 (2011).
Richman, J. S. & Moorman, J. R. Physiological time-series analysis using approximate entropy and sample entropy. Am. J. Physiol. Heart Circ. Physiol. 278, H2039–H2049 (2000).
Khandoker, A. H., . & Jelinek, H. F. Evaluating cardiovascular risk using the tone-entropy algorithm. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2013, 6139–6141 (2013).
Chiu, H. C. et al. Complexity of cardiac signals for predicting changes in alpha-waves after stress in patients undergoing cardiac catheterization. Sci. Rep. 5, 13315 (2015).
Hazra, A. & Gogtay, N. Biostatistics series module 6: correlation and linear regression. Indian J. Dermatol. 61, 593–601 (2016).
Massin, M. & von Bernuth, G. Clinical and haemodynamic correlates of heart rate variability in children with congenital heart disease. Eur. J. Pediatr. 157, 967–971 (1998).
Kardelen, F. et al. Heart rate variability and circadian variations in type 1 diabetes mellitus. Pediatr. Diabetes 7, 45–50 (2006).
Dahlqvist, J. A. et al. Heart rate variability in children with fontan circulation: lateral tunnel and extracardiac conduit. Pediatr. Cardiol. 33, 307–315 (2012).
Drawz, P. E. et al. Heart rate variability is a predictor of mortality in chronic kidney disease: a report from the CRIC Study. Am. J. Nephrol. 38, 517–528 (2013).
Stein, P. K. & Reddy, A. Non-linear heart rate variability and risk stratification in cardiovascular disease. Indian. Pacing Electrophysiol. J. 5, 210–220 (2005).
Brouwer, J. et al. Prognostic value of heart rate variability during long-term follow-up in patients with mild to moderate heart failure. The Dutch Ibopamine Multicenter Trial Study Group. J. Am. Coll. Cardiol. 28, 1183–1189 (1996).
Silva, L. E. et al. Multiscale entropy analysis of heart rate variability in heart failure, hypertensive, and sinoaortic-denervated rats: classical and refined approaches. Am. J. Physiol. Regul. Integr. Comp. Physiol. 311, R150–R156 (2016).
Tewari, H. K. et al. Sympathetic-parasympathetic activity and reactivity in central serous chorioretinopathy: a case-control study. Invest. Ophthalmol. Vis. Sci. 47, 3474–3478 (2006).
DeGiorgio, C. M. et al. RMSSD, a measure of vagus-mediated heart rate variability, is associated with risk factors for SUDEP: the SUDEP-7 Inventory. Epilepsy Behav. 19, 78–81 (2010).
Schechtman, V. L., Harper, R. M. & Kluge, K. A. Development of heart rate variation over the first 6 months of life in normal infants. Pediatr. Res. 26, 343–346 (1989).
Hunt, B. E., Fahy, L., Farquhar, W. B. & Taylor, J. A. Quantification of mechanical and neural components of vagal baroreflex in humans. Hypertension 37, 1362–1368 (2001).
Zamuner, A. R. et al. The influence of motor impairment on autonomic heart rate modulation among children with cerebral palsy. Res. Dev. Disabil. 32, 217–221 (2011).
Cohen, J. Statistical Power Analysis for the Behavioral Sciences. (Academic Press Inc, New York, 1977).
Acknowledgements
The authors thank the “Corporación de Rehabilitation Club of Leones Cruz del Sur” of Punta Arenas and its personnel for allowing the use of their facilities and for their excellent disposition and collaboration.
Funding
FONDECYT 1180172 grant from the National Fund for Scientific and Technological Development of Chile and Interinstitutional Project PI1804 from U. Católica Silva Henríquez.
Author information
Authors and Affiliations
Contributions
A.A.-A., D.C.A. and C.T. perform data analysis and interpretation of data; C.M. perform acquisition of data, analysis and interpretation of data; A.A.-A., C.M., D.C.A., C.T., N.J.M. and R.D.R. draft the article and review it critically for important intellectual content. R.D.R. perform conception and design of the study. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arce-Alvarez, A., Melipillán, C., Andrade, D.C. et al. Heart rate variability alterations in infants with spontaneous hypertonia. Pediatr Res 86, 77–84 (2019). https://doi.org/10.1038/s41390-019-0318-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0318-7
- Springer Nature America, Inc.