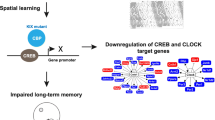
Abstract
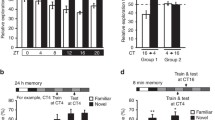
The circadian system influences many different biological processes, including memory performance. While the suprachiasmatic nucleus (SCN) functions as the brain’s central pacemaker, downstream “satellite clocks” may also regulate local functions based on the time of day. Within the dorsal hippocampus (DH), for example, local molecular oscillations may contribute to time-of-day effects on memory. Here, we used the hippocampus-dependent Object Location Memory task to determine how memory is regulated across the day/night cycle in mice. First, we systematically determined which phase of memory (acquisition, consolidation, or retrieval) is modulated across the 24 h day. We found that mice show better long-term memory performance during the day than at night, an effect that was specifically attributed to diurnal changes in memory consolidation, as neither memory acquisition nor memory retrieval fluctuated across the day/night cycle. Using RNA-sequencing we identified the circadian clock gene Period1 (Per1) as a key mechanism capable of supporting this diurnal fluctuation in memory consolidation, as learning-induced Per1 oscillates in tandem with memory performance in the hippocampus. We then show that local knockdown of Per1 within the DH impairs spatial memory without affecting either the circadian rhythm or sleep behavior. Thus, Per1 may independently function within the DH to regulate memory in addition to its known role in regulating the circadian system within the SCN. Per1 may therefore exert local diurnal control over memory consolidation within the DH.





Similar content being viewed by others
References
Richards J, Gumz ML. Advances in understanding the peripheral circadian clocks. FASEB J. 2012;26:3602–13.
Gerstner JR, Yin JCP. Circadian rhythms and memory formation. Nat Rev Neurosci. 2010;11:577–88.
Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:6901. 2002;418:935–941
Jilg A, Lesny S, Peruzki N, Schwegler H, Selbach O, Dehghani F, et al. Temporal dynamics of mouse hippocampal clock gene expression support memory processing. Hippocampus. 2010;20:377–88.
Hartsock MJ, Spencer RL. Memory and the circadian system: Identifying candidate mechanisms by which local clocks in the brain may regulate synaptic plasticity. Neurosci Biobehav Rev. 2020;118:134–62.
Smies CW, Bodinayake KK, Kwapis JL. Time to learn: The role of the molecular circadian clock in learning and memory. Neurobiol Learn Mem. 2022;193:107651.
Kwapis JL, Alaghband Y, Kramár EA, López AJ, Vogel Ciernia A, White AO, et al. Epigenetic regulation of the circadian gene Per1 contributes to age-related changes in hippocampal memory. Nat Commun. 2018;9:3323.
Urban MW, Lo C, Bodinayake KK, Brunswick CA, Murakami S, Heimann AC, et al. The circadian clock gene Per1 modulates context fear memory formation within the retrosplenial cortex in a sex-specific manner. Neurobiol Learn Mem. 2021;185:107535.
Rawashdeh O, Jilg A, Maronde E, Fahrenkrug J, Stehle JH. Period1 gates the circadian modulation of memory-relevant signaling in mouse hippocampus by regulating the nuclear shuttling of the CREB kinase pP90RSK. J Neurochem. 2016;138:731–45.
Woodruff ER, Chun LE, Hinds LR, Varra NM, Tirado D, Morton SJ, et al. Coordination between prefrontal cortex clock gene expression and corticosterone contributes to enhanced conditioned fear extinction recall. ENeuro. 2018;5:455–73.
Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacner LD, King DP, et al. Role of the CLOCK protein in the mammalian circadian mechanism. Science. 1998;280:1564–9.
Kume K, Zylka MJ, Sriram S, Shearman LP, Weaver DR, Jin X, et al. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell. 1999;98:193–205.
Griffin EA, Staknis D, Weitz CJ. Light-independent role of CRY1 and CRY2 in the mammalian circadian clock. Science. 1999;286:768–71.
Sato TK, Yamada RG, Ukai H, Baggs JE, Miraglia LJ, Kobayashi TJ, et al. Feedback repression is required for mammalian circadian clock function. Nat Genet. 2006;38:312–9.
Albrecht U, Sun ZS, Eichele G, Lee CC. A differential response of two putative mammalian circadian regulators, mper1 and mper2, to light. Cell. 1997;91:1055–64.
Rawashdeh O, Jilg A, Jedlicka P, Slawska J, Thomas L, Saade A, et al. PERIOD1 coordinates hippocampal rhythms and memory processing with daytime. Hippocampus. 2014;24:712–23.
Alberini CM. Transcription factors in long-term memory and synaptic plasticity. Physiol Rev. 2009;89:121–45.
Alberini CM, Kandel ER. The regulation of transcription in memory consolidation. Cold Spring Harb Perspect Biol. 2015;7:a021741.
Chatterjee S, Angelakos CC, Bahl E, Hawk JD, Gaine ME, Poplawski SG, et al. The CBP KIX domain regulates long-term memory and circadian activity. BMC Biollogy. 2020;18:155.
Nadel L, Hupbach A, Gomez R, Newman-Smith K. Memory formation, consolidation and transformation. Neurosci Biobehav Rev. 2012;36:1640–5.
Tapp WN, Holloway FA. Phase shifting circadian rhythms produces retrograde amnesia. Science. 1981;211:1056–8.
Davies JA, Navaratnam V, Redfern PH. A 24-h rhythm in passive-avoidance behaviour in rats. Psychopharmacologia. 1973;32:211–4.
Vogel-Ciernia A, Wood MA. Examining object location and object recognition memory in mice. Curr Protoc Neurosci. 2014;2014:8.31.1–8.31.17.
Brown LA, Hasan S, Foster RG, Peirson SN. COMPASS: Continuous open mouse phenotyping of activity and sleep status. Wellcome Open Res. 2017;1:1–18.
Chaudhury D, Colwell CS. Circadian modulation of learning and memory in fear-conditioned mice. Behavioural Brain Research. 2002;133:95–108.
Eckel-Mahan KL, Phan T, Han S, Wang H, Chan GCK, Scheiner ZS, et al. Circadian oscillation of hippocampal MAPK activity and cAMP: Implications for memory persistence. Nat Neurosci. 2008;11:1074–82.
Davis HP, Squire LR. Protein synthesis and memory: A review. Psychol Bull. 1984;96:518–59.
Igaz LM, Bekinschtein P, Vianna MMR, Izquierdo I, Medina JH. Gene expression during memory formation. Neurotox Res. 2004;6:189–203.
Hughes ME, Hogenesch JB, Kornacker K. JTK-CYCLE: An efficient nonparametric algorithm for detecting rhythmic components in genome-scale data sets. J Biol Rhythms. 2010;25:372–80.
Rao-Ruiz P, Couey JJ, Marcelo IM, Bouwkamp CG, Slump DE, Matos MR, et al. Engram-specific transcriptome profiling of contextual memory consolidation. Nat Commun. 2019;10:1–14.
Rawashdeh O, Parsons R, Maronde E. Clocking in time to gate memory processes: The circadian clock is part of the Ins and outs of memory. Neural Plast. 2018;2018:6238989.
Brunswick CA, Baldwin DJ, Bodinayake KK, McKenna AR, Lo C-Y, Bellfy L, et al. The clock gene Per1 is necessary in the retrosplenial cortex—but not in the suprachiasmatic nucleus—for incidental learning in young and aging male mice. Neurobiol Aging. 2023. https://doi.org/10.1016/j.neurobiolaging.2023.02.009.
Yeo NC, Chavez A, Lance-Byrne A, Chan Y, Menn D, Milanova D, et al. An enhanced CRISPR repressor for targeted mammalian gene regulation. Nat Methods. 2018;15:611–6.
Neve RL, Neve KA, Nestler EJ, Carlezon WA. Use of herpes virus amplicon vectors to study brain disorders. Biotechniques. 2005;39:381–9.
Sarno E, Robison AJ. Emerging role of viral vectors for circuit-specific gene interrogation and manipulation in rodent brain. Pharmacol Biochem Behav. 2018;174:2–8.
Krishnan HC, Lyons LC. Synchrony and desynchrony in circadian clocks: Impacts on learning and memory. Learning and Memory. 2015;22:426–37.
Cho K. Chronic ‘jet lag’ produces temporal lobe atrophy and spatial cognitive deficits. Nat Neurosci. 2001;4:567–8.
Marquié JC, Tucker P, Folkard S, Gentil C, Ansiau D. Chronic effects of shift work on cognition: Findings from the VISAT longitudinal study. Occup Environ Med. 2015;72:258–64.
Horsey EA, Maletta T, Turner H, Cole C, Lehmann H, Fournier NM. Chronic jet lag simulation Decreases Hippocampal Neurogenesis and Enhances Depressive Behaviors and Cognitive Deficits in Adult Male Rats. Front Behav Neurosci. 2020;13:272.
Valentinuzzi VS, Neto SPD, Carneiro BTS, Santana KS, Araújo JF, Ralph MR. Memory for time of training modulates performance on a place conditioning task in marmosets. Neurobiol Learn Mem. 2008;89:604–7.
Valentinuzzi VS, Menna-Barreto L, Xavier GF. Effect of circadian phase on performance of rats in the Morris water maze task. J Biol Rhythms. 2004;19:312–24.
Phan TH, Chan GCK, Sindreu CB, Eckel-Mahan KL, Storm DR. The diurnal oscillation of MAP (Mitogen-Activated Protein) kinase and adenylyl cyclase activities in the hippocampus depends on the suprachiasmatic nucleus. J Neurosci. 2011;31:10640–7.
Liu D, Li J, Lin H, Lorsung E, Le N, Singla R, et al. Circadian activities of the brain MNK-eIF4E signalling axis contribute to diurnal rhythms of some cognitive functions. Eur J Neurosci. 2022;56:1–17.
Loss CM, Binder LB, Muccini E, Martins WC, de Oliveira PA, Vandresen-Filho S, et al. Influence of environmental enrichment vs. time-of-day on behavioral repertoire of male albino Swiss mice. Neurobiol Learn Mem. 2015;125:63–72.
Tsao CH, Flint J, Huang GJ. Influence of diurnal phase on behavioral tests of sensorimotor performance, anxiety, learning and memory in mice. Sci Rep. 2022;12:1–10.
Colwell CS. Linking neural activity and molecular oscillations in the SCN. Nat Rev Neurosci. 2011;12:553–69.
Acknowledgements
We would like to thank Dr. Rachael Neve and the Gene Delivery Technology Core at Massachusetts General Hospital for help designing and packaging all HSV viruses described here. We would also like to thank the Huck Institutes of the Life Sciences at Penn State for funding, Dr. Craig Praul and the Huck Genomics Core Facility for library construction, Dr. Yuka Imamura and the Penn State Hershey Genome Sciences Facility for sequencing, and the Penn State Bioinformatics Consulting Center for assistance with the RNA-seq.
Funding
This research was funded by NIH grants R01AG074041 (JLK), K99/R00AG056586 (JLK) and R21AG068444 (JLK), Whitehall Foundation Grant #2020-05-06 (JLK), American Federation for Aging Research Grant #A21105 (JLK), startup funds from the Eberly College of Science and Department of Biology at Pennsylvania State University (JLK) and the National Institute on Aging under Grant T32 AG049676 to The Pennsylvania State University (LB).
Author information
Authors and Affiliations
Contributions
Study concept and design: LB and JLK. Acquisition of data: LB, CWS, ARB, KKB, EMS, DSW, CL, SM, HMB, and MJV. Analysis of data: LB, ARB, KKB, MJV, AS, and IA. Drafting of manuscript: LB and JLK. Final approval of manuscript: All authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bellfy, L., Smies, C.W., Bernhardt, A.R. et al. The clock gene Per1 may exert diurnal control over hippocampal memory consolidation. Neuropsychopharmacol. 48, 1789–1797 (2023). https://doi.org/10.1038/s41386-023-01616-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-023-01616-1
- Springer Nature Switzerland AG