Abstract
Background
The rate of gastric emptying and glucoregulatory hormones are key determinants of postprandial glycaemia. Intragastric administration of L-tryptophan slows gastric emptying and reduces the glycaemic response to a nutrient drink in lean individuals and those with obesity. We investigated whether tryptophan decreases postprandial glycaemia and slows gastric emptying in type 2 diabetes (T2D).
Methods
Twelve men with T2D (age: 63 ± 2 years, HbA1c: 49.7 ± 2.5 mmol/mol, BMI: 30 ± 1 kg/m2) received, on three separate occasions, 3 g (‘Trp-3’) or 1.5 g (‘Trp-1.5’) tryptophan, or control (0.9% saline), intragastrically, in randomised, double-blind fashion, 30 min before a mixed-nutrient drink (500 kcal, 74 g carbohydrates), containing 3 g 3-O-methyl-D-glucose (3-OMG) to assess glucose absorption. Venous blood samples were obtained at baseline, after tryptophan, and for 2 h post-drink for measurements of plasma glucose, C-peptide, glucagon and 3-OMG. Gastric emptying of the drink was quantified using two-dimensional ultrasound.
Results
Tryptophan alone stimulated C-peptide (P = 0.002) and glucagon (P = 0.04), but did not affect fasting glucose. In response to the drink, Trp-3 lowered plasma glucose from t = 15–30 min and from t = 30–45 min compared with control and Trp-1.5, respectively (both P < 0.05), with no differences in peak glucose between treatments. Gastric emptying tended to be slower after Trp-3, but not Trp-1.5, than control (P = 0.06). Plasma C-peptide, glucagon and 3-OMG increased on all days, with no major differences between treatments.
Conclusions
In people with T2D, intragastric administration of 3 g tryptophan modestly slows gastric emptying, associated with a delayed rise, but not an overall lowering of, postprandial glucose.
Similar content being viewed by others
Introduction
Gastric emptying and the release of the glucoregulatory hormones, insulin and glucagon, are important determinants of the postprandial blood glucose response to carbohydrate-containing meals, in health, obesity and type 2 diabetes (T2D)1. For example, gastric emptying, which exhibits a substantial inter-individual variation, accounts for about 35% of the variance in peak postprandial blood glucose1. Slowing of gastric emptying, by dietary or pharmacological means, attenuates postprandial glycaemic excursions, particularly during the first 30–60 min postprandially2,3. Gastric emptying and the release of glucoregulatory hormones can be influenced by nutrients4,5. For example, in T2D, whey protein, when given as a preload 30 min before a high-carbohydrate meal, slowed gastric emptying and stimulated insulin, leading to a reduction in the glycaemic response6. These effects of protein appear to be mediated, at least in part, by their digestion products, amino acids — both circulating amino acids and as a result of the interaction of amino acids with the small intestine7,8,9,10.
A number of amino acids have been shown to reduce the blood glucose response to glucose or mixed-nutrient drinks11,12,13,14,15. The aromatic amino acid, tryptophan, is of particular interest; it potently stimulates pyloric motility and plasma cholecystokinin (CCK) concentrations, which are both central to the slowing of gastric emptying16,17. Solutions containing tryptophan, when administered intragastrically or orally, empty from the stomach more slowly than control solutions18,19. We reported that intragastric administration of 3 g tryptophan slowed gastric emptying of a mixed-nutrient drink, consumed 15 min later, associated with a reduced glycaemic response in the first 30 min, in both lean individuals and those with obesity who do not have T2D15. That intragastric tryptophan had a minimal16, or no19, effect to stimulate insulin, and may reduce insulin15, suggests that the attenuation in postprandial blood glucose is unlikely to reflect an insulinotropic effect of tryptophan. Like other amino acids, tryptophan has been reported to stimulate glucagon15,16, which may potentially counteract glucose lowering by stimulating glycogenolysis and gluconeogenesis. Hence, slowing of gastric emptying is likely to be fundamental to a reduction in postprandial glycaemia by tryptophan. The above considerations are of relevance to the management of T2D; it is now appreciated that postprandial glycaemic excursions are a major determinant of overall glycaemic control, as assessed by measurement of glycated haemoglobin, and the consequent risk of microvascular complications (i.e. retinopathy, nephropathy and neuropathy). However, there is no information about the effects of tryptophan in T2D. The observed reduction in postprandial glucose resulting from slowing of gastric emptying, induced by tryptophan, should intuitively be associated with a reduction in the rate of carbohydrate absorption, as supported by animal studies20. Glucose absorption can be measured in humans using plasma concentrations of the non-metabolisable glucose analogue, 3-orthomethyl-glucose (3-OMG)21.
The aim of the current study was to evaluate the effects of intragastric administration (to avoid potential confounding effects of taste) of tryptophan on the glycaemic response to, as well as glucoregulatory hormones and glucose absorption, and gastric emptying of, a mixed-nutrient drink in people with T2D.
Materials and methods
Participants
Twelve men with T2D (mean age: 63 ± 2 years, BMI: 30 ± 1 kg/m2, HbA1c: 49.7 ± 2.5 mmol/mol (6.7 ± 0.1%), duration of diabetes: 10 ± 2 years) participated in the study. Their diabetes was managed by metformin (500–2000 mg/day) alone (n = 7), or in combination with a DPP-4 inhibitor (n = 2) or a sodium-glucose transport inhibitor (n = 3). Participants were recruited by flyers placed at the Royal Adelaide Hospital and Diabetes South Australia, and through advertisements on online sites (University of Adelaide and Gumtree). Participants were excluded if they had significant gastrointestinal disorders or symptoms, cardiovascular or respiratory diseases or surgery, used medication known to affect gastrointestinal function and/or appetite, were lactose-intolerant, consumed protein supplements or >2 standard drinks (20 g) alcohol on >5 days a week, were vegetarians or smokers, or had an estimated glomerular filtration rate <45 mL/min, or low serum ferritin (<30 μg/L) or iron (<8 μmol/L) levels (a requirement by the Human Research Ethics Committee). In all cases, autonomic nerve function was evaluated using standardised cardiovascular reflex tests, and the presence of autonomic neuropathy (i.e. a score ≥3) represented an exclusion22. After inclusion, each participant was allocated to a treatment order of balanced randomisation (www.randomization.com) by a research officer who was not involved in data analysis. The Human Research Ethics Committee of the Central Adelaide Local Health Network approved the study protocol, and the study was performed in accordance with the Declaration of Helsinki and the National Health and Medical Research Council of Australia Statement on Ethical Conduct in Human Research. All participants provided written informed consent before inclusion. The study was registered as a clinical trial with the Australia and New Zealand Clinical Trials Registry (www.anzctr.org.au) (trial number: ACTRN12613000899741).
Study design
We investigated the effects of intragastric administration of (i) 3 g (Trp-3) or (ii) 1.5 g (Trp-1.5) tryptophan, or (iii) control (0.9% saline) on plasma glucose (the primary outcome), C-peptide (a measure of insulin secretion), and glucagon concentrations and glucose absorption in response to, and gastric emptying of, a mixed-nutrient drink, consumed 30 min after the intragastric treatments.
Control and tryptophan treatments
Tryptophan treatments were prepared by dissolving 1.5 g or 3 g food-grade tryptophan (PureBulk Inc., Roseburg, Oregon, USA), 58 mg CaCl2xH2O, and 1.75 g or 1.65 g NaCl, respectively, in 200-mL water for irrigation. Control solutions consisted of 58 mg CaCl2xH2O and 1.85 g NaCl in 200-mL water. The solutions were approximately iso-osmolar (control: 296, 1.5 g: 318, 3 g: 335 mOsm/kg) and had a pH of ~7 and a temperature of ~23 °C. Solutions were prepared by a research officer, who was not involved in the performance of the studies or data analysis, on the morning of each study and administered via a nasogastric catheter directly into the stomach. Syringes were covered, so that both study participants and the investigators performing the study were blinded to the nature of the solution. The doses of tryptophan were chosen based on our previous work and were well tolerated15.
Study protocol
Participants were studied in a randomised, double-blind, cross-over fashion on three occasions each separated by 3–7 days. Participants were provided with a standardised meal (Beef lasagne, McCain Food, Wendouree, Victoria, Australia; energy content: 603 kcal), to be consumed between 6.30 p.m. and 7 p.m. on the night prior to each study. All participants were instructed to maintain their normal eating habits between study days and to discontinue their glucose-lowering medication for 48 h, and refrain from strenuous exercise and alcohol for 24 h, before each study. Participants were also asked to abstain from any solids or liquids, except water (which was allowed until 7 a.m.), after the evening meal until they attended the Clinical Research Facility at the University of Adelaide at 8.30 a.m. the next morning. An intravenous cannula was placed into a forearm vein, and the arm was kept warm with a heat pad for regular sampling of ‘arterialised’ blood. A baseline blood sample for measurement of plasma glucose and hormones, and a baseline antral area measurement, using two-dimensional (2D) ultrasound, with the participant seated in an upright position, were taken. Participants also completed a visual analogue scale (VAS) questionnaire to assess gastrointestinal symptoms. Each participant was then intubated with a soft-silicon feeding tube (outer diameter: 4 mm; Dentsleeve, Mississauga, Ontario, Canada), which was inserted through an anaesthetised nostril into the stomach. Immediately thereafter (t = −31 min, ~8.45 a.m.), participants received the 200-mL intragastric bolus of one of the two doses of tryptophan, or control, within 1 min. The tube was then removed. Further blood samples and VAS ratings were collected at t = −20, −10 and −1 min. At t = −1 min, each participant consumed, within 1 min, a mixed-nutrient drink (350 mL, 500 kcal, 74 g carbohydrates, including maltodextrin and sucrose, 18 g protein and 15 g fat) consisting of 325-mL Nestle Resource Plus® vanilla (Nestle Healthcare Nutrition, Tongala, Victoria, Australia) plus 25-mL water to make up the final volume, containing 3 g 3-OMG (Sigma-Aldrich, Milwaukee, Wisconsin, USA). Blood samples and VAS ratings were collected subsequently at 15-min intervals from t = 15 to 60 min and at 30-min intervals from t = 60 to 120 min. Measurements of antral area were obtained immediately after the drink (t = 0 min), at 5-min intervals from t = 0 to 60 min, and at 15-min intervals from t = 60 to 120 min. At t = 120 min, the cannula was removed, and participants were provided with a light lunch, after which they were free to leave the laboratory.
Measurements
Plasma glucose, C-peptide, glucagon and serum 3-OMG concentrations
For glucose and hormones, venous blood samples (7 mL) were collected into ice-chilled ethylenediaminetetraacetic acid-containing tubes. For serum 3-OMG, 3-mL venous samples were collected into untreated tubes and allowed to clot. Plasma and serum were each separated by centrifugation at 3200 rpm for 15 min at 4 °C within 15 min of collection and stored at −80 °C until subsequent analysis.
Plasma glucose concentrations (mmol/L) were quantified by the glucose oxidase method using a glucose analyser (YSI 2300 Stat Plus, Yellow Springs Instruments, Yellow Springs, Ohio, USA). Intra- and inter-assay coefficient variations (CVs) were ≤2%.
Plasma C-peptide concentrations (pmol/L) were measured by ELISA immunoassay (10-1136-01, Mercodia, Uppsala, Sweden). The minimum detectable limit was 15 pmol/L and the inter- and intra-assay CVs were 8.3% and 2.9%, respectively. C-peptide reflects endogenous insulin secretion, since it is not extracted by the liver and its half-life is longer than that of insulin23.
Plasma glucagon concentrations (pg/mL) were measured by radioimmunoassay (GL-32K, Millipore, Billerica, Massachusetts, USA). The minimum detectable limit was 15 pg/mL, and inter- and intra-assay CVs were 6.9% and 4.2%, respectively.
Serum 3-OMG concentrations (mmol/L) were measured by liquid chromatography and mass spectrometry, with a sensitivity of 0.0103 mmol/L24.
Gastric emptying
Gastric emptying was evaluated by measuring antral area using 2D ultrasound (Aloka SSD-650 CL; ALOKA Co. Ltd., Tokyo, Japan). Imaging was performed with the transducer positioned vertically to obtain a sagittal image of the antrum, with the superior mesenteric vein and the abdominal aorta in a longitudinal section25. Images were taken at the end of inspiration to minimise the effects of the motion of the stomach that occurs with regular breathing26. A region-of-interest was drawn around the cross-section of the antrum using in-built software. The intragastric retention of the meal, expressed as a percentage of the meal at t = 0 min, at any given time25, and the gastric half-emptying time (T50), defined as the time taken for 50% of the meal to empty from the stomach27, were calculated.
GI symptoms
Nausea and bloating were quantified using a VAS questionnaire28. The strength of each sensation was rated on a 100-mm horizontal line, where 0 mm represented ‘sensation not felt at all’ and 100 mm ‘sensation felt the greatest’. Participants were asked to indicate how they were feeling at each time point by placing a vertical mark on the 100-mm line.
Data and statistical analysis
The number of participants included was determined by power calculations based on our previous studies15,16. We calculated that n = 12 participants would allow detection of a 1.0 mmol/L reduction in blood glucose at α = 0.05 with a power of 80%15.
Areas under the curve (AUCs) for plasma glucose, C-peptide, glucagon and VAS ratings were calculated using the trapezoidal rule, from t = −31 to −1 min to assess the effects of tryptophan alone, and from t = −1 to 120 min (t = 0 to 120 min for gastric emptying and serum 3-OMG) to assess the responses to the mixed-nutrient drink. AUCs were analysed using general linear model mixed-model analysis, with baseline values as a covariate. C-peptide and glucagon concentrations at t = −1 min (i.e. immediately pre-drink), which we reported previously to correlate with subsequent glycaemic control15, peak plasma glucose, time to peak glucose and gastric half-emptying time (T50) were analysed using repeated-measures analysis of variance (ANOVA). Post-hoc comparisons, adjusted for multiple comparisons by Bonferroni correction, using parametric tests, were performed when significant ANOVA effects were found. Statistical analysis was performed using SPSS software (version 25, IBM, Chicago, Illinois, USA). Differences were considered statistically significant at P ≤ 0.05, and 0.05 < P ≤ 0.1 as trend. All data are reported as mean ± SEM.
Results
All participants completed the study and tolerated the study conditions well. There were no effects of treatment on nausea or bloating, neither in response to tryptophan alone nor after the mixed-nutrient drink (Table 1). No participant had evidence of autonomic nerve dysfunction (mean total score: 1.1 ± 0.1; range: 0–2).
Plasma glucose
Effect of tryptophan alone
There was no effect of treatment on fasting plasma glucose.
Effect following the mixed-nutrient drink
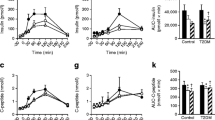
There was a substantial rise in plasma glucose after the drink on all days. While there was no effect of treatment on overall plasma glucose AUC−1 to 120 min, there was a treatment * time interaction for plasma glucose (P = 0.05). Plasma glucose was lower after Trp-3 from t = 15–30 min compared with control, and from t = 30–45 min compared with Trp-1.5 (P < 0.05 for all). There was no effect of treatment on peak glucose concentration or the time to peak concentration (Fig. 1 and Table 1).
After tryptophan alone (i.e. t = −31 to −1 min), there was no effect on fasting plasma glucose. Following the mixed-nutrient drink (i.e. t = 0 to 120 min), there was no effect on overall plasma glucose AUC−1 to 120 min. However, there was a significant treatment * time interaction for plasma glucose (P = 0.05); Plasma glucose after Trp-3 was lower from t = 15 to 30 min compared with control (*P < 0.05), and from t = 30 to 45 min compared with Trp-1.5 (**P < 0.05). Data are expressed as mean ± SEM; n = 12. Data were analysed using general linear model mixed-model analysis, with baseline as covariate, and repeated-measures ANOVA, and adjustments for multiple comparisons were made using Bonferroni correction.
Gastric emptying
There was an effect of treatment on gastric retention AUC0 to 120 min (P = 0.04), which tended to be greater after Trp-3 compared with control (P = 0.06). While there was also an effect of treatment on T50 (P = 0.05), following post-hoc comparisons the difference between treatments was not significant (Fig. 2 and Table 1).
There was an effect of treatment on gastric emptying AUC0 to 120 min (P = 0.04), which tended to be greater after Trp-3 compared with control (*P = 0.06). There was also an effect of treatment on T50 (P = 0.05), although this was lost on post-hoc comparisons. Data are expressed as mean ± SEM; n = 12. Data were analysed using general linear model mixed-model analysis, with baseline as covariate, and adjustments for multiple comparisons made using Bonferroni correction.
Plasma C-peptide
Effect of tryptophan alone
There was an effect, albeit very small, of treatment on fasting C-peptide AUC−31 to −1 min (P = 0.002), which tended to be greater after Trp-3 (P = 0.06), and was greater after Trp-1.5 (P = 0.002), compared with control. There was also an effect on C-peptide at t = −1 min (P = 0.01), which was greater after both Trp-3 and Trp-1.5 compared with control (P < 0.05 for both).
Effect following the mixed-nutrient drink
C-peptide rose substantially after the drink on all days. There was an effect of treatment on C-peptide AUC−1 to 120 min (P = 0.01), which tended to be greater after Trp-1.5 (P = 0.06), but not Trp-3, compared with control. There was no treatment * time interaction (Fig. 3A and Table 1).
(A) After tryptophan alone (i.e. t=−31 to −1min), there was an effect of treatment on C-peptide AUC−31 to −1min (P=0.002), which tended to be greater after Trp-3 (*P=0.06), and was greater after Trp-1.5 (**P=0.002), compared with control. There was also an effect on C-peptide at t=−1min (P=0.01), which was greater after both Trp-3 and Trp-1.5 compared with control (*P<0.05 for both). Following the mixed-nutrient drink (i.e. t=0 to 120min), there was an effect of treatment on C-peptide AUC−1 to 120min (P=0.01), which tended to be greater after Trp-1.5, but not Trp-3 (***P=0.06). (B) After tryptophan alone, there was an effect of treatment on fasting glucagon AUC−31 to −1min (P=0.04), however, this was lost on post-hoc comparisons. There was also an effect on glucagon at t=−1min (P=0.007), which tended to be greater after Trp-3 compared with control (*P=0.07). Following the mixed-nutrient drink, plasma glucagon rose on all days. However, there was no effect of treatment on glucagon AUC−1 to 120min, or a treatment*time interaction. (C) There was no effect of treatment on serum 3-OMG AUC0 to 120min, or a treatment*time interaction. Data are expressed as mean±SEM; n=12. Data were analysed using general linear model mixed-model analysis, with baseline as covariate, and repeated-measures ANOVA, adjusted for multiple comparisons using Bonferroni correction.
Plasma glucagon
Effect of tryptophan alone
There was an effect of treatment on fasting glucagon AUC−31 to −1 min (P = 0.04), however, this was lost on post-hoc comparisons. There was also an effect on glucagon at t = −1 min (P = 0.007), which tended to be greater after Trp-3 compared with control (P = 0.07).
Effect following the mixed-nutrient drink
There was a marked rise in plasma glucagon after the drink on all days. However, there was no effect of treatment on glucagon AUC−1 to 120 min, or a treatment * time interaction (Fig. 3B and Table 1).
Serum 3-OMG
There was no effect of treatment on serum 3-OMG AUC0 to 120 min, or a treatment * time interaction (Fig. 3C and Table 1).
Discussion
Our study indicates that in T2D, intragastric administration of tryptophan, at a dose of 3 g, but not 1.5 g, delays the rise in, but does not result in an overall lowering of, the plasma glucose response to a carbohydrate-containing mixed-nutrient drink, contrasting our previous observation in lean individuals or those with obesity15. Tryptophan slowed gastric emptying modestly, in line with our recent findings in lean people and normoglycaemic people with obesity15, which was likely responsible for delaying the rise in postprandial plasma glucose. Tryptophan also stimulated C-peptide, reflecting insulin secretion (although this effect was very small), as well as glucagon; the latter, therefore, most likely counteracted any glucose-lowering effect of insulin.
Protein ‘preloads’ containing whey6 and oral administration of specific amino acids29 have been shown to diminish postprandial glycaemic excursions in T2D and, accordingly, have the potential to be used in the management of T2D. We have reported that Trp-3, but not Trp-1.5, modestly lowered blood glucose in response to a mixed-nutrient drink in lean people and those with obesity15. We, therefore, hypothesised that, in people with T2D, in whom postprandial blood glucose is characteristically elevated, the blood glucose-lowering effect of tryptophan would be enhanced. Contrary to our hypothesis, tryptophan did not diminish the overall postprandial blood glucose, at either dose. However, Trp-3 modestly reduced the initial glycaemic response to the drink, by delaying the rise in blood glucose.
Gastric emptying is a major determinant of the glycaemic response to carbohydrate-containing meals in health, obesity and T2D1, and our previous study showed that Trp-3, administered intragastrically, slowed gastric emptying in both lean people and those with obesity15. Accordingly, the finding that tryptophan, in the same dose, slowed gastric emptying in people with T2D is consistent with these observations and is likely to account for the delayed rise in blood glucose. It needs to be recognised, though, that the magnitude of the slowing of gastric emptying was modest, which may explain the lack of effect on blood glucose lowering. The slowing of gastric emptying after Trp-3 may explain the apparent lack of effect of tryptophan on glucose absorption, as measured by serum 3-OMG. That is, any effect of Trp-3 on glucose absorption may have been masked by its effect to slow gastric emptying. Because our study did not include healthy participants as controls, the effect of tryptophan on glucose absorption in health, and whether any effect of tryptophan on glucose absorption may be altered in T2D, remains unclear.
Trp-3 tended to stimulate, and Trp-1.5 had a small effect to increase, plasma C-peptide immediately before the nutrient drink. We have reported that intraduodenal tryptophan, at a load of 0.15 kcal/min, providing ~3.3 g over 90 min, had a small effect to stimulate insulin in health16. This stimulation of insulin may reflect a direct effect of circulating amino acids29 or be secondary to the secretion of the incretin hormones, glucagon-like peptide-1 (GLP-1) or glucose-dependent insulinotropic polypeptide (GIP), which both have glucose-dependent insulinotropic effects30,31. The insulin-stimulatory effect of GIP is known to be markedly attenuated in T2D32. In healthy participants, the small insulinotropic effect of tryptophan is likely to be independent of GLP-1 and GIP16, as insulin stimulation occurred at blood glucose levels of <8 mmol/L33. After the drink, Trp-1.5, but not Trp-3, tended to increase C-peptide compared with control; this may reflect the lack of effect of Trp-1.5 to slow gastric emptying. Nevertheless, overall the insulinotropic capacity of tryptophan in the doses examined is modest. Therefore, the absence of overall glucose lowering by tryptophan is most likely due to only small insulin stimulation and modest slowing of gastric emptying, combined with relatively more potent stimulation of glucagon, possibly as a direct response to circulating amino acids29. A progressive rise in glucagon for ~30 min, followed by a sustained elevation, was evident on all three days, consistent with the well-established failure of postprandial glucagon suppression characteristic of T2D34.
The potential mechanism(s) underlying the differences in effects of tryptophan observed in this study, compared with those in healthy people15, deserve some consideration. Tryptophan may modulate GI motility, and, thus, gastric emptying, locally via its metabolite, serotonin35, or via CCK16,36. Tryptophan-induced stimulation of CCK most likely involves Ca2+-sensing receptors (CaSR) on enteroendocrine L cells37. Whether tryptophan-induced stimulation of CCK is altered in T2D warrants evaluation in future studies. The effect of tryptophan to stimulate insulin may be directly at the pancreatic beta-cell via activation of G protein-coupled receptor 142 (GPR142) and/or CaSR38,39,40, and may also involve serotonin41. Since people with T2D have beta-cell dysfunction, it is possible that the effects of tryptophan on one or more of these mechanisms may be attenuated, or the expression of these receptors altered, in T2D42. This warrants further investigation.
Some potential limitations of our study should be appreciated. We deliberately studied patients with well-controlled T2D (i.e. glycated haemoglobin is more dependent on postprandial glucose) without autonomic neuropathy and managed on oral hypoglycaemic drugs, but not insulin, as we considered that a glucose-lowering effect of tryptophan would be most evident in this group. It would be anticipated that in the cohort studied, the rate of gastric emptying would be normal, or slightly accelerated1. The two doses of tryptophan were based on our previous studies; while it is tempting to speculate that larger doses may have more potent effects, our previous studies have indicated that these may be associated with adverse effects16. Our study utilised intragastric administration of tryptophan, however, administration in capsules is also possible43, providing for a more practical application outside the laboratory setting. We only included men to avoid any effects of the menstrual cycle on gastric emptying44, thus, extrapolation of our observations to females should, of necessity, be circumspect.
In conclusion, our study suggests that in T2D, administration of tryptophan at a dose of 3 g before a carbohydrate-containing drink delays the rise in plasma glucose, probably as a result of slowing of gastric emptying, but does not affect the overall blood glucose response.
References
Phillips, L. K., Deane, A. M., Jones, K. L., Rayner, C. K. & Horowitz, M. Gastric emptying and glycaemia in health and diabetes mellitus. Nat. Rev. Endocrinol. 11, 112–128 (2015).
Phillips, W. T., Schwartz, J. G. & McMahan, C. A. Reduced postprandial blood glucose levels in recently diagnosed non-insulin-dependent diabetics secondary to pharmacologically induced delayed gastric emptying. Dig. Dis. Sci. 38, 51–58 (1993).
Gonlachanvit, S. et al. Effect of altering gastric emptying on postprandial plasma glucose concentrations following a physiologic meal in type-II diabetic patients. Dig. Dis. Sci. 48, 488–497 (2003).
Ryan, A. T. et al. Effects of intraduodenal lipid and protein on gut motility and hormone release, glycemia, appetite, and energy intake in lean men. Am. J. Clin. Nutr. 98, 300–311 (2013).
Krebs, M. et al. Direct and indirect effects of amino acids on hepatic glucose metabolism in humans. Diabetologia 46, 917–925 (2003).
Ma, J. et al. Effects of a protein preload on gastric emptying, glycemia, and gut hormones after a carbohydrate meal in diet-controlled type 2 diabetes. Diabetes Care 32, 1600–1602 (2009).
Elovaris, R. A. et al. Plasma free amino acid responses to whey protein and their relationships with gastric emptying, blood glucose- and appetite-regulatory hormones and energy intake in lean healthy men. Nutrients 11, e2465 (2019).
Hajishafiee, M., Bitarafan, V. & Feinle-Bisset, C. Gastrointestinal sensing of meal-related signals in humans, and dysregulations in eating-related disorders. Nutrients 11, e1298 (2019).
Potier, M., Darcel, N. & Tome, D. Protein, amino acids and the control of food intake. Curr. Opin. Clin. Nutr. Metab. Care 12, 54–58 (2009).
Fromentin, G. et al. Peripheral and central mechanisms involved in the control of food intake by dietary amino acids and proteins. Nutr. Res Rev. 25, 29–39 (2012).
Kalogeropoulou, D., Lafave, L., Schweim, K., Gannon, M. C. & Nuttall, F. Q. Leucine, when ingested with glucose, synergistically stimulates insulin secretion and lowers blood glucose. Metabolism 57, 1747–1752 (2008).
Nuttall, F. Q., Schweim, K. J. & Gannon, M. C. Effect of orally administered isoleucine with and without glucose on insulin, glucagon and glucose concentrations in non‐diabetic subjects. Eur. J. Clin. Nutr. Metab. 3, e152–e158 (2008).
Ullrich, S. S. et al. Intragastric lysine lowers the circulating glucose and insulin responses to a mixed-nutrient drink without slowing gastric emptying in healthy adults. J. Nutr. 147, 1275–1281 (2017).
Ullrich, S. S. et al. Intragastric administration of leucine or isoleucine lowers the blood glucose response to a mixed-nutrient drink by different mechanisms in healthy, lean volunteers. Am. J. Clin. Nutr. 104, 1274–1284 (2016).
Ullrich, S. S. et al. Effects of intragastric administration of tryptophan on the blood glucose response to a nutrient drink and energy intake, in lean and obese men. Nutrients 10, e463 (2018).
Steinert, R. E. et al. Effects of intraduodenal infusion of L-tryptophan on ad libitum eating, antropyloroduodenal motility, glycemia, insulinemia, and gut peptide secretion in healthy men. J. Clin. Endocrinol. Metab. 99, 3275–3284 (2014).
Edelbroek, M. et al. Stereospecific effects of intraduodenal tryptophan on pyloric and duodenal motility in humans. Scand. J. Gastroenterol. 29, 1088–1095 (1994).
Carney, B. I. et al. Stereospecific effects of tryptophan on gastric emptying and hunger in humans. J. Gastroenterol. Hepatol. 9, 557–563 (1994).
Meyer-Gerspach, A. C. et al. Effect of L-tryptophan and L-leucine on gut hormone secretion, appetite feelings and gastric emptying rates in lean and non-diabetic obese participants: a randomized, double-blind, parallel-group trial. PLoS One 11, e0166758 (2016).
Inubushi, T. et al. L-tryptophan suppresses rise in blood glucose and preserves insulin secretion in type-2 diabetes mellitus rats. J. Nutr. Sci. Vitaminol. (Tokyo) 58, 415–422 (2012).
Fordtran, J. S., Clodi, P. H., Soergel, K. H. & Ingelfinger, F. J. Sugar absorption tests, with special reference to 3-0-methyl-d-glucose and d-xylose. Ann. Intern. Med. 57, 883–891 (1962).
Ewing, D. J. & Clarke, B. F. Diagnosis and management of diabetic autonomic neuropathy. Br. Med. J. (Clin. Res. Ed.) 285, 916–918 (1982).
Saisho, Y. Postprandial C-peptide to glucose ratio as a marker of β cell function: implication for the management of type 2 diabetes. Int. J. Mol. Sci. 17, 744 (2016).
Pham, H. et al. The effects of a whey protein and guar gum-containing preload on gastric emptying, glycaemia, small intestinal absorption and blood pressure in healthy older subjects. Nutrients 11, 2666 (2019).
Hveem, K., Jones, K. L., Chatterton, B. E. & Horowitz, M. Scintigraphic measurement of gastric emptying and ultrasonographic assessment of antral area: relation to appetite. Gut 38, 816–821 (1996).
Jones, K. L. et al. Relation between postprandial satiation and antral area in normal subjects. Am. J. Clin. Nutr. 66, 127–132 (1997).
Collins, P. J., Horowitz, M., Cook, D. J., Harding, P. E. & Shearman, D. J. Gastric emptying in normal subjects–a reproducible technique using a single scintillation camera and computer system. Gut 24, 1117–1125 (1983).
Parker, B. A. et al. Relation between food intake and visual analogue scale ratings of appetite and other sensations in healthy older and young subjects. Eur. J. Clin. Nutr. 58, 212–218 (2004).
Gannon, M. C. & Nuttall, F. Q. Amino acid ingestion and glucose metabolism–a review. IUBMB Life 62, 660–668 (2010).
Müller, T. D. et al. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 30, 72–130 (2019).
Wu, T., Rayner, C. K. & Horowitz, M. Incretins. Handb. Exp. Pharmacol. 233, 137–171 (2016).
Nauck, M. A. et al. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J. Clin. Invest. 91, 301–307 (1993).
Nauck, M. A. et al. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7–36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia 36, 741–744 (1993).
Raskin, P. & Unger, R. H. Hyperglucagonemia and its suppression. Importance in the metabolic control of diabetes. N. Engl. J. Med 299, 433–436 (1978).
Gershon, M. D. & Tack, J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology 132, 397–414 (2007).
Brennan, I. M. et al. Dose-dependent effects of cholecystokinin-8 on antropyloroduodenal motility, gastrointestinal hormones, appetite, and energy intake in healthy men. Am. J. Physiol. Endocrinol. Metab. 295, E1487–E1494 (2008).
Young, S. H., Rey, O., Sternini, C. & Rozengurt, E. Amino acid sensing by enteroendocrine STC-1 cells: role of the Na+-coupled neutral amino acid transporter 2. Am. J. Physiol. Cell Physiol. 298, C1401–C1413 (2010).
Lin, H. V. et al. GPR142 controls tryptophan-induced insulin and incretin hormone secretion to improve glucose metabolism. PLoS One 11, e0157298 (2016).
Acar, I., Cetinkaya, A., Lay, I. & Ileri-Gurel, E. The role of calcium sensing receptors in GLP-1 and PYY secretion after acute intraduodenal administration of L-Tryptophan in rats. Nutr. Neurosci. 23, 481–489 (2020).
Rudenko, O. et al. The aromatic amino acid sensor GPR142 controls metabolism through balanced regulation of pancreatic and gut hormones. Mol. Metab. 19, 49–64 (2019).
Cataldo Bascuñan, L. R., Lyons, C., Bennet, H., Artner, I. & Fex, M. Serotonergic regulation of insulin secretion. Acta Physiol. (Oxf.) 225, e13101 (2019).
Riddy, D. M., Delerive, P., Summers, R. J., Sexton, P. M. & Langmead, C. J. G protein-coupled receptors targeting insulin resistance, obesity, and type 2 diabetes mellitus. Pharm. Rev. 70, 39–67 (2018).
Caballero, B., Finer, N. & Wurtman, R. J. Plasma amino acids and insulin levels in obesity: response to carbohydrate intake and tryptophan supplements. Metabolism 37, 672–676 (1988).
Brennan, I. M. et al. Effects of the phases of the menstrual cycle on gastric emptying, glycemia, plasma GLP-1 and insulin, and energy intake in healthy lean women. Am. J. Physiol. Gastrointest. Liver Physiol. 297, G602–G610 (2009).
Acknowledgements
We thank Kylie Lange, Centre of Research Excellence in Translating Nutritional Science to Good Health, University of Adelaide, a biostatistician, for her statistical support, and Scott Standfield and Judith Wishart, University of Adelaide, for performing the hormone assays. This work was supported by Research Training Program Scholarships funded by the Australian Commonwealth Government (2018–2021, to M.H.), and The University of Adelaide (2017–2020, to R.A.E.), a William Southcott Fellowship from The University of Adelaide (2019–2020, to K.L.J.), a National Health and Medical Research Council of Australia (NHMRC) Project grant (grant no. 1158296, 2019–2022, to C.F-.B.), and an NHMRC Senior Research Fellowship (grant no. 1103020, 2016–2021, to C.F-.B.). The sponsors had no role in the design of the study, the collection, analyses or interpretation of data, writing of the manuscript, or the decision to publish the results.
Author information
Authors and Affiliations
Contributions
M.H., R.A.E. and C.F-.B. designed the research; M.H. and R.A.E. conducted the research; M.H. and C.F-.B. analysed the data and conducted the statistical analysis; M.H., R.A.E., K.L.J., L.K.H., M.H., S.D.P. and C.F-.B. interpreted the data and prepared the manuscript; C.F-.B. had primary responsibility for the final content; all authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hajishafiee, M., Elovaris, R.A., Jones, K.L. et al. Effects of intragastric administration of L-tryptophan on the glycaemic response to a nutrient drink in men with type 2 diabetes — impacts on gastric emptying, glucoregulatory hormones and glucose absorption. Nutr. Diabetes 11, 3 (2021). https://doi.org/10.1038/s41387-020-00146-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41387-020-00146-9
- Springer Nature Limited