Abstract
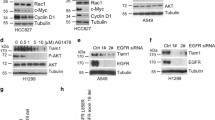
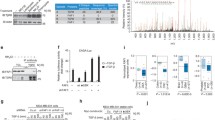
Protein-tyrosine kinases regulate a broad range of intracellular processes occurring primarily just beneath the plasma membrane. With the greatest care to prevent dephosphorylation, we have shown that nuclear tyrosine phosphorylation regulates global chromatin structural states. However, the roles for tyrosine phosphorylation in the nucleus are poorly understood. Here we identify transcriptional intermediary factor 1-γ (TIF1γ/TRIM33/Ectodermin), which suppresses transforming growth factor-β (TGF-β) signaling through the association with Smad2/3 transcription factor, as a new nuclear substrate of c-Abl tyrosine kinase. Replacement of the three tyrosine residues Tyr-524, -610, and -1048 with phenylalanine (3YF) inhibits c-Abl-mediated phosphorylation of TIF1γ and enhances TIF1γ’s association with Smad3. Importantly, knockdown-rescue experiments show that 3YF strengthens TIF1γ’s ability to suppress TGF-β signaling. Intriguingly, activation of c-Abl by epidermal growth factor (EGF) induces desuppression of TGF-β signaling via enhancing the tyrosine phosphorylation level of TIF1γ. TGF-β together with EGF synergistically provokes desuppressive responses of epithelial-to-mesenchymal transition through tyrosine phosphorylation of TIF1γ. These results suggest that nuclear c-Abl-mediated tyrosine phosphorylation of TIF1γ has a desuppressive role in TGF-β–Smad2/3 signaling.









Similar content being viewed by others
References
Hubbard SR, Till JH. Protein tyrosine kinase structure and function. Annu Rev Biochem. 2000;69:373–98.
Hunter T. Tyrosine phosphorylation: thirty years and counting. Curr Opin Cell Biol. 2009;21:140–6.
Taagepera S, McDonald D, Loeb JE, Whitaker LL, McElroy AK, Wang JYJ, et al. Nuclear-cytoplasmic shuttling of c-Abl tyrosine kinase. Proc Natl Acad Sci USA. 1998;95:7457–62.
Greuber EK, Smith-Pearson P, Wang J, Pendergast AM. Role of Abl family kinases in cancer: from leukaemia to solid tumours. Nat Rev Cancer. 2013;13:559–71.
Khatri A, Wang J, Pendergast AM. Multifunctional Abl kinases in health disease. J Cell Sci. 2016;129:9–16.
Wang JYJ. The capable Abl: what is its biological function? Mol Cell Biol. 2014;34:1188–97.
Woodring PJ, Hunter T, Wang JYJ. Regulation of F-actin-dependent processes by the Abl family of tyrosine kinases. J Cell Sci. 2003;116:2613–26.
Aoyama K, Fukumoto Y, Ishibashi K, Kubota S, Morinaga T, Horiike Y, et al. Nuclear c-Abl-mediated tyrosine phosphorylation induces chromatin structural changes through histone modifications that include H4K16 hypoacetylation. Exp Cell Res. 2011;317:2874–903.
Aoyama K, Yuki R, Horiike Y, Kubota S, Yamaguchi N, Morii M, et al. Formation of long winding nuclear F-actin bundles by nuclear c-Abl tyrosine kinase. Exp Cell Res. 2013;319:3251–68.
Baskaran R, Wood LD, Whitaker LL, Canman CE, Morgan SE, Xu Y, et al. Ataxia telangiectasia mutant protein activates c-Abl tyrosine kinase in response to ionizing radiation. Nature. 1997;387:516–9.
Shafman T, Khanna KK, Kedar P, Spring K, Kozlov S, Yen T, et al. Interaction between ATM protein c-Abl in response to DNA damage. Nature. 1997;387:520–3.
Herquel B, Ouararhni K, Davidson I. The TIF1α-related TRIM cofactors couple chromatin modifications to transcriptional regulation, signaling and tumor suppression. Transcription. 2011;2:231–6.
Massagué J. TGFβ signalling in context. Nat Rev Mol Cell Biol. 2012;13:616–30.
Wang S, Wilkes MC, Leof EB, Hirschberg R. Noncanonical TGF-β pathways mTORC1 and Abl in renal interstitial fibrogenesis. Am J Physiol Ren Physiol. 2010;298:F142–F149.
He W, Dorn DC, Erdjument-Bromage H, Tempst P, Moore MA, Massagué J. Hematopoiesis controlled by distinct TIF1γ and Smad4 branches of the TGFβ pathway. Cell. 2006;125:929–41.
Hantschel O, Nagar B, Guettler S, Kretzschmar J, Dorey K, Kuriyan J, et al. A myristoyl/phosphotyrosine switch regulates c-Abl. Cell. 2003;112:845–57.
Dennler S, Itoh S, Vivien D, ten Dijke P, Huet S, Gauthier JM. Direct binding of Smad3 and Smad4 to critical TGFβ-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. EMBO J. 1998;17:3091–3100.
Sirvent A, Benistant C, Roche S. Cytoplasmic signalling by the c-Abl tyrosine kinase in normal and cancer cells. Biol Cell. 2008;100:617–31.
Nasrollahi S, Pathak A. Topographic confinement of epithelial clusters induces epithelial-to-mesenchymal transition in compliant matrices. Sci Rep. 2016;6:18831.
Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial–mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–96.
Sundqvist A, Zieba A, Vasilaki E, Herrera Hidalgo C, Söderberg O, Koinuma D, et al. Specific interactions between Smad proteins and AP-1 components determine TGFβ-induced breast cancer cell invasion. Oncogene. 2013;32:3606–15.
Bergeron JJ, Di Guglielmo GM, Dahan S, Dominiguez M, Posner BI. Spatial and temporal regulation of receptor tyrosine kinase activation and intracellular signal transduction. Annu Rev Biochem. 2016;85:573–97.
Hunter T. The genesis of tyrosine phosphorylation. CSH Perspect Biol. 2014;6:a020644.
Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68.
Schlessinger J. Receptor tyrosine kinases: legacy of first two decades. CSH Perspect Biol. 2014;6:a008912.
Cans C, Mangano R, Barilá D, Neubauer G, Superti-Furga G. Nuclear tyrosine phosphorylation: the beginning of a map. Biochem Pharmacol. 2000;60:1203–15.
Moorhead GB, Trinkle-Mulcahy L, Ulke-Lemée A. Emerging roles of nuclear protein phosphatases. Nat Rev Mol Cell Biol. 2007;8:234–44.
Yamaguchi N, Nakayama Y, Urakami T, Suzuki S, Nakamura T, Suda T, et al. Overexpression of the Csk homologous kinase (Chk tyrosine kinase) induces multinucleation: a possible role for chromosome-associated Chk in chromosome dynamics. J Cell Sci. 2001;114:1633–41.
Morii M, Kubota S, Honda T, Yuki R, Morinaga T, Kuga T, et al. Src acts as an effector for Ku70-dependent suppression of apoptosis through phosphorylation of Ku70 at Tyr-530. J Biol Chem. 2017;292:1648–65.
Ishibashi K, Fukumoto Y, Hasegawa H, Abe K, Kubota S, Aoyama K, et al. Nuclear ErbB4 signaling through H3K9me3 is antagonized by EGFR-activated c-Src. J Cell Sci. 2013;126:625–37.
Kubota S, Fukumoto Y, Aoyama K, Ishibashi K, Yuki R, Morinaga T, et al. Phosphorylation of KRAB-associated protein 1 (KAP1) at Tyr-449, Tyr-458, and Tyr-517 by nuclear tyrosine kinases inhibits the association of KAP1 and heterochromatin protein 1α (HP1α) with heterochromatin. J Biol Chem. 2013;288:17871–83.
Kubota S, Fukumoto Y, Ishibashi K, Soeda S, Kubota S, Yuki R, et al. Activation of the pre-replication complex is blocked by mimosine through reactive oxygen species-activated Ataxia telangiectasia mutated (ATM) protein without DNA damage. J Biol Chem. 2015;290:10891–904.
Takahashi A, Obata Y, Fukumoto Y, Nakayama Y, Kasahara K, Kuga T, et al. Nuclear localization of Src-family tyrosine kinases is required for growth factor-induced euchromatinization. Exp Cell Res. 2009;315:1117–41.
Yamaguchi N, Shibazaki M, Yamada C, Anzai E, Morii M, Nakayama Y, et al. Tyrosine phosphorylation of the pioneer transcription factor FoxA1 promotes activation of estrogen signaling. J Cell Biochem. 2017;118:1453–61.
Kuki K, Yamaguchi N, Iwasawa S, Takakura Y, Aoyama K, Yuki R, et al. Enhancement of TGF-β-induced Smad3 activity by c-Abl-mediated tyrosine phosphorylation of its coactivator SKI-interacting protein (SKIP). Biochem Biophys Res Commun. 2017;490:1045–51.
Deininger M, Buchdunger E, Druker BJ. The development of imatinib as a therapeutic agent for chronic myeloid leukemia. Blood. 2005;105:2640–53.
Quéré R, Saint-Paul L, Carmignac V, Martin RZ, Chrétien ML, Largeot A, et al. Tif1γ regulates the TGF-β1 receptor and promotes physiological aging of hematopoietic stem cells. Proc Natl Acad Sci USA. 2014;111:10592–7.
Macias MJ, Martin-Malpartida P, Massagué J. Structural determinants of Smad function in TGF signaling. Trends Biochem Sci. 2015;40:296–308.
Agricola E, Rall RA, Gaarenstroom T, Dupont S, Hill CS. Recruitment of TIF1γ to chromatin via its PHD finger bromodomain activates its ubiquitin ligase and transcriptional repressor activities. Mol Cell. 2011;43:85–96.
Plattner R, Kadlec L, DeMali KA, Kazlauskas A, Pendergast AM. c-Abl is activated by growth factors and Src family kinases and has a role in the cellular response to PDGF. Genes Dev. 1999;13:2400–11.
Grände M, Franzen A, Karlsson JO, Ericson LE, Heldin NE, Nilsson M. Transforming growth factor-β and epidermal growth factor synergistically stimulate epithelial to mesenchymal transition (EMT) through a MEK-dependent mechanism in primary cultured pig thyrocytes. J Cell Sci. 2002;115:4227–36.
He J, Bazan HE. Epidermal growth factor synergism with TGF-β1 via PI-3 kinase activity in corneal keratocyte differentiation. Invest Ophthalmol Vis Sci. 2008;49:2936–45.
Krainock M, Toubat O, Danopoulos S, Beckham A, Warburton D, Kim R. Epicardial epithelial-to-mesenchymal transition in heart development and disease. J Clin Med. 2016;19:E27.
Morabito CJ, Dettman RW, Kattan J, Collier JM, Bristow J. Positive and negative regulation of epicardial-mesenchymal transformation during avian heart development. Dev Biol. 2001;234:204–15.
Jia M, Souchelnytstkyi S. Comments on the cross-talk of TGFβ and EGF in cancer. Exp Oncol. 2011;33:170–3.
Kretzschmar M, Doody J, Timokhina I, Massagué J. A mechanism of repression of TGFβ/Smad signaling by oncogenic Ras. Genes Dev. 1999;13:804–16.
Docherty NG, O’Sullivan OE, Healy DA, Murphy M, O’Neill AJ, Fitzpatrick JM, et al. TGF-β1-induced EMT can occur independently of its proapoptotic effects and is aided by EGF receptor activation. Am J Physiol Ren Physiol. 2006;290:F1202–F1212.
Saha D, Datta PK, Sheng H, Morrow JD, Wada M, Moses H, et al. Synergistic induction of cyclooxygenase-2 by transforming growth factor-β1 and epidermal growth factor inhibits apoptosis in epithelial cells. Neoplasia. 1999;1:508–17.
Uttamsingh S, Bao X, Nguyen KT, Bhanot M, Gong J, Chan JLK, et al. Synergistic effect between EGF and TGF-β1 in inducing oncogenic properties of intestinal epithelial cells. Oncogene. 2008;27:2626–34.
Fukumoto Y, Obata Y, Ishibashi K, Tamura N, Kikuchi I, Aoyama K, et al. Cost-effective gene transfection by DNA compaction at pH 4.0 using acidified long shelf-life polyethylenimine. Cytotechnology. 2010;62:73–82.
Shtivelman E, Lifshitz B, Gale RP, Canaani E. Fused transcript of abl and bcr genes in chronic myelogenous leukaemia. Nature. 1985;315:550–4.
Aoyama K, Yamaguchi N, Yuki R, Morii M, Kubota S, Hirata K, et al. c-Abl induces stabilization of histone deacetylase 1 (HDAC1) in a kinase activity-dependent manner. Cell Biol Int. 2015;39:446–56.
Hasegawa H, Ishibashi K, Kubota S, Yamaguchi C, Yuki R, Nakajo H. et al. Cdk1-mediated phosphorylation of human ATF7 at Thr-51 and Thr-53 promotes cell-cycle progression into M phase. PLoS One. 2014;9:e116048
Kasahara K, Nakayama Y, Sato I, Ikeda K, Hoshino M, Endo T, et al. Role of Src-family kinases in formation and trafficking of macropinosomes. J Cell Physiol. 2007;211:220–32.
Dupont S, Mamidi A, Cordenonsi M, Montagner M, Zacchigna L, Adorno M, et al. FAM/USP9x a deubiquitinating enzyme essential for TGFβ signaling controls Smad4 monoubiquitination. Cell. 2009;136:123–35.
Obata Y, Fukumoto Y, Nakayama Y, Kuga T, Dohmae N, Yamaguchi N. The Lyn kinase C-lobe mediates Golgi export of Lyn through conformation-dependent ACSL3 association. J Cell Sci. 2010;123:2649–62.
Morii M, Fukumoto Y, Kubota S, Yamaguchi N, Nakayama Y, Yamaguchi N. Imatinib inhibits inactivation of the ATM/ATR signaling pathway and recovery from Adriamycin/doxorubicin-induced DNA damage checkpoint arrest. Cell Biol Int. 2015;39:923–32.
Yuki R, Aoyama K, Kubota S, Yamaguchi N, Kubota S, Hasegawa H, et al. Overexpression of Zinc-finger protein 777 (ZNF777) inhibits proliferation at low cell density through down-regulation of FAM129A. J Cell Biochem. 2015;116:954–68.
Tamura T, Kunimatsu T, Yee ST, Igarashi O, Utsuyama M, Tanaka S, et al. Molecular mechanism of the impairment in activation signal transduction in CD4 T cells from old mice. Int Immunol. 2000;12:1205–15.
Sato I, Obata Y, Kasahara K, Nakayama Y, Fukumoto Y, Yamasaki T, et al. Differential trafficking of Src, Lyn, Yes, and Fyn is specified by the state of palmitoylation in the SH4 domain. J Cell Sci. 2009;122:965–75.
Fattet L, Ay AS, Bonneau B, Jallades L, Mikaelian I, Treilleux I, et al. TIF1γ requires sumoylation to exert its repressive activity on TGFβ signaling. J Cell Sci. 2013;126:3713–23.
Soeda S, Nakayama Y, Honda T, Aoki A, Tamura N, Abe K. et al. v-Src causes delocalization of Mklp1,Aurora B, and INCENP from the spindle midzone during cytokinesis failure. Exp Cell Res. 2013;319:1382–97.
Honda T, Morii M, Nakayama Y, Suzuki K, Yamaguchi N, Yamaguchi N. v-Src-driven transformation is due to chromosome abnormalities but not Src-mediated growth signaling. Sci Rep. 2018;8:1063.
Yamaguchi N, Yuki R, Kubota S, Aoyama K, Kuga T, Hashimoto Y, et al. c-Abl-mediated tyrosine phosphorylation of JunB is required for Adriamycin-induced expression of p21. Biochem J. 2015;471:67–77.
Acknowledgements
We are grateful to Dr. Eli Canaani (Weizmann Institute of Science), Dr. Hiroyuki Miyoshi (RIKEN BRC, Tsukuba), Dr. Toshiki Tamura (National Institute of Infectious Diseases, Tokyo), and Dr. Masatoshi Tagawa (Chiba Cancer Center Research Institute, Chiba) for their materials. This work was supported in part by grants-in-aid for Scientific Research 15K07922 (to Naoto Y) and 16K08227 (to Noritaka Y), Global COE Program, and Program for Leading Graduate School (LGS) from the MEXT. KA and SK were G-COE Research Assistants. TH and M.M. were LGS Research Assistants. SK, TH, and MM are JSPS Research Fellows.
Author contributions
RY and Naoto Y conceived the study, designed the experiments, and wrote the manuscript. RY, T Tatewaki, Noritaka Y, and TH performed experiments. KA, SK, TK, and T Tomonaga performed phosphoproteomic analysis. RY, T Tatewaki, KA, TH, SK, MM, IM, and Naoto Y analyzed and discussed the data. All authors approved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Yuki, R., Tatewaki, T., Yamaguchi, N. et al. Desuppression of TGF-β signaling via nuclear c-Abl-mediated phosphorylation of TIF1γ/TRIM33 at Tyr-524, -610, and -1048. Oncogene 38, 637–655 (2019). https://doi.org/10.1038/s41388-018-0481-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-018-0481-z
- Springer Nature Limited
This article is cited by
-
SH2D4A promotes centrosome maturation to support spindle microtubule formation and mitotic progression
Scientific Reports (2023)
-
Src-mediated tyrosine phosphorylation of PRC1 and kinastrin/SKAP on the mitotic spindle
Scientific Reports (2021)