Abstract
Critical period-like plasticity (iPlasticity) can be reinstated in the adult brain by several interventions, including drugs and optogenetic modifications. We have demonstrated that a combination of iPlasticity with optimal training improves behaviors related to neuropsychiatric disorders. In this context, the activation of TrkB, a receptor for BDNF, in Parvalbumin-positive (PV+) interneurons has a pivotal role in cortical network changes. However, it is unknown if the activation of TrkB in PV+ interneurons is important for other plasticity-related behaviors, especially for learning and memory. Here, using mice with heterozygous conditional TrkB deletion in PV+ interneurons (PV-TrkB hCKO) in IntelliCage and fear erasure paradigms, we show that chronic treatment with fluoxetine, a widely prescribed antidepressant drug that is known to promote the activation of TrkB, enhances behavioral flexibility in spatial and fear memory, largely depending on the expression of the TrkB receptor in PV+ interneurons. In addition, hippocampal long-term potentiation was enhanced by chronic treatment with fluoxetine in wild-type mice, but not in PV-TrkB hCKO mice. Transcriptomic analysis of PV+ interneurons after fluoxetine treatment indicated intrinsic changes in synaptic formation and downregulation of enzymes involved in perineuronal net formation. Consistently, immunohistochemistry has shown that the fluoxetine treatment alters PV expression and reduces PNNs in PV+ interneurons, and here we show that TrkB expression in PV+ interneurons is required for these effects. Together, our results provide molecular and network mechanisms for the induction of critical period-like plasticity in adulthood.
Similar content being viewed by others
Introduction
Learning and memory dysfunction is a common neuropsychological symptom of neuropsychiatric and neurological diseases. It has been proposed that chronic treatment with antidepressants (ADs) improves impaired learning and memory in animal models [1,2,3] via increased neuronal plasticity, by promoting neurogenesis [4, 5], and long-term potentiation (LTP) in the hippocampus [6,7,8]. However, it is still not clear how AD treatments improve the dysfunction.
The activation of the brain-derived neurotrophic factor (BDNF) and its receptor TrkB is a key factor in neuronal plasticity. The binding of BDNF to TrkB causes the autophosphorylation of TrkB and leads to the activation of intracellular signaling pathways involved in neuronal differentiation, survival, and growth, as well as synaptic plasticity in neurons [9, 10]. This pathway also regulates gene transcription and LTP [9]. Previous studies demonstrated that chronic treatment with AD, such as the selective serotonin reuptake inhibitor (SSRI) fluoxetine, increases the plastic state of Parvalbumin-positive (PV+) fast-spiking interneurons primarily targeting the perisomatic area of pyramidal neurons [11,12,13]. Donato et al. showed that PV+ fast-spiking basket cells exhibit plasticity by dynamically changing their states in response to recent experience: a state characterized by low PV expression in the PV+ interneurons is involved in plastic networks while a state with high PV-expression in PV cells promotes memory consolidation in the hippocampal CA3 region. This leads to a lower and a higher number of excitatory synaptic inputs onto PV interneurons, respectively, regulating experience-dependent network plasticity [14]. Furthermore, perineuronal nets (PNN) [15], an extracellular matrix surrounding PV interneurons, are known to be a plastic structure regulated by iPlasticity in the amygdala, hippocampus and visual cortex [13, 16, 17].
We have demonstrated that ADs induce a critical period-like plasticity in the adult brain (iPlasticity), which allows brain networks to better adapt to environmental stimuli, such as training or rehabilitation, and consequently ameliorate neuropsychiatric symptoms [7, 10, 18]. iPlasticity occurs in a variety of brain areas and can be induced by different interventions to modulate behaviors when combined with appropriate training. We have proposed the “network hypothesis” of neuropsychiatric diseases, according to which neuropsychiatric diseases reflect malfunctioning information processing within particular neural networks, and interventions, including ADs, act by providing an opportunity for the neuronal activity to improve this processing [19]. Our laboratory recently demonstrated that ADs directly bind to TrkB through a lipid binding motif and activate TrkB to promote neural plasticity [20]. We also recently showed that TrkB activation in PV+ interneurons is necessary and sufficient for iPlasticity in the visual cortex [16]. Therefore, the treatment with ADs is a good tool to directly activate TrkB to study the mechanisms of iPlasticity. However, it is still unknown whether elevated plasticity by ADs in PV+ interneurons combined with learning processes can improve learning and memory more generally.
In order to assess the effects of TrkB in PV+ interneurons on reversal learning, we treated PV+ interneuron-specific heterozygous TrkB knockout (PV-TrkB hCKO) mice with fluoxetine in the fear extinction paradigm and in IntelliCage apparatus. We also examined the dependency of LTP on the expression of TrkB in PV+ interneurons by studying local field potential activity in the hippocampal CA1 of PV-TrkB hCKO mice. We then performed a transcriptomic analysis specifically for PV+ interneurons using translating ribosome affinity purification (TRAP) after chronic treatment with fluoxetine, and found intrinsic changes in PV+ interneurons, especially in genes related to the formation of PNNs. Finally, we immunohistologically confirmed the plastic changes in PV+ interneurons after chronic treatment with fluoxetine.
Material and methods
Details of material and methods are provided in the Supplementary Note.
Animals and experimental design
Heterozygous mice with reduced expression of TrkB specifically in PV+ interneurons (PV-TrkB hCKO; PVcre/wt, TrkBflx/wt), and littermate control mice that normally express TrkB (PVwt/wt, TrkBflx/wt; hereafter indicated as “wild-type”) were produced by mating females from a heterozygous PV-specific Cre line [21] (PVcre/wt; Pvalb-IRES-Cre, JAX: 008069, Jackson Laboratory) with males from a homozygous floxed TrkB mouse line (TrkBflx/flx) [22] (Fig. 1a). Due to frequent fights among males, only females (5 months old) were used for IntelliCage and the males (2 months old) were used for the fear extinction paradigm. Transgenic mice harboring FLEX-L4 conjugating GFP [23] were crossed with homozygous PV-specific Cre mice (PVcre/wt) to obtain the mice (2 months old) expressing GFP-L4 specifically in PV interneurons. The room temperature was kept at 23 ± 2°C, and all mice were kept in a room with a 12-h light/dark cycle (lights on at 6:00 a.m.) with access to food and water ad libitum. All experiments were carried out in accordance with the European Communities Council Directive 86/6609/EEC and the guidelines of the Society for Neuroscience and were approved by the County Administrative Board of Southern Finland (License number: ESAVI/38503/2019).
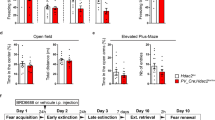
a Mating strategy to obtain wild-type and PV-specific heterozygous TrkB knockout (PV-TrkB hCKO) mice. b Scheme of the fear-conditioning paradigm. Mice were conditioned by pairing a tone and an electric shock in context A (c), and then one group was treated with fluoxetine (24 mg/kg/day). After 2 weeks, mice were subjected to 2 days of fear extinction training: day 1 (Ext1), day 2 (Ext2) wt (d), PV-TrkB hCKO (e). After 1 week, mice were tested for spontaneous recovery (SR) in context B (g, h) and fear renewal (FR) in context A (i, j). c Freezing was similarly increased during the conditioning/acquisition phase in both WT and hCKO mice and both genotypes reached a similar level of acquisition (two-way ANOVA, Conditioning, F (4, 270) = 21.94, P < 0.0001). However, PV-TrkB hCKO mice showed significantly higher freezing compared to wild-type mice (Genotype, F (1, 270) = 4.049, P < 0.0452; Sidak’s post hoc, wild-type vs PV-TrkB hCKO in Trial 3, P = 0.0037). d In wild-type mice, fear extinction trials significantly reduced freezing in both Ext1 (F (11, 336) = 1.988, P = 0.0288) and Ext2 (F (11, 336) = 9.624, P < 0.0001) and an effect of fluoxetine treatment on both days (Treatment, Ext1, F (1, 336) = 34.34, P < 0.0001, Sidak’s post hoc test for trial 5, P = 0.0034; Ext2, F (1, 336) = 39.68, P < 0.0001, Sidak’s test for trial 1, P = 0.0061; trial 6, P = 0.0025; **P < 0.01). e In PV-TrkB hCKO mice, extinction training significantly reduced the freezing in all PV-TrkB-hCKO mice only on day 2 (Ext1, trials, F (11, 288) = 0.8743, P = 0.5660; Ext2, trials, F (11, 288) = 0.8726, P < 0.0001) but fluoxetine treatment failed to significantly influence extinction (two-way ANOVA, treatment, Ext1, F (11, 288) = 3.866, P = 0.0502; Ext2, F (1, 288) = 3.776, P = 0.0530). f The effect of fluoxetine (delta: freezing in control (%) – fluoxetine (%)) between wild-type and PV-TrkB hCKO mice on each session in 2 days. The effect of fluoxetine on extinction was significantly more pronounced in wild-type than in PV-TrkB hCKO mice on both Ext1 (t-test, F = 1.616, DFn = 11, Dfd = 11, P = 0.0071) and Ext2 (t-test, F = 1.887, DFn = 11, Dfd = 11, P = 0.0108). g In SR, fluoxetine treatment significantly decreased freezing throughout sessions in wild-type mice (two-way ANOVA, Treatment, F (1, 112) = 14.05, P = 0.0003; Sidak’s post hoc test “water-treated vs Fluoxetine-treated” Trial 1, P = 0.0387) but not in PV-TrkB hCKO mice (Treatment, F (1, 96) = 1.876, P = 0.1739, Sidak’s post hoc test “water-treated vs Fluoxetine-treated” Trial 1, P = 0.9822). h Fluoxetine treatment significantly reduced spontaneous recovery in the first session of SR in the WT mice (Treatment, F (1, 52) = 4.585, P = 0.0370; Sidak’s post hoc test “water-treated vs Fluoxetine-treated” for wild-type P = 0.0229) but not in PV-TrkB hCKO mice (Sidak’s post hoc test “water-treated vs Fluoxetine-treated” for PV-TrkB hCKO P = 0.8618). i Freezing was reduced in repeated sessions after fear renewal by fluoxetine treatment in both wt (Treatment, F (1, 112) = 14.56, P = 0.0002; Sidak’s post hoc test “water-treated vs Fluoxetine-treated” Trial 1, P = 0.0159) and PV-TrkB hCKO mice (Treatment, F (1, 96) = 16.61, P < 0.0001; Sidak’s post hoc test “water-treated vs Fluoxetine-treated” Trial 1, P = 0.7996, Trial 2, P = 0.0195, Trial 3, P = 0.0243). j Fluoxetine attenuated fear renewal in the first session in wild-type (Treatment, F (1, 52) = 7.465, P = 0.0086; Sidak’s post hoc test “water-treated vs Fluoxetine-treated” P = 0.0122) but not PV-TrkB hCKO mice (Sidak’s post hoc test “water-treated vs Fluoxetine-treated” P = 0.4948). Error bars designate SEM. *P < 0.05; **P < 0.01.
Fear extinction paradigm
The fear-conditioning paradigm was conducted following a protocol described previously [13].
IntelliCage with chronic fluoxetine treatment
IntelliCage (NewBehavior AG, Zurich, Switzerland) is an automated device that allows housing, performance and measurements of specific tasks in a fully automated manner, removing the need for a human operator and operator-derived bias [24, 25]. We used a number of mice that, according to the method described by Charan and Knetharia [26], leads to a more than adequate degree of freedom of the analysis of variance. Mice were divided into two groups: the “control” treated with 0.1% (w/v) saccharine in the drinking water and the “experimental group” with 0.1% (w/v) saccharin supplemented with 0.08% (w/v) fluoxetine in the drinking water. Previous studies showed that using the increase in hippocampal neurogenesis as an indicator of the beginning of fluoxetine effects showed that 2 weeks of fluoxetine treatment was enough to increase neurogenesis [27]. We have previously demonstrated that 2 weeks of fluoxetine after fear conditioning promotes fear erasure, which is more clinically relevant than providing fluoxetine before or during conditioning [13]. We performed patrolling tasks, where the water bottles were made accessible (doors would open for 4 s) only if the mouse nose-poked the “active” door area which, once discovered and used by the mouse, would switch to the one immediately next to it, in a clockwise direction. During the reversal phase, the direction was switched counter-clockwise.
Electrophysiology in acute slices
Population spike-free field excitatory postsynaptic currents (fEPSPs) were recorded in an interface chamber using ACSF-filled electrodes (2–4 MΩ) positioned within the CA1 stratum radiatum (Supplementary Note). Notably, LTP was induced through tetanic stimulation (10 ms pulse interval; 100 pulses; 0.1 ms pulse duration) and recorded for 45 min.
Immunohistochemistry
Animals treated with control or fluoxetine were perfused transcardially with PBS followed by 4% PFA in PBS and the brains were isolated. The brains were post-fixed overnight and stored in PBS with 0.02% NaN3 until cutting on a vibratome (VT 1000E, Leica). Free-floating sections (40 μm) were processed for fluorescence immunohistochemistry following a protocol described previously [29]. The staining for PV was achieved using a Guinea Pig anti-PV antibody (Synaptic Systems, Göttingen, Germany) (#195004), with a dilution of 1:500, whereas the staining for PNN was obtained using Lectin WFA conjugated with biotin (Sigma-Aldrich St. Louis, MO, USA) (#L1516) with a dilution of 1:400. All the used antibodies are listed in Supplementary Table 1.
Confocal imaging and imaging analysis on PV and PNN intensity
Immunohistologically stained sections were imaged with a confocal microscope (Zeiss LSM 700). PV/PNN was imaged in different sections containing CA3b region in the dorsal hippocampus (between −1.94 and −2.18 mm in the anterior-posterior axis relative to Bregma). The multilayer confocal images were stacked (z-stack, maximum intensity), and the fluorescence intensity of PV, PNN and TdTomato were analyzed with Fiji software (National Institute of Health, USA) (https://fiji.sc/) [30]. We arbitrarily set the borderlines of PV intensity to check the proportion of PV+ interneurons subgroups expressing low, intermediate low, intermediate high, and high PV according to the distribution pattern (Supplementary Note and Supplementary Fig. 2).
TRAP sample preparation and sequencing
TRAP analysis was performed according to a previously published protocol [31]. Briefly, we isolated the hippocampus from mice expressing GFP tagged to the ribosomal subunit L10a specifically in PV+ interneuron (see above), after 2 weeks of treatment with 0.1% (w/v) saccharine in drinking water (control) or 0.1% (w/v) saccharine and 0.080% (w/v) fluoxetine in drinking water (experimental group). The isolated hippocampi were stored at −80 °C until the TRAP experiments. After lysating the hippocampus, the tagged ribosomes were precipitated using magnetic beads covered with anti-GFP antibodies. Actively translated mRNA from tagged ribosomes co-precipitated and were sequenced using HiSeq2500 (Illumina, CA, USA) and SMART-Seq v4 Ultra Low Input RNA Kit (Takara, Japan) for making cDNA library. Primary statistical analysis was carried out with Negative Binomial Distribution with a significance limit of P < 0.05 and the pathway analysis was done from these significant genes using Fisher’s exact test in DAVID with a significance limit of P < 0.1.
Experimental design and statistical analysis
Biochemical and behavioral data were analyzed by two-way ANOVA, taking sessions, genotype, and treatment with fluoxetine, followed by post hoc test. All results of two-way ANOVA and t-test are shown in Supplementary Table 4. All statistical analyses were performed using Prism 6 or 8 (GraphPad Software). A P value < 0.05 was considered statistically significant.
Results
Expression of TrkB in PV+ interneurons is important for fear erasure induced by fluoxetine treatment
We have previously demonstrated that chronic treatment with fluoxetine combined with fear extinction training promotes the erasure of previously acquired fear memory and alters the configuration of PV+ interneurons thereby reducing the proportion of PV+ interneurons expressing PNN [13]. We first tested whether this promoted fear erasure might depend on TrkB expressed in PV+ interneurons and would therefore be blunted in PV-TrkB hCKO mice (Fig. 1a). In a fear-conditioning paradigm (Fig. 1b), all mice were conditioned with a shock paired with a sound cue in context A during the fear-conditioning/acquisition phase, resulting in an increased freezing that was comparable in duration across all groups, although PV-TrkB hCKO mice conditioned faster than wild-type mice (two way ANOVA, Trials, F (4, 270) = 21.94, P < 0.0001; Genotype, F (1, 270) = 4.049, P < 0.0452; post hoc, wild-type vs PV-TrkB hCKO in Trial 3, P = 0.0037) (Fig. 1c). The wild-type and PV-TrkB hCKO mice were then assigned equally and randomly into groups receiving either water or water supplied with 0.08% (w/v) of fluoxetine, both enriched with 0.1 % (w/v) saccharin. Two weeks later, the mice were exposed to the conditioned stimulus (“beep” sound) in context B during 2 days of extinction training. In the wild-type group, two-way ANOVA showed a significant trial effect in both control and fluoxetine-treated mice (Effect of trials, Ext1, F (11, 336) = 1.988, P = 0.0288; Ext2, F (11, 336) = 9.624, P < 0.0001), indicating that the freezing response decreased during the extinction training (Fig. 1d). Moreover, the fluoxetine treatment showed a significantly stronger effect on the extinction training compared to water during both Ext1 and Ext2 (Effect of treatment, Ext1, F (1, 336) = 34.34, P < 0.0001; Ext2, F (1, 336) = 39.68, P < 0.0001) (Fig. 1d). Both control and fluoxetine-treated PV-TrkB hCKO mice showed decreased freezing after the second day of extinction training (Effect of trials, Ext2, F (11, 288) = 0.8726, P < 0.0001), but not after day 1 (Effect of trials, Ext1 F (11, 288) = 0.8743, P = 0.5660). In PV-TrkB hCKO mice, fluoxetine treatment failed to significantly enhance extinction when compared to water-treated mice (Effect of treatment, Ext1, F (1, 288) = 3.866, P = 0.0502; Ext2, F (1, 288) = 3.776, P = 0.0530) (Fig. 1e). Moreover, the effect of fluoxetine treatment on extinction was significantly better in the wild-type mice than in the PV-TrkB hCKO mice during both days of extinction (difference in freezing (delta) between water and fluoxetine-treated mice in each extinction session, t-test, Ext1, F = 1.616, DFn = 11, P = 0.0071; Ext2, F = 1.887, DFn = 11, P = 0.0108) (Fig. 1f), suggesting that in the absence of TrkB in PV neurons, the effects of fluoxetine are significantly reduced. One week later, the fluoxetine-treated wild-type mice showed decreased freezing compared to the water-treated wild-type mice throughout the whole session in context B (spontaneous recovery, SR) (Treatment, F (1, 112) = 14.05, P = 0.0003; Sidak’s post hoc test “water-treated vs Fluoxetine-treated” Trial 1, P = 0.0387) (Fig. 1g), as well as in the first session of this test (Treatment, F (1, 52) = 4.585, P = 0.0370; Sidak’s post hoc test “water-treated vs Fluoxetine-treated” P = 0.0229) (Fig. 1h). The freezing in the first session of SR and FR has been used for estimating cued and contextual memory, respectively [13, 32], as it is the only session that has not been influenced by a possible gradual habituation or extinction-training effect due to the test itself. However, the PV-TrkB hCKO mice failed to show similar effects of the fluoxetine treatment throughout the trials of the test (two-way ANOVA, SR, Treatment, F (1, 96) = 1.876, P = 0.1739) (Fig. 1g), and in the first trial (two-way ANOVA, treatment, F (1, 52) = 4.585, P = 0.0370, Sidak’s post hoc test “water-treated vs Fluoxetine-treated” for PV-TrkB hCKO P = 0.8618) (Fig. 1h). In addition, the treatment significantly reduced the freezing in the fear renewal test (FR) in context A in wild-type mice, especially in the first session of (Treatment, F (1, 112) = 14.56, P = 0.0002; Sidak’s post hoc test “water-treated vs Fluoxetine-treated” Trial 1, P = 0.0159) (Fig. 1i) (Treatment, F (1, 52) = 7.465, P = 0.0086; Sidak’s post hoc test “water-treated vs Fluoxetine-treated” P = 0.0122) (Fig. 1j). Interestingly, the treatment with fluoxetine decreased the overall freezing of PV-TrkB hCKO mice in the fear renewal test (Treatment, F (1, 96) = 16.61, P < 0.0001, Sidak’s post hoc test “water-treated vs Fluoxetine-treated” Trial 1, P = 0.7996, Trial 2, P = 0.0195, Trial 3, P = 0.0243) (Fig. 1i), but there was no difference in the first session (Treatment, F (1, 52) = 7.465, P = 0.0086; Sidak’s post hoc test “water-treated vs Fluoxetine-treated” P = 0.4948) (Fig. 1j). The absence of an effect of Fluoxetine on the PV-TrkB hCKO mice in the SR and a presence of a smaller effect in the FR suggest a role of TrkB expression in PV neurons in the extinction-enhancing effects of fluoxetine in cued fear conditioning, but a less pronounced role in the contextual component of the paradigm (FR).
Expression of TrkB in PV+ interneurons is important for the improvement of reversal spatial learning induced by fluoxetine treatment
The IntelliCage experiments were conducted to test the effect of chronic fluoxetine treatment on spatial learning, as depicted in Fig. 2a. Mice were implanted with transponders and were treated with fluoxetine-containing water for 2 weeks before the experiments. During the adaptation to freely accessible water bottles in the corners (FA), nose pokes (NPA), and drinking sessions (DSA), six mice were excluded because they could not learn the adaptation tasks [Control group (wild-type, 1; PV-TrkB hCKO, 2), fluoxetine-treated group (wild-type, 1; PV-TrkB hCKO, 2)]. In the acquisition phase of the patrolling task, the location of the open corner changed after each visit, and the water-deprived mice had to patrol the corners in a “clockwise” order to receive a water reward (Fig. 2a, left panel). The percentages of error ratios were calculated as the number of visits in the incorrect corner divided by the number of total visits and expressed as an average of each mouse of the 2-h session. The wild-type mice decreased the error ratio during sessions, and there was no effect of fluoxetine treatment in the acquisition phase (Acquisition (days), F (7, 208) = 8,520, P < 0.0001; Treatment, F (1, 208) = 1.021, P = 0.3133) (Fig. 2c–e). The PV TrkB hCKO mice also decreased the error ratio during sessions (Fig. 2f–h), but interestingly the fluoxetine treatment decreased the error ratio faster than in water-treated mice (Acquisition (days), F (7, 183) = 9.462, P < 0.0001; Treatment, F (1, 183) = 16.37, P < 0.0001) (Fig. 2f). The water-treated PV TrkB hCKO mice had significantly higher error ratios compared to wild-type mice treated with control water (Acquisition (days), F (7, 200) = 6.015, P < 0.0001; Genotype, F (1, 200) = 30.09, P < 0.0001) (Supplementary Fig. 1a). These results indicate that PV TrkB hCKO mice have lower spatial learning skills in acquisition compared to wild-type mice, but the fluoxetine treatment recovers them to a level comparable to wild-type mice.
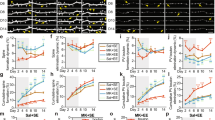
a Scheme of the IntelliCage system during the chronic treatment with fluoxetine. All mice were implanted with transponders, and were treated with fluoxetine in water. Mice adapted gradually to the tasks in the IntelliCage (FA free adaptation, NPA nose poke adaptation, DAA drinking session adaptation), followed by the actual leaning tasks (Patrolling). b Scheme of the patrolling task. Error ratio in acquisition (c–h) and reversal phase (i–n) in wild-type (c–e, i–k) and PV-TrkB-hCKO mice (f–h, l–n) (n = 12–15 per group). c Wild-type mice decreased the error ratio during the acquisition days (two-way ANOVA, Acquisition (days), F (7,208) = 8.520, P < 0.0001), but there was no difference caused by the treatment (two-way ANOVA, treatment, F (1, 208) = 1.021, P = 0.3133). Significant differences were found in pairwise comparisons between the first and last sessions in control mice (pairwise t-test, t = 3,562, df = 14, P = 0.0031) (d) and fluoxetine water-treated mice (t = 6,626, df = 13, P < 0.0001) (e). Fluoxetine treatment reduced the error ratio during sessions in PV-TrkB-hCKO mice (treatment, F (1, 183) = 16.37, P < 0.0001; Acquisition (days), F (7, 183) = 9.462, P < 0.0001). There was a significant difference in the error ratio between the initial and the last sessions in control (pairwise t-test, t = 6,747, df = 10, P < 0.0001) (g) and fluoxetine-treated mice (t = 5,527, df = 12, P = 0.0001) (h). In the reversal phase, wild-type mice showed a significant effect in days (F (7, 208) = 4.212, P = 0.0002) and treatment (F (1, 208) = 6.794, P = 0.0098) (i). There were significant differences between the error ratio in the initial session and the last one in both control (pairwise t-test, t = 2,845, df = 14, P = 0.0130) (j) and fluoxetine-treated mice (t = 4,543, df = 12, P = 0.0007) (k). (l) In PV hTrkB cKO mice, fluoxetine treatment did not have an effect during sessions (two-way ANOVA, Treatment, F (1, 184) = 0.2608, P = 0.6102; Reversal (days), F (7, 184) = 3.550, P = 0.0013). There was a significant difference in error ratios between first and last session in control (pairwise t-test, t = 3,123, df = 11, P = 0.0097) (m) and fluoxetine treatment (t = 4,628, df = 12, P = 0.0006) (n). Error bars designate SEM. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001 (in post hoc test: f, I, l; pairwise t-test: d, e, g, h, j, k, m, n).
In the reversal phase, wild-type mice significantly reduced the error ratio during sessions in both control and fluoxetine-treated groups (Reversal (days), F (7, 208) = 4.212, P = 0.0002) (Fig. 2i–k), but the treatment with fluoxetine facilitated the decrease of the error ratio during sessions (Treatment, F (1, 208) = 6.794, P = 0.0098) (Fig. 2i). These results indicate that the fluoxetine treatment improves the reversal learning in wild-type mice. The PV hTrkB CKO mice also improved their performance during sessions (Reversal (days), F (7, 184) = 3.550, P = 0.0013) (Fig. 2l–n). These results suggest that TrkB expression in PV interneurons is important for the effect of fluoxetine on the reversal learning in spatial tasks.
Fluoxetine treatment potentiates hippocampal LTP through expression of TrkB in PV interneurons
In order to understand whether the improved behavioral flexibility after fluoxetine treatment reflects enhanced neural plasticity in the hippocampus, the main region involved in contextual fear and spatial memory [32], we recorded fEPSPs in acute hippocampal slices of wild-type and PV TrkB hCKO mice after chronic fluoxetine treatment (Fig. 3). As previously reported [8, 28] we observed a significant enhancement of LTP at 45 min after tetanic stimulation in wild-type mice treated with fluoxetine compared to mice treated with water (two-way ANOVA, treatment, wild-type, F (1, 414) = 50.20, P < 0.0001). There was, however, no effect of fluoxetine treatment on LTP in hPV-TrkB CKO mice (treatment, F (1, 40) = 0.2726, P = 0.6019) (Fig. 3). These results indicate that the chronic treatment with fluoxetine enhances the expression of LTP in the Shaffer collateral-CA1 synapses of the hippocampus, in a manner dependent on the expression of TrkB in PV-interneurons.
LTP induction after chronic treatment with fluoxetine. LTP was significantly enhanced 45 min after tetanic stimulation in wild-type mice treated with fluoxetine compared to control (two-way ANOVA, treatment, wild-type, F (1, 414) = 50.20, P < 0.0001) but not in PV-TrkB hCKO mice (treatment, F (1, 40) = 0.2726, P = 0.6019). Bars indicate mean ± SEM.
PV-specific transcriptomic analysis through TRAP
In order to investigate gene expression in PV interneurons after chronic treatment with fluoxetine, we conducted a TRAP analysis, a system that allows the precipitation mRNA bound to ribosomes, to investigate ongoing protein translation specifically in PV+ interneurons (Fig. 4a) (see Material and method, and Supplementary note). After chronic treatment with fluoxetine, the whole hippocampus of mice expressing EGFP-tagged L10a ribosomal subunits specifically in PV interneurons (Fig. 4b) was used for TRAP followed by next-generation sequencing. We found 879 genes that were differentially expressed after chronic treatment with fluoxetine (P < 0.05) (Fig. 4c and Supplementary Table 2), and these were further studied by pathway analysis using Fisher’s exact test for up- and downregulated genes separately. The chronic fluoxetine treatment significantly affected several of these pathways in the hippocampus (P < 0.1) (Supplementary Table 3), and differentially expressed genes from enriched pathways are shown in Fig. 4d. Interestingly, genes in glycosaminoglycan chondroitin sulfate (Fisher’s exact test, P = 0.0268) and heparan biosynthesis (Fisher’s exact test, P = 0.0377) pathways (B4galt7, Extl3, B3gat3 and Chst3) were significantly downregulated. These are associated with chondroitin sulfate proteoglycans, which are an integral part of PNNs [15]. Also, genes related to glycerolipid (Fisher’s exact test, P = 0.0384) and glycerophospholipid metabolism (Fisher’s exact test, P = 0.0013) were downregulated. These pathways are involved in the regulation of lipid composition of the cellular membrane, which is highly related to AD effects [20]. Furthermore, the fluoxetine treatment significantly changed the expression of genes in the GABAergic synapse pathway (Fisher’s exact test, P = 0.0863), including G Protein Alpha Inhibiting Activity Polypeptide 3 (Gnai3), G protein subunit gamma 4, 8, and 13 (Gng4, Gng8, and Gng13), which are coupled with GABA type B receptor, and mediate slow and prolonged inhibitory action [33]. Huntingtin-associated protein 1 (Hap1) directly interacts with GABA type A (GABAA) receptors and influences the recycling of the receptor by inhibiting its degradation [34]. Such modulation of the expression and localization of GABAA receptors are thought to be a plastic event resulting in the maintenance of the excitatory/inhibitory balance [35].
a Ribosome-tagged transgenic mice were treated with fluoxetine or control water for 2 weeks, and their hippocampi were isolated and lysated. Ribosomes bound to mRNA were immunoprecipitated with beads coated with GFP-antibody and the mRNA was purified for cDNA synthesis followed by next-generation sequencing (NGS). b Immunohistochemistry analysis with anti-PV antibody. Parvalbumin is co-localized with GFP indicating that the cells expressing GFP-tag in ribosomes are PV cells. Scale bars, 50 µm. c Volcano plot showing log2 of fold change of all genes after fluoxetine treatment in x-axis and negative log10 of P value in y-axis. Downregulated genes that had significantly differential expression are marked in blue and upregulated in red. d Heatmap of significant genes and pathways detected by GO analysis. The expression of genes in a sample is scaled to values between −2 and 2, and these correlate with colors in the heatmap according to the panel on the right. GAGs C glycosaminoglycan biosynthesis chondroitin sulfate, GAGs H glycosaminoglycan biosynthesis heparan sulfate, GPL glycerophospholipid metabolism, GL glycerolipid metabolism.
Overall, our TRAP analysis provides insight into the observed phenomena of increased neural plasticity in PV+ interneurons, such as synaptic formation and turnover of PNNs through the regulation of gene expression after fluoxetine treatment.
Decreased intensity of PV and PNN after fluoxetine treatment depending on TrkB expression in PV+ interneurons
TRAP analysis showed decreased expression of genes related to the formation of PNN. In addition, it has been reported that PV configurations in the CA3 region of the hippocampus are dynamically regulated by experiences, such as environmental enrichment and fear conditioning [36]. We used immunohistochemistry to analyze the intensities of PV, and PNNs surrounding PV interneurons as a measure of their expression levels in the hippocampal CA3 region after chronic fluoxetine treatment (Fig. 5a). After fluoxetine treatment the proportion of low-intensity PV cells increased, and the high-intensity PV cells were reduced in wild-type mice, while there was no obvious difference in the proportions of PV intensity in PV-TrkB hCKO mice (Fig. 5b). In addition, the proportion of PV-positive cells among cells positive for PNN was significantly reduced after fluoxetine treatment in wild-type mice as shown previously [13], but not in PV TrkB hCKO mice (two-way ANOVA, Treatment, F (1, 36) = 1.757, P = 0.1934; Genotype, F (1, 36) = 1.203, P = 0.2801; Fisher’s LSD post hoc test, control vs Flx: wild-type, P = 0.0138; CKO, P = 0.7571) (Fig. 5c). Interestingly, when the intensity of PNNs were separately measured in lower and higher PV-expressing PV interneurons, the fluoxetine treatment significantly reduced the intensity of PNN only in high but not in low PV-expressing cells in wild-type mice (two-way ANOVA, interaction between PV intensity and Treatment, F (1, 208) = 4.785, P = 0.0298; PV intensity, F (1, 208) = 16.92, P < 0.0001; Treatment, F (1, 208) = 3.103, P = 0.0796; Fisher’s LSD post hoc test, control vs Flx: Low, P = 0.6365; High, P = 0.0281) (Fig. 5d). However, the treatments showed no effect on the PNN intensity in either low or high PV-expressing cells in PV-TrkB hCKO mice (two-way ANOVA, interaction, F (1, 108) = 1.326, P = 0.2520; PV intensity, F (1, 108) = 28.88, P < 0.0001; Treatment, F (1, 108) = 2.564, P = 0.1122; Fisher’s LSD post hoc test, control vs Flx: Low, P = 0.6722; High, P = 0.1075) (Fig. 5e). These results strongly suggest that chronic fluoxetine treatment shifts the configuration of PV interneurons toward lower PV and PNN expressing cell state through TrkB signaling. Taken together with the TRAP analysis, the decreased gene expressions of the extracellular matrix might be involved in the reduced PNN formation after chronic treatment with fluoxetine.
a–e Image analysis on PV and PNN expression in the dorsal hippocampus of control and fluoxetine-treated wild-type and PV-TrkB hCKO mice. a Representative image of PV and PNN staining. Immunostaining with PV and PNN followed by intensity analysis on PV and PNN. SP stratum pyramidale, SO stratum oriens, SR stratum radiatum. Scale bar, 50 µm. b Intensity analysis of PV expression in PV interneurons. The ratio of high and intermediate-high PV was lower after fluoxetine treatment in wild-type mice, but this difference was not observed in PV-TrkB hCKO mice. c Fluoxetine-treated wild-type mice have significantly lower percentages of PV interneurons also expressing PNNs, but this effect is blunted in PV-TrkB hCKO mice (two-way ANOVA, Treatment, F (1, 36) = 1.757, P = 0.1934; Genotype, F (1, 36) = 1.203, P = 0.2801; Fisher’s LSD post hoc test, control vs Flx: wild-type, P = 0.0138; CKO, P = 0.7571). d, e PNN intensity analysis in cells separated by PV-intensity. Fluoxetine treatment reduces PNN intensities in high (intermediate-high and high) PV-expressing cells only in WT mice (two-way ANOVA, the interaction between PV intensity and Treatment, F (1, 208) = 4.785, P = 0.0298; PV intensity, F (1, 208) = 16.92, P < 0.0001; Treatment, F (1, 208) = 3.103, P = 0.0796; Fisher’s LSD post hoc test, control vs Flx: Low, P = 0.6365; High, P = 0.0281), but not in PV-TrkB hCKO mice (two-way ANOVA, interaction, F (1, 108) = 1.326, P = 0.2520; PV intensity, F (1, 108) = 28.88, P < 0.0001; Treatment, F (1, 108) = 2.564, P = 0.1122; Fisher’s LSD post hoc test, control vs Flx: Low, P = 0.6722; High, P = 0.1075). WT, control, low, number of cells (n) = 88; WT, Flx, low, n = 79; WT, control, high, n = 28; WT, control, high,17; CKO, control, low, n = 32; CKO, Flx, low, n = 48; CKO, control, high, n = 12; CKO, control, high, 20. Bars indicate mean + SEM. *P < 0.05.
Discussion
Here, we demonstrate that iPlasticity induced by pharmacological activation of TrkB in PV+ interneurons promotes reversal learning in fear and spatial memory. We also showed that both the potentiation of LTP in Schaffer collaterals to CA1 synapses, and the shift in the configuration of the PV and PNN network to a more plastic state require TrkB expression in hippocampal PV+ interneurons. These alterations involve changes in the gene expression patterns related to GABAergic synapses and PNN formation.
Impaired fear extinction consolidation of heterozygous PV-TrkB CKO mice
It has previously been shown that heterozygous PV-hTrkB CKO male mice exhibit slightly impaired extinction consolidation but not fear acquisition/conditioning in the auditory fear paradigm [37]. In this study, PV-hTrkB CKO mice showed normal or even faster fear acquisition, but control heterozygous PV-TrkB CKO mice kept a higher level of freezing in fear renewal, compared to that of wild-type mice. Altogether, a reduced expression of TrkB in PV interneurons blunts the effect of fluoxetine on short-term fear erasure, whereas fluoxetine seems to have an independent effect from TrkB expression in PV interneurons on long-lasting fear erasure. Further studies are needed to elucidate the details of this mechanism.
Roles of TrkB in PV+ interneurons for contextual fear erasure
We show here that fluoxetine treatment decreases cued and contextual fear responses in wild-type mice as previously shown [13], but not in heterozygous PV-TrkB CKO mice. However, fluoxetine-treated heterozygous PV-TrkB CKO mice showed a blunted effect only in the initial phase, but then reduced their fear responses in later phases during fear renewal. Interestingly, optical activation of TrkB in the pyramidal neurons of the ventral hippocampus showed a similar pattern: decreased contextual fear response except for the initial phase [29]. One of the possibilities raised by these observations is that the expression of TrkB in PV interneurons is important, but that TrkB expression in pyramidal neurons is also involved in the formation of a new inhibitory memory to overwrite a conditioned memory or to erase a fear memory.
PV interneurons are involved in the reversal learning phase in spatial learning
To test whether fluoxetine treatment can improve behavioral flexibility or spatial reversal learning through TrkB receptors in PV interneurons, we used the IntelliCage system. IntelliCage is an automated setup that allows behavioral experiments without direct handling of mice except when changing cages and water bottles, which results in higher reproducibility and reliability [38]. Previous studies have shown that Calcium/calmodulin-dependent protein kinase type II subunit alpha (CaMKIIa) specific TrkB heterozygous CKO mice had normal spatial memory in the classical Morris water maze test, but had lower behavioral flexibility in naturalistic settings [22, 39]. Chronic treatment with fluoxetine did not affect acquisition but it enhanced reversal spatial memory in wild-type mice. It has been observed previously that fluoxetine can affect the reversal without influencing the acquisition in the fear-conditioning paradigm [13]. In contrast, PV-TrkB hCKO mice showed improved spatial memory in acquisition but not in the reversal phase after fluoxetine treatment. In addition, PV-TrkB CKO mice showed a higher error ratio in acquisition but not in the reversal phase, when compared to the wild-type mice. The effect of Fluoxetine on the acquisition in the PV-TrkB hCKO mice might be due to the drug acting on the residual TrkB present on PV cells (the mice are heterozygote for the knockout), which might be enough to induce an observable improvement in the performance. Alternatively, it is possible that in the absence of TrkB in PV cells, fluoxetine might be acting on TrkB present in other neurons (e.g., pyramidal neurons), rescuing the impaired spatial memory of PV-TrkB CKO mice. We therefore suggest that TrkB in PV+ interneurons plays a role in the basal level of spatial learning as well as in the reversal learning improvement promoted by fluoxetine. However, we cannot exclude the possibility of the TrkB-independent effects of fluoxetine, acting on spatial memory. For instance, serotonin receptor inhibitors might shed light on possible SSRI effects. More research is needed to elucidate the detailed mechanism of the effect of chronic fluoxetine treatment on spatial memory.
PV and PNN as makers of plasticity in PV interneurons
We found that fluoxetine treatment mainly affects the high PV-expressing PV interneurons, which have been demonstrated to be born earlier during embryonic development and to be responsible for memory formation of recent experiences [14, 36]. For instance, fear conditioning increases the number of high PV-expressing cells, while environmental enrichment increases the fraction of PV basket cells with low levels of PV [36]. These observations suggest that chronic fluoxetine treatment and environmental enrichment show similar effects, promoting the more plastic state of PV configuration. In addition, we demonstrate that the intensity of PNN was reduced after fluoxetine treatment only in high PV-expressing but not in low PV-expressing interneurons. Lower expression of PNN is known to represent a plastic state of PV interneurons [40], and it is interesting that chronic fluoxetine treatment regulates both PV and PNNs via TrkB expression. The TRAP analysis also points toward the regulation of PNNs as the target of fluoxetine action. We have previously shown that plasticity promoted by the reduction of PNNs by chondroitinase treatment is also dependent on the expression of TrkB in PV interneurons and that this effect is mediated by the inhibition of the PNN receptor, receptor-type tyrosine-protein phosphatase Sigma (PTPRS) [41]. In addition, fluoxetine was shown to disrupt the interaction between TrkB and PTPRS, functionally mimicking the effects of PNN disruption [41]. Furthermore, it appears that fluoxetine specifically targets PV+ interneurons, which was also observed in visual cortex plasticity [16]. These effects of fluoxetine treatment on the TrkB receptors expressed in PV interneurons might be involved in behavioral flexibility, which would promote the exchange or renewal of consolidated memories.
LTP is increased through TrkB activation in PV interneurons
Hippocampal LTP is widely regarded as the cellular substrate underlying learning and memory, enabling plasticity processes to take place [42]. Previous research has shown that chronic fluoxetine treatment increases LTP in the hippocampus, amygdala and visual cortex [8, 13, 16, 43]. The TrkB receptor and particularly its signaling through phospholipase Cγ has emerged as a potent regulator of LTP [9]. We now show that the fluoxetine-mediated increase in hippocampal LTP is prevented when TrkB expression is reduced in PV interneurons.
Gene regulation in fluoxetine-induced plasticity in PV+ interneurons
In addition to the genes related to the formation of PNNs, we found regulation of genes related to the composition of the cellular membrane, regulatory proteins of GABArgic receptors and genes related to axonal growth guidance.
In a previous study from our lab, we observed that the optogenetic activation of TrkB in PV neurons modulates the expression of genes related to GABAergic signaling [16]. This result fits with our observation that fluoxetine upregulates genes involved in GABAergic synapses.
Among our data, we also found upregulation of Hap1, which is known to co-localize with TrkB and takes part in the reuptake of BDNF [44]. Literature suggests that the activation of the BDNF/TrkB pathway is involved in guiding axonal growth [45]. Consistently with this notion, we found that fluoxetine upregulated Limk1, which is a crucial link in axonal growth induced by BDKF/TrkB signaling [46].
Moreover, several studies show how the Trk family is involved in the development of thyroid cancer [47] and TrkB specifically seems to be involved in tumor pathology in neuroblastoma [48].
Overall, the observation of the upregulation of genes involved in TrkB signaling or in processes regulated by TrkB supports the notion that the BDNF/TrkB pathway has indeed been activated by the fluoxetine treatment.
All significant DE genes related to axon guidance were upregulated. For instance, Sem3D is a receptor of Sema3A, which is known to be localized in PNNs [49], and Srgap2 is localized in synapses and regulates synaptic densities through Rac1-GAP activity [50, 51]. Since the Rac1 signaling regulates the density of inhibitory synapses within dendrites and their subcellular distribution, Srgap2 is considered to coordinate excitatory/inhibitory balance [52]. These results suggest that axon regeneration and sprouting actively occurred and can potentially rewire neuronal networks involving PV+ interneurons responding to environmental stimuli.
Mechanisms of fluoxetine-induced plasticity
We have recently shown that fluoxetine directly binds to the transmembrane domain of TrkB dimers and increases TrkB retention in the plasma membrane, thereby allosterically promoting BDNF signaling [20]. Furthermore, ADs disrupt the interaction between TrkB and the AP2 complex involved in endocytosis, promoting TrkB localization in the plasma membrane [53]. Consistently with the present findings, we have observed that the activation of TrkB specifically in the PV interneurons is necessary and sufficient for iPlasticity in the visual cortex [16]. We found here that TrkB activation by fluoxetine regulates PNNs encasing PV neurons and our previous findings suggest that reduction in PNNs further promotes TrkB activity within PV+ interneurons [17]. Taken together, a positive feedback loop between the reduction of PNN and TrkB activation may explain the critical role of TrkB in the PV+ neurons in iPlasticity. Importantly, while the activation of TrkB in pyramidal neurons promotes their excitability, in PV+ neurons TrkB activation reduces excitability through the downregulation of Kv3-family potassium channels [16]. Therefore, the activation of TrkB in PV+ interneurons does not counteract the concomitant TrkB activation in pyramidal neurons but synergizes with it by disinhibiting pyramidal neurons, thereby orchestrating an enhanced state of cortical plasticity that underlies iPlasticity. Our present data suggest that a similar kind of state of enhanced plasticity, involving reformation of GABAergic signaling and reduction in PNNs, is underlying the effects of fluoxetine on behavioral flexibility and reversal learning.
References
Barkas L, Redhead E, Taylor M, Shtaya A, Hamilton DA, Gray WP. Fluoxetine restores spatial learning but not accelerated forgetting in mesial temporal lobe epilepsy. Brain. 2012;135:2358–74.
Marwari S, Dawe GS. (R)-fluoxetine enhances cognitive flexibility and hippocampal cell proliferation in mice. J Psychopharmacol. 2018;32:441–57.
Gan H, Zhang Q, Zhu B, Wu S, Chai D. Fluoxetine reverses brain radiation and temozolomide-induced anxiety and spatial learning and memory defect in mice. J Neurophysiol. 2019;121:298–305.
Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–10.
Malberg JE, Duman RS. Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology. 2003;28:1562–71.
Rubio FJ, Ampuero E, Sandoval R, Toledo J, Pancetti F, Wyneken U. Long-term fluoxetine treatment induces input-specific LTP and LTD impairment and structural plasticity in the CA1 hippocampal subfield. Front Cell Neurosci. 2013;0:66.
Castrén E, Hen R. Neuronal plasticity and antidepressant actions. Trends Neurosci. 2013;36:259–67.
Popova D, Castrén E, Taira T. Chronic fluoxetine administration enhances synaptic plasticity and increases functional dynamics in hippocampal CA3-CA1 synapses. Neuropharmacology. 2017;126:250–6.
Minichiello L, Calella AM, Medina DL, Bonhoeffer T, Klein R, Korte M. Mechanism of TrkB-mediated hippocampal long-term potentiation. Neuron. 2002;36:121–37.
Castrén E, Antila H. Neuronal plasticity and neurotrophic factors in drug responses. Mol Psychiatry. 2017;22:1085–95.
Ohira K, Takeuchi R, Iwanaga T, Miyakawa T. Chronic fluoxetine treatment reduces parvalbumin expression and perineuronal nets in gamma-aminobutyric acidergic interneurons of the frontal cortex in adult mice. Mol Brain. 2013;6:43.
Guirado R, Perez-Rando M, Sanchez-Matarredona D, Castrén E, Nacher J. Chronic fluoxetine treatment alters the structure, connectivity and plasticity of cortical interneurons. Int J Neuropsychopharmacol. 2014;17:1635–46.
Karpova NN, Pickenhagen A, Lindholm J, Tiraboschi E, Kulesskaya N, Ágústsdóttir A, et al. Fear erasure in mice requires synergy between antidepressant drugs and extinction training. Science. 2011;334:1731–4.
Donato F, Chowdhury A, Lahr M, Caroni P. Early-and late-born parvalbumin basket cell subpopulations exhibiting distinct regulation and roles in learning. Neuron. 2015;85:770–86.
Fawcett JW, Oohashi T, Pizzorusso T. The roles of perineuronal nets and the perinodal extracellular matrix in neuronal function. Nat Rev Neurosci. 2019;20:451–65.
Winkel F, Ryazantseva M, Voigt MB, Didio G, Lilja A, Llach Pou M, et al. Pharmacological and optical activation of TrkB in Parvalbumin interneurons regulate intrinsic states to orchestrate cortical plasticity. Mol Psychiatry. 2021;2021:1–10.
Lesnikova A, Casarotto PC, Fred SM, Voipio M, Winkel F, Steinzeig A, et al. Chondroitinase and antidepressants promote plasticity by releasing TRKB from dephosphorylating control of PTPσ in Parvalbumin neurons. J Neurosci. 2021;41:972–80.
Umemori J, Winkel F, Didio G, Llach Pou M, Castrén E. iPlasticity: induced juvenile-like plasticity in the adult brain as a mechanism of antidepressants. Psychiatry Clin Neurosci. 2018;72:633–53. https://doi.org/10.1111/pcn.12683.
Castrén E. Is mood chemistry? Nat Rev Neurosci. 2005;6:241.
Casarotto PC, Girych M, Fred SM, Kovaleva V, Moliner R, Enkavi G, et al. Antidepressant drugs act by directly binding to TRKB neurotrophin receptors. Cell. 2021;184:1299–313.e19.
Hippenmeyer S, Vrieseling E, Sigrist M, Portmann T, Laengle C, Ladle DR, et al. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol. 2005;3:e159.
Minichiello L, Korte M, Wolfer D, Kühn R, Unsicker K, Cestari V, et al. Essential role for TrkB receptors in hippocampus-mediated learning. Neuron. 1999;24:401–14.
Liu J, Krautzberger AM, Sui SH, Hofmann OM, Chen Y, Baetscher M, et al. Cell-specific translational profiling in acute kidney injury. J Clin Invest. 2014;124:1242–54.
Kiryk A, Janusz A, Zglinicki B, Turkes E, Knapska E, Konopka W, et al. IntelliCage as a tool for measuring mouse behavior – 20 years perspective. Behav Brain Res. 2020;388:112620.
Lipp HP. High-throughput and automated behavioural screening of normal and genetically modified mice. Business Briefing: future drug discovery. 2005;5.
Charan J, Kantharia ND. How to calculate sample size in animal studies? J Pharmacol Pharmacother. 2013;4:303–6. https://doi.org/10.4103/0976-500X.119726.
Wu X, Castrén E. Co-treatment with diazepam prevents the effects of fluoxetine on the proliferation and survival of hippocampal dentate granule cells. Biol Psychiatry. 2009;66:5–8.
Vetencourt JFM, Sale A, Viegi A, Baroncelli L, De Pasquale R, O’leary OF, et al. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science. 2008;320:385–8.
Umemori, J. Optical activation of TrkB neurotrophin receptor in mouse ventral hippocampus promotes plasticity and facilitates fear extinction. BioRxiv [Preprint]. 2021:2021.02.14.431126.
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–82.
Heiman M, Kulicke R, Fenster RJ, Greengard P, Heintz N. Cell type–specific mRNA purification by translating ribosome affinity purification (TRAP). Nat Protoc. 2014;9:1282–91.
Tovote P, Fadok JP, Lüthi A. Neuronal circuits for fear and anxiety. Nat Rev Neurosci. 2015;16:317–31.
Padgett CL, Slesinger PA. GABAB receptor coupling to G-proteins and ion channels. Adv Pharm. 2010;58:123–47.
Kittler JT, Thomas P, Tretter V, Bogdanov YD, Haucke V, Smart TG, et al. Huntingtin-associated protein 1 regulates inhibitory synaptic transmission by modulating γ-aminobutyric acid type A receptor membrane trafficking. Proc Natl Acad Sci USA. 2004;101:12736–41.
Mele M, Leal G, Duarte CB. Role of GABA A R trafficking in the plasticity of inhibitory synapses. J Neurochem. 2016;139:997–1018.
Donato F, Rompani SB, Caroni P. Parvalbumin-expressing basket-cell network plasticity induced by experience regulates adult learning. Nature. 2013;504:272.
Lucas EK, Jegarl A, Clem RL. Mice lacking TrkB in parvalbumin-positive cells exhibit sexually dimorphic behavioral phenotypes. Behav Brain Res. 2014;274:219–25.
Puścian A, Łeski S, Górkiewicz T, Meyza K, Lipp HP, Knapska E. A novel automated behavioral test battery assessing cognitive rigidity in two genetic mouse models of autism. Front Behav Neurosci. 2014;8:140.
Vyssotski AL, Dell’Omo G, Poletaeva II, Vyssotski DL, Minichiello L, Klein R. et al. Long-term monitoring of hippocampus-dependent behavior in naturalistic settings: mutant mice lacking neurotrophin receptor TrkB in the forebrain show spatial learning but impaired behavioral flexibility. Hippocampus. 2002;12:27–38.
Lensjø KK, Lepperød ME, Dick G, Hafting T, Fyhn M. Removal of perineuronal nets unlocks juvenile plasticity through network mechanisms of decreased inhibition and increased gamma activity. J Neurosci. 2017;37:1269–83.
Lesnikova A, Casarotto P, Moliner R, Fred SM, Biojone C, Castrén E. Perineuronal net receptor PTPσ regulates retention of memories. Front Synaptic Neurosci. 2021;13:37.
Minichiello L. TrkB signalling pathways in LTP and learning. Nat Rev Neurosci. 2009;10:850–60.
Wang JW, David DJ, Monckton JE, Battaglia F, Hen R. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J Neurosci. 2008;28:1374–84.
Lim Y, Wu LLY, Chen S, Sun Y, Vijayaraj SL, Yang M, et al. HAP1 is required for endocytosis and signalling of BDNF and its receptors in neurons. Mol Neurobiol. 2018;55:1815–30.
Song HJ, Ming GL, Poo MM. cAMP-induced switching in turning direction of nerve growth cones. Nature. 1997;388:275–9.
Dong Q, Ji YS, Cai C, Chen ZY. LIM kinase 1 (LIMK1) interacts with tropomyosin-related kinase B (TrkB) and mediates brain-derived neurotrophic factor (BDNF)-induced axonal elongation. J Biol Chem. 2012;287:41720.
Mcgregor LM, Mccune BK, Graff JR, Mcdowell PR, Romans KE, Yancopoulos GD, et al. Roles of trk family neurotrophin receptors in medullary thyroid carcinoma development and progression. Proc Natl Acad Sci USA. 1999;96:4540–5.
Thiele CJ, Li Z, McKee AE. On Trk – the TrkB signal transduction pathway is an increasingly important target in cancer biology. Clin Cancer Res. 2009;15:5962–7.
Vo T, Carulli D, Ehlert EME, Kwok JCF, Dick G, Mecollari V, et al. The chemorepulsive axon guidance protein semaphorin3A is a constituent of perineuronal nets in the adult rodent brain. Mol Cell Neurosci. 2013;56:186–200.
Okada H, Uezu A, Mason FM, Soderblom EJ, Moseley MA, Soderling SH. SH3 domain-based phototrapping in living cells reveals rho family GAP signaling complexes. Sci Signal. 2011;4:rs13.
Fossati M, Pizzarelli R, Schmidt ER, Kupferman JV, Stroebel D, Polleux F, et al. SRGAP2 and its human-specific paralog co-regulate the development of excitatory and inhibitory synapses. Neuron. 2016;91:356–69.
Maffei A, Charrier C, Caiati MD, Barberis A, Mahadevan V, Woodin MA, et al. Emerging mechanisms underlying dynamics of GABAergic synapses. J Neurosci. 2017;37:10792–9.
Fred SM, Laukkanen L, Brunello CA, Vesa L, Göös H, Cardon I, et al. Pharmacologically diverse antidepressants facilitate TRKB receptor activation by disrupting its interaction with the endocytic adaptor complex AP-2. J Biol Chem. 2019;294:18150–61.
Acknowledgements
We thank Sulo Kolehmainen and Outi Nikkila for their assistance in all experiments. We also thank the caretakers in the F-building in UH for assistance with animal care, and Sequencing unit of Institute for Molecular Medicine Finland FIMM Technology Centre, University of Helsinki supported by Biocenter Finland. Behavioral testing was carried out at the Mouse Behavioural Phenotyping Facility (MBPF) supported by Biocenter Finland and HiLIFE. We also thank Anu Suoranta, and Pirkko Mattila for FIMM Transcriptomics services supported by UH and Biocenter Finland.
Funding
The original research in our laboratory was supported by the ERC grant # 322742 – iPLASTICITY, the Sigrid Jusélius foundation, Jane & Aatos Erkko Foundation, the EU Joint Programme–Neurodegenerative Disease Research (JPND) project # JPCOFUND_FP-829-007, the HiLife Fellows program, the Academy of Finland grants #294710, 303124, 307416 and 347358, Bilateral exchange program between the Academy of Finland and JSPS (Japan Society for the Promotion of Science), the Brain & Mind grants, The Finnish Medical Foundation and the University of Helsinki Research Foundation. Open Access funding provided by University of Helsinki including Helsinki University Central Hospital.
Author information
Authors and Affiliations
Contributions
JU and EC conceived of and designed the project. CB, GD, VV and JU performed behavioral experiments. MLP, LK-N, and JU conducted immunohistochemistry and imaging analysis under the supervision of RG. FW performed electrophysiological experiments under the supervision of TT and SEL. EJ performed TRAP analysis under the supervision of JU. All authors were involved in writing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jetsonen, E., Didio, G., Winkel, F. et al. Activation of TrkB in Parvalbumin interneurons is required for the promotion of reversal learning in spatial and fear memory by antidepressants. Neuropsychopharmacol. 48, 1021–1030 (2023). https://doi.org/10.1038/s41386-023-01562-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-023-01562-y
- Springer Nature Switzerland AG