Abstract
The lateral habenula (LHb) is an epithalamic nuclei that has been shown to signal the aversive properties of ethanol. The present study tested the hypothesis that activity of the LHb is required for the acquisition and/or expression of dependence-induced escalation of ethanol drinking and somatic withdrawal symptoms. Male Sprague–Dawley rats completed 4 weeks of baseline drinking under a standard intermittent access two-bottle choice (2BC) paradigm before undergoing 2 weeks of daily chronic intermittent ethanol (CIE) via vapor inhalation. Following this CIE exposure period, rats resumed 2BC drinking to assess dependence-induced changes in voluntary ethanol consumption. CIE exposed rats exhibited a significant increase in ethanol drinking that was associated with high levels of blood alcohol and a reduction in somatic symptoms of ethanol withdrawal. However, despite robust cFos activation in the LHb during ethanol withdrawal, chemogenetic inhibition of the LHb did not alter either ethanol consumption or somatic signs of ethanol withdrawal. Consistent with this observation, ablating LHb outputs via electrolytic lesions of the fasciculus retroflexus (FR) did not alter the acquisition of somatic withdrawal symptoms or escalation of ethanol drinking in CIE-exposed rats. The LHb controls activity of the rostromedial tegmental nucleus (RMTg), a midbrain nucleus activated by aversive experiences including ethanol withdrawal. During ethanol withdrawal, both FR lesioned and sham control rats exhibited similar cFos activation in the RMTg, suggesting that RMTg activation during ethanol withdrawal does not require LHb input. These data suggest that, at least in male rats, the LHb is not necessary for the acquisition or expression of escalation of ethanol consumption or expression of somatic symptoms of ethanol withdrawal. Overall, our findings provide evidence that the LHb is dispensable for some of the negative consequences of ethanol withdrawal.
Similar content being viewed by others
Introduction
Alcohol use disorder (AUD) is a chronic relapsing disorder that involves the transition from recreational drinking to alcohol dependence, which is characterized by the emergence of a negative affective state, excessive alcohol intake, and a persistent motivation to consume alcohol despite negative consequences [1]. A wealth of research has demonstrated that rodent models of ethanol dependence recapitulate many aspects of dependence in humans, including somatic withdrawal symptoms and escalated ethanol intake associated with the relief of negative withdrawal symptoms [2,3,4]. For example, following induction of dependence, rodents exhibit significantly higher ethanol intake than pre-dependence levels, and this escalated consumption corresponds with reduced somatic and affective withdrawal symptoms [5,6,7]. Furthermore, escalated intake and alleviation of withdrawal symptoms represents a critical component of dependence as it has been linked with increased future ethanol consumption even after withdrawal has subsided [8]. Thus, there is an urgent need to identify neural targets associated with negative affect and alcohol dependence to facilitate the development of improved therapeutic strategies.
A candidate brain region in mediating the ethanol withdrawal-induced negative affective state is the lateral habenula (LHb). The LHb is an epithalamic nuclei primarily comprised of glutamatergic neurons [9,10,11] that project to midbrain structures involved in affect and motivation, including the rostromedial tegmental nucleus (RMTg), ventral tegmental area (VTA), and raphe nuclei [12,13,14,15]. The LHb encodes aversive stimuli [16, 17] and promotes aversion/avoidance behaviors via input to the GABAergic RMTg nucleus [18,19,20,21,22] and midbrain monoaminergic circuitry [23,24,25,26]. Moreover, the LHb undergoes neuroadaptations following exposure to drugs of abuse and has been implicated in playing a critical role in a number of addiction-related behaviors [27,28,29,30]. Consistent with this, the LHb has emerged as a key structure for signaling the aversive properties of alcohol. For example, the LHb had been reported to mediate both ethanol conditioned taste aversion [31,32,33] and conditioned place aversion [34] to ethanol. Furthermore, manipulations of LHb activity have been reported to regulate ethanol consumption as a function of the stage of ethanol drinking. During early stages of ethanol drinking, rats with LHb lesions exhibit increased ethanol consumption [31, 35], suggesting that the LHb may limit drinking by signaling ethanol’s aversive properties. However, long-term ethanol drinking induces heightened LHb activity and excitability, which promote various negative affective behaviors [36,37,38,39]. Following long-term ethanol drinking and withdrawal, pharmacological or chemogenetic inhibition of the LHb reduces ethanol intake and negative affective behaviors [36,37,38]. Together these findings suggest that signaling by the LHb may act to limit ethanol consumption during the early stages of drinking, but following chronic exposure, LHb hyperactivity may promote negative affective behaviors and facilitate increased ethanol consumption [40].
Despite the accumulating evidence that the LHb is involved in ethanol-related behaviors, its role in regulation of behaviors associated with ethanol dependence and withdrawal is unclear. Therefore, the goal of the present study was to determine whether the LHb is necessary for the expression and/or acquisition of escalated ethanol consumption and somatic withdrawal symptoms associated with ethanol dependence. Using a rat model of ethanol dependence and withdrawal drinking, we provide evidence that the LHb is active during ethanol withdrawal. However, despite robust cFos immunoreactivity as an established indicator of neuronal recruitment, chemogenetic and lesion approaches revealed that the LHb is not necessary for the acquisition or development of dependence-induced escalation of drinking or somatic withdrawal symptoms. Moreover, withdrawal-induced cFos immunoreactivity in the RMTg, a primary recipient of LHb projections also involved in negative affect, was not altered by lesioning LHb outputs. Overall, our findings support the hypothesis that the LHb is not required for the acquisition and expression of enhanced ethanol consumption and somatic withdrawal signs during ethanol dependence. Alternatively, these ethanol dependence phenotypes appear to be mediated by other negative affect-associated neurocircuitry.
Materials & methods
Animals
Adult male Sprague–Dawley rats (n = 33; Envigo, Indianapolis, IN) were used for all experiments. All animal procedures were conducted with the approval of the Institutional Animal Care and Use Committee at the Medical University of South Carolina and adhered to the guidelines set forth by the National Research Council’s Guide for the Care and Use of Laboratory Animals.
Chronic intermittent ethanol vapor exposure and withdrawal drinking
Adult male Sprague Dawley rats underwent a well-characterized procedure for induction of ethanol dependence and withdrawal to assess dependence-induced escalation of ethanol drinking and somatic withdrawal symptoms [7, 41]. See Supplemental Methods for additional details.
Chemogenetic LHb inhibition
Chemogenetic LHb inhibition was performed using inhibitory DREADDs and systemic Clozapine-N-oxide (CNO; NIDA Drug Supply Program) administration. See Supplemental Methods for further details.
Fasciculus retroflexus electrolytic lesioning
Fasciculus retroflexus lesions were performed to determine whether LHb outputs are necessary for the acquisition of dependence-induced escalation of ethanol drinking or somatic withdrawal. See Supplemental Methods for further details.
Immunofluorescence & image analysis
Immunolabeling and image analysis was performed to measure cFos expression, viral expression, and surgical accuracy. See Supplemental Methods for additional details.
Statistical analysis
Unless stated otherwise, all data was analyzed in GraphPad Prism 9.0 software using mixed-effects ANOVAs with Dunnett’s multiple comparisons test. Data are presented as mean ± sem, and effects were considered statistically significant at p ≤ 0.05.
Results
CIE exposure produces behavioral characteristics of ethanol dependence
The schematic in Fig. 1A depicts each phase of the ethanol dependence drinking procedure. During an initial ethanol drinking phase, rats were subjected to 4 weeks of baseline 2BC drinking during which time they gradually increase their ethanol consumption and preference (Fig. S1). During the next phase of the procedure that involves CIE exposure, rats exhibited blood ethanol concentrations (BECs) that significantly correlated with their behavioral intoxication scores (r = 0.7174, p < 0.0001; BEC: 250.2 ± 19.90 mg/dl, mean intoxication score: 2.15 ± 0.13) (Fig. 1B). In addition, CIE exposure produced withdrawal symptoms in CIE exposed rats (n = 7) that persisted throughout ethanol dependence, whereas AIR exposed rats (n = 7) did not display somatic signs of ethanol withdrawal (Fig. 1C). A mixed effects ANOVA of withdrawal scores revealed a main effect of CIE exposure day (F(2.617,15.70) = 6.851, p = 0.0046). Follow-up multiple comparison tests revealed that CIE exposed rats exhibited significant withdrawal symptoms on days 4–14 relative to day 1 (all p values < 0.05). During the final CIE + 2BC withdrawal drinking phase, CIE exposed rats exhibited robust escalation of ethanol consumption that was associated with a reduction in both withdrawal symptoms and BECs similar to those observed in CIE exposed rats. A mixed-effects ANOVA of 2 hr ethanol consumption (Fig. 1D) with treatment (AIR vs CIE) and session as factors revealed significant main effects of treatment (F(1,12) = 13.55, p = 0.0031) and session (F(2.492,29.90) = 6.578, p = 0.0025), and a significant group x session interaction (F(3,36) 6.266; p = 0.0016). Multiple comparisons revealed that CIE exposed rats significantly escalated their ethanol consumption on sessions 2 and 3 relative to baseline drinking (all p values <0.05), whereas consumption in AIR exposed rats remained unchanged from baseline (all p values >0.05). A mixed-effects ANOVA of total (14 h) ethanol consumption (Fig. 1E) with treatment (AIR vs CIE) and session as factors revealed significant main effects of treatment (F(1,12) = 23.01, p = 0.0004) and session F(1.575,18.90) = 13.85, p = 0.0004), and a significant group x session interaction (F(3,36) 24.54; p < 0.0001). Multiple comparisons revealed that CIE exposed rats significantly escalated their ethanol consumptions on sessions 1–3 relative to baseline (all p values <0.05), whereas AIR exposed rats exhibited a slight, but significant, reduction of consumption on sessions 2 and 3 relative to baseline drinking (all p values <0.05). The slight reduction in consumption in AIR control rats may be attributed to the shorter session time during this drinking phase (14 h) compared to baseline (24 h). As shown in Fig. 1F, the increase in ethanol consumption in CIE exposed rats was associated with an alleviation of somatic withdrawal symptoms. Pre and post-2BC withdrawal scores from sessions 2 and 3, in which statistically significant escalated drinking is observed, were averaged and used for this analysis. A paired t-test revealed that following 2BC drinking, the CIE exposed rats exhibited a significant reduction in somatic withdrawal symptoms relative to before ethanol access (pre-2BC: 2.64 ± 0.23, post-2BC: 1.00 ± 0.24; t6 = 5.421, p = 0.0016). In addition, immediately following 14 h of voluntary drinking, CIE-exposed rats exhibited BECs similar to those achieved during ethanol vapor exposure (CIE: 250.2 ± 19.90 mg/dl vs. Drinking: 220.2 ± 38.70 mg/dl; Fig. 1G). An unpaired t-test revealed that CIE exposed rats exhibited greater BECs than AIR controls (CIE: 220.2 ± 38.70 mg/dl, AIR: 10.54 ± 0.67 mg/dl, t6.004 = 5.417, p = 0.0016), and overall ethanol consumption was significantly correlated with BEC (r = 0.97, p < 0.0001).
A Schematic of the experimental design and timeline depicting the dependence and withdrawal drinking procedure. B During CIE exposure, the degree of behavioral intoxication was positively correlated with the BECs. C Over the time-course of CIE exposure, rats rapidly develop somatic symptoms assessed after 10 h of withdrawal. Bar graph represents the mean withdrawal score across all exposure days Following induction of ethanol dependence, CIE exposed rats exhibited significant escalations of ethanol consumption assessed at both the 2 h (D) and 14 h (E) withdrawal time-points relative to AIR controls and to their average intake during the baseline phase of the 2BC procedure. Bar graphs represent group mean ethanol consumption across all drinking sessions. F Escalated ethanol consumption in CIE exposed rats is associated with significant attenuation of somatic signs of withdrawal. G Following 14 h of voluntary ethanol consumption, CIE exposed rats exhibited high BECs that were significantly correlated with the level of consumption. All values in C–F represent means ± sem. *p < 0.05; n = 7/group.
LHb inactivation has no effect on dependent drinking or somatic withdrawal
To determine whether the LHb is necessary for the expression of dependence-induced escalation of ethanol consumption, we chemogenetically inhibited the LHb prior to withdrawal drinking according to the experimental schema in Fig. 2A. In the subsequent sessions following the initial escalation of drinking (3 sessions), rats received either vehicle (VEH) or CNO in a counterbalanced order prior to drinking. A mixed-effects ANOVA of 2 hr ethanol intake (Fig. 2B) with group (AIR vs CIE) and treatment (VEH, CNO dose) as factors revealed a significant main effect of group (F(1,12) = 21.33, p = 0.0006), indicating that CIE exposed rats continued to exhibit greater levels of ethanol consumption than AIR controls. However, there was no significant main effect of treatment (F(1.819, 21.83) = 1.064, p = 0.3566) or interaction (F(2,24) = 0.3530, p = 0.7061). Similarly, a mixed-effects ANOVA of ethanol consumption after 14 h (Fig. 2C) revealed a main effect of group (F(1,12) = 26.20, p = 0.0003), but no main effect of treatment (F(1.182, 14.18) = 1.295, p = 0.2828) or interaction (F(2,24) = 0.8598, p = 0.4359). Ethanol preference was also unaltered by chemogenetic inhibition of the LHb (Fig. S2). Consistent with a lack of effect on ethanol intake, rats continued to display a reduction in withdrawal symptoms following 2BC drinking during the LHb inhibition sessions. Pre- and post-2BC withdrawal from vehicle (Pre: 1.857 ± 0.3401, Post: 0.8571 ± 0.09221) and CNO (Pre: 1.857 ± 0.2827, Post: 0.5000 ± 0.1543) sessions (data not shown) were analyzed using a mixed-effects ANOVA with drug (VEH vs CNO) and session (pre-2BC vs post-BC) as factors. This revealed a significant main effect of session (F(1, 24) = 24.38, p < 0.0001), but no significant main effect of drug (F(1,24) = 0.5597, p = 0.4616), or interaction (F(1,24) = 0.5597, p = 0.4616), indicating that both groups exhibited a drinking-mediated reduction in withdrawal symptoms regardless of LHb inhibition.
A Schematic of the experimental design and timeline of the chemogenetic LHb inhibition portion of the withdrawal drinking model. Following the initial escalation of CIE + 2BC drinking, rats received counterbalanced injections of either VEH or CNO prior each drinking session. Ethanol consumption at the 2 h (B) and 14 h (C) time-points during vehicle (VEH) and CNO sessions. D Schema of the procedural timeline for testing the effects of repeated LHb inhibition on somatic signs of withdrawal and escalated drinking. As depicted in the schema, rats were assessed for somatic signs of withdrawal (indicated by WD) 3, 6, and 9 h into withdrawal. Immediately after each assessment, rats received an injection of either VEH or CNO (10 mg/kg). E Normalized withdrawal scores across the time-course of withdrawal revealed that chemogenetic LHb inhibition did not alter the emergence of somatic withdrawal symptoms as both VEH and CNO treated rats exhibited significant withdrawal scores at 6 and 9 h, relative to the 3 h baseline. F Ethanol consumption assessed before (2 h) and after (14 h) the 3 VEH/CNO injections indicated inhibition of LHb did not alter ethanol intake. All values represent the means ± sem. *p < 0.05; n = 7/group.
The next set of studies examined whether the LHb activity is necessary for the expression of somatic withdrawal symptoms following the experimental schema in Fig. 2D. Upon removal from the vapor chambers after CIE, rats were scored for withdrawal symptoms at 3, 6, and 9 h, and each assessment was followed by either a VEH or CNO injection (10 mg/kg). To determine whether repeated LHb inhibition altered dependent drinking, rats had 2BC access 10 h into withdrawal like a normal CIE + 2BC drinking session. A mixed-effects ANOVA of withdrawal scores with withdrawal time and drug as factors revealed a significant main effect of time (F(1.675, 20.10) = 12.56, p = 0.0005), but no significant main effect of drug (F(1,12) = 0.2935, p = 0.5979), or interaction (F(2, 24) = 1.068, p = 0.3595). Multiple comparisons indicated that rats in both groups exhibited significantly increased withdrawal scores at 6 and 9 hr time points, relative to the 3 h baseline (all p values <0.05; Fig. 2E). For ethanol consumption associated with the repeated CNO injection sessions (Fig. 2F), paired t-tests of 2 h (t6 = 0.3950, p = 0.7065) and 14 h (t6 = 1.916, p = 0.1969) ethanol consumption revealed no significant difference between vehicle and CNO treated rats, suggesting that repeated LHb inhibition during withdrawal did not alter ethanol consumption in CIE exposed rats. Following the chemogenetic studies, slice electrophysiology confirmed that bath application of CNO produced the expected inhibition of infected LHb neurons (Fig. S3). Additional behaviors were also assessed in the withdrawal drinking model that included post-dependent and quinine-resistant drinking (See supplemental results, Figs. S4 and S5). This revealed that following cessation of CIE exposure, withdrawal symptoms in post-dependent rats gradually decline and dissipate, which mirrors their decrease in ethanol intake. Moreover, dependent and post-dependent rats exhibited quinine-resistant ethanol intake without apparent alterations in quinine taste sensitivity.
The LHb is recruited during acute ethanol withdrawal
Previous studies have utilized cFos immunohistochemistry to demonstrate that the LHb is activated during the acute phase of ethanol withdrawal [41, 42]. To confirm LHb activation during acute withdrawal in the ethanol dependence model used in the present study, cFos immunohistochemistry was carried out in slices obtained from ethanol dependent rats sacrificed 11.5 h into withdrawal, (Fig. 3A). A separate group of rats (n = 6) that had undergone the same ethanol exposure procedures (baseline 2BC, followed by CIE exposure, followed by CIE + 2BC drinking) were left undisturbed in the homecage for 4–6 weeks after cessation of CIE + 2BC drinking. This protracted withdrawal group served as a control for ethanol exposure and allowed for examination of cFos induction specifically associated with the acute withdrawal state (Fig. 3B). An unpaired t-test of the number of cFos+ cells revealed that rats in acute withdrawal exhibited significantly greater cFos reactivity in the LHb compared to the protracted withdrawal controls (t11 = 8.405, p < 0.0001; Fig. 3C). In contrast, an unpaired t-test of the number of cFos+ cells in the dorsal raphe revealed no significant group differences (t11 = 0.5995, p = 0.5610; Fig. 3D) between the acute and protracted withdrawal groups. The results of this indicate that the LHb is active during ethanol withdrawal but is not necessary for escalated drinking and withdrawal symptoms, and are consistent with the results from the LHb chemogenetic inhibition study.
A Representative image of cFos expression in the LHb assessed 11.5 h into withdrawal from CIE exposure. The inset shows higher magnification of a cFos “hotspot” in the medial portion of the LHb. B Representative image of cFos expression in the LHb from a rat with a history of CIE exposure that was sacrificed after 4–6 weeks of protracted withdrawal. C Quantification of cFos immunoreactivity revealed significantly greater numbers of cFos+ cells in the LHb during acute withdrawal compared to protracted withdrawal. D In contrast, quantification of cFos immunoreactivity in the dorsal raphe revealed no differences in cFos immunoreactivity during acute withdrawal compared to protracted withdrawal. All values represent the means ± sem. *p < 0.05; n = 6 for the protracted withdrawal group, 7 for the acute withdrawal group. 3 V, 3rd ventricle; mHb medial habenula, LHb lateral habenula.
Lesioning the FR has no effect on dependence-induced drinking or somatic withdrawal symptoms
The results of the chemogenetic inactivation study indicated that the LHb region is not necessary for the expression of dependent drinking or somatic signs of withdrawal. However, a potential confound to the interpretation of these results is that chemogenetic LHb inactivation occurred after rats were rendered ethanol dependent and had already escalated their ethanol consumption. Therefore, the next set of studies assessed whether LHb output is necessary for the acquisition of escalated ethanol drinking and somatic signs of withdrawal in CIE exposed rats. The experimental approach (Fig. 4A and S6) involved lesioning of the FR, a white matter tract that carries all of the habenular projections to midbrain areas, including the RMTg, VTA, and raphe nuclei [43, 44]. Following 2BC baseline drinking, rats received either bilateral electrolytic lesions of the FR (Fig. 4B) or sham lesions and were then transitioned to CIE exposure after a surgical recovery period. As in the previous experiment, somatic signs of ethanol withdrawal were assessed across the CIE exposure period (Fig. 4C). A mixed-effects ANOVA of withdrawal scores with treatment (sham vs lesion) and CIE exposure day as factors revealed a significant main effect of day (F(5.441, 70.73) = 41.55, p < 0.0001) and treatment x day interaction (F(14, 182) = 0.1.844, p = 0.0353), but no main effect of treatment (F(1,13) = 2.955, p = 0.1093). Bonferroni multiple comparisons indicated no significant differences between withdrawal scores in sham and lesion animals on any exposure day (all p values >0.05), indicating that both sham and lesioned rats exhibited similar acquisition of somatic signs of withdrawal across the CIE exposure period. Withdrawal symptoms were also measured before and after 2BC drinking to determine whether sham or lesion groups exhibited reduced withdrawal symptoms following voluntary ethanol drinking (data not shown). A mixed-effects ANOVA of 2BC withdrawal scores with treatment and time (pre vs post) as factors revealed a significant main effect of time (F(1,26) = 176.9, p < 0.0001), but no main effect of treatment (F(1,26) = 0.09273, p = 0.7632) or interaction (F(1,26) = 0.7312, p = 0.4003), indicating that both groups consumed sufficient amounts of ethanol to reduce somatic withdrawal symptoms.
A Schematic of experimental design and timeline for lesioning. B Representative image of bilateral electrolytic lesions of the fasciculus retroflexus (FR). C Lesioning of the FR did not alter the development of somatic signs of withdrawal when assessed across the time-course of CIE exposure. There was also no signification effect of FR lesioning on either baseline drinking (BL), or dependence-induced escalation of drinking assessed at 2 h (D) and 14 h (E) over the 6 drinking sessions. All values represent the means ± sem. *p < 0.05; n = 6 for the sham group, 8 for the lesioned group.
Upon initiation of CIE + 2BC ethanol drinking (see experimental schema in Fig. 4A), ethanol consumption was assessed in the sham and FR lesioned animals to determine whether LHb output is required for the development of dependence-induced escalation of ethanol drinking. A mixed-effects ANOVA of 2 h ethanol consumption (Fig. 4D) with treatment and drinking session as factors revealed a significant main effect of session (F(3.930, 47.17) = 16.21, p < 0.0001), but no main effect of treatment (F(1,12) = 4.276, p = 0.0609) or interaction (F(6.72) = 0.5672, p = 0.7551). A mixed-effects ANOVA of 14 h ethanol consumption (Fig. 4E) with treatment and drinking session as factors revealed significant main effects of session (F(2.862, 34.34) = 26.57, p < 0.0001) and treatment (F(1,12) = 8.405, p = 0.0133), but no interaction (F(6,72) = 1.880, p = 0.0959). These results indicate that both sham and lesion animals similarly escalated their ethanol consumption over time. Although overall intake at the 14 h measurement was significantly higher in lesion animals, this minor effect should be interpreted with caution due to the relatively higher variability in consumption in sham animals during sessions 1 and 2. Furthermore, visual inspection of subsequent drinking sessions indicates no group differences.
To determine whether FR lesions impacted other aspects of ethanol drinking phenotypes, we also assessed quinine-resistant ethanol drinking and post-dependent drinking. This revealed that lesioning the FR had no effect on quinine-resistant drinking but did result in a modest increase in ethanol consumption in post-dependent rats following the cessation of CIE exposure (See Supplemental Results, Figs. S7 and S8).
The RMTg exhibits withdrawal-induced cFos activity despite FR lesion
The initial working hypothesis of the present study was that LHb projections to the RMTg are critically involved in the development and/or expression of ethanol dependence due to the role of this circuit in aversive signaling [18, 22] and in promoting negative affective states during withdrawal from other drugs of abuse [27, 30, 41, 45]. However, given that our results did not support this idea, we then considered the opposing hypothesis that the LHb is not necessary for modulating activity in the RMTg or other circuits that may have more proximal control over the ethanol-related behaviors [18]. To explore this possibility, CIE exposure was resumed in the sham and lesion animals following post-dependent drinking (Fig. S8) for 5 days to re-establish dependence (Fig. 5A). Across this CIE re-exposure period, withdrawal scores were measured to ensure rats displayed typical somatic signs of withdrawal prior to sacrifice (Fig. 5B). A mixed effects ANOVA of withdrawal scores with treatment and exposure day as factors revealed a significant main effect of day (F(3.582, 33.67) = 7.570, p < 0.0003), but no main effect of treatment (F(1,10) = 0.09468, p = 0.7646) or interaction (F(5,47) = 0.7304, p = 0.6042). Multiple comparisons revealed that both groups exhibited a significant increase in withdrawal symptoms on the 2nd exposure day and onward, relative to the 1st exposure day following resumption of CIE exposure (all p values <0.05).
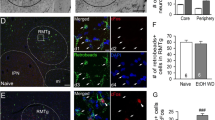
A Schematic of experimental design and timeline. Rats that had previously undergone CIE exposure and withdrawal drinking were re-exposed to CIE to reestablish ethanol dependence. B Lesioning of the FR did not alter the development somatic signs of withdrawal when assessed across the time-course of CIE re-exposure. C–E After the last day of CIE re-exposure, rats were sacrificed during withdrawal for assessment of cFos activity in the rostromedial tegmental nucleus (RMTg). Representative images of FoxP1 expression in the RMTg of sham (C) and lesioned (C’) rats. C” Quantification of the number of FoxP1+ cells in the RMTg revealed no differences between the sham and lesioned animals. Representative images of cFos expression in the RMTg of sham (D) and lesioned (D’) rats. D” Quantification of the number of cFos+ cells in the RMTg revealed no differences between the sham and lesioned animals. Overlay of the representative images of cFos+ and FoxP1+ expression in the RMTg of sham (E) and lesioned (E’) rats. E3” Quantification of the number of cells in the RMTg that were both cFos+ and FoxP1+ revealed no differences between the sham and lesioned animals. All values represent the means ± sem. *p < 0.05; n = 5 rats for the sham group, 7 rats for the lesioned group.
After completion of CIE re-exposure, both the sham and FR lesioned rats were sacrificed 11.5 h into acute withdrawal to determine whether the RMTg still exhibited enhanced cFos expression despite a lack of LHb input in the FR lesioned rats. Following sacrifice, tissue was processed for cFos and FoxP1 (an established marker of RMTg neurons [20, 46, 47]) in order to assess withdrawal-induced cFos in the RMTg. Unpaired t-tests indicated that sham and FR lesion rats exhibited similar numbers of FoxP1+ (t10 = 0.3294, p = 0.7487; Fig. 5C), cFos+ (t10 = 0.1590, p = 0.8769; Fig. 5D), and cFos+/FoxP1+ cells (t10 = 0.3767, p = 0.7143; Fig. 5E) in the RMTg. These results provide evidence that activation of the RMTg during dependence withdrawal is similar in both sham and FR lesioned rats, suggesting that the RMTg can still be recruited during ethanol withdrawal despite the loss of input from the LHb. Together, these results reveal that the LHb is not required for the dependence-induced escalation of ethanol drinking and does not contribute to acute withdrawal symptomology.
Discussion
The present study used chemogenetic and lesion approaches in a voluntary ethanol drinking model to exam the role of the LHb in the dependence-induced escalation of ethanol drinking and somatic signs of withdrawal. In contrast to our initial working hypothesis that the LHb plays a critical role in these dependence-related behaviors, our results instead revealed that activation of the LHb during ethanol withdrawal is not required for the acquisition and expression of these dependence/withdrawal-related behaviors. Instead, these findings suggest that other aversion-related neural circuits mediate these effects.
Several prior studies demonstrated that exposure to drugs of abuse produces LHb hyperactivity, leading to negative affective behaviors that are alleviated by attenuating LHb activity [27, 30, 36, 45]. Thus, our initial working hypothesis was that increased LHb activity during ethanol withdrawal promotes escalated ethanol intake and somatic withdrawal symptoms as a means of self-medication to mitigate the negative affective state of withdrawal. However, while LHb activity was increased following chronic ethanol exposure as expected, acute chemogenetic inhibition of the LHb prior to drinking, or repeated inhibition prior to withdrawal onset, had no effect on either dependence-related behavior. Previous research has reported increased LHb activity following non-dependent ethanol drinking and observed reduced ethanol intake and/or negative affective behaviors following pharmacological or chemogenetic LHb inhibition. However, those findings were obtained in non-dependent rats [36,37,38], and thus these observations may not extend to dependence/withdrawal-related drinking as was modeled in the present study. Together with the extant literature, our results instead support the suggestion that while the LHb can regulate ethanol intake and negative affective behaviors in non-dependent rats, it appears to be dispensable for withdrawal-related behaviors in the dependent state.
A potential concern with our chemogenetic approach was that LHb inhibition did not occur until after rats were rendered ethanol dependent and had already established escalated drinking behavior. Therefore, as a complementary approach we used electrolytic lesions of the FR [43, 44] to ablate LHb outputs to assess whether the LHb is necessary for the acquisition/expression of escalated ethanol consumption and somatic signs of withdrawal. Consistent with the chemogenetic inhibition approach, these experiments revealed that FR lesioned rats also escalate their ethanol consumption and exhibited enhanced somatic signs of withdrawal similar to that observed in the sham controls. Moreover, FR lesioning had no effect on quinine-resistant ethanol drinking, quinine taste sensitivity, or post-dependent ethanol drinking, further suggesting that LHb outputs are not necessary for these dependence-related behaviors.
Our observation that the LHb is not required for the development of dependence-induced drinking and expression of somatic signs of withdrawal lead us to consider an alternative hypothesis that these dependence/withdrawal-related behaviors are regulated by other brain regions that mediate aversion [1, 7]. A potential candidate brain region we considered was the RMTg as prior research indicates that this brain area is critically involved in regulating ethanol withdrawal-associated negative-affect [41]. The LHb provides a prominent input to the RMTg, and this LHb-RMTg circuit has been shown to mediate aversive signaling in response to a variety of stimuli [22, 44, 48]. Therefore, we examined cFos expression in the RMTg of FR lesioned rats to determine whether this aversive brain nucleus is recruited during ethanol withdrawal even in the absence of LHb inputs that are severed by FR lesioning. These studies revealed similar levels of cFos induction in the RMTg of FR lesioned and sham controls, supporting the suggestion that during ethanol withdrawal the RMTg can be activated independent of LHb input. This result aligns with a previous report demonstrating that RMTg neurons can respond to aversive stimuli at a faster latency than LHb neurons [18], suggesting that RMTg neurons can signal aversion independent of LHb input. Moreover, a previous study demonstrated that rats with FR lesions subjected to low or high-intensity electric footshocks exhibited differential cFos expression in the RMTg [43]. In the low-intensity shock condition, it was observed that FR lesions significantly attenuated cFos relative to sham controls. In contrast, robust cFos expression was observed in the RMTg of FR lesioned and sham controls in the high-intensity shock condition, suggesting that high stimulus intensities engage RMTg signaling regardless of LHb input. Together, these findings complement our observation that ethanol withdrawal is a potent stimulus that is sufficient to recruit RMTg activation independent of LHb input and supports our overall findings that the LHb is not necessary for the acquisition and expression of escalated ethanol drinking and somatic withdrawal during ethanol dependence.
The negative affective state during withdrawal is a key antecedent of excessive ethanol intake during ethanol dependence, and brain areas involved in negative affect and aversion have been shown to mediate dependence-related phenotypes [1, 3, 8]. Therefore, we tested the hypothesis that the LHb plays a key role in ethanol withdrawal and dependence-related behaviors due to its involvement in negative affect and aversion. However, contrary to this hypothesis, our results indicate that the LHb is dispensable for dependence-induced escalation of ethanol consumption and somatic symptoms of withdrawal despite showing elevated cFos reactivity during withdrawal. Additional research exploring other aversion-related circuits and their regulation of the negative aspects of ethanol dependence may provide insight towards identifying therapeutic targets for the treatment of AUD.
References
Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–38.
Gilpin NW, et al. Operant behavior and alcohol levels in blood and brain of alcohol-dependent rats. Alcohol Clin Exp Res. 2009;33:2113–23.
Vendruscolo LF, Roberts AJ. Operant alcohol self-administration in dependent rats: Focus on the vapor model. Alcohol. 2014;48:277–86.
O’Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res. 2004;28:1676–82.
Rassnick S, Heinrichs SC, Britton KT, Koob GF. Microinjection of a corticotropin-releasing factor antagonist into the central nucleus of the amygdala reverses anxiogenic-like effects of ethanol withdrawal. Brain Res. 1993;605:25–32.
Roberts AJ, Cole M, Koob GF. Intra-amygdala muscimol decreases operant ethanol self-administration in dependent rats. Alcohol Clin Exp Res. 1996;20:1289–98.
de Guglielmo G, et al. Inactivation of a CRF-dependent amygdalofugal pathway reverses addiction-like behaviors in alcohol-dependent rats. Nat Commun. 2019;10:1238.
Cunningham CL, Fidler TL, Murphy KV, Mulgrew JA, Smitasin PJ. Time-dependent negative reinforcement of ethanol intake by alleviation of acute withdrawal. Biol Psychiatry. 2013;73:249–55.
Aizawa H, Kobayashi M, Tanaka S, Fukai T, Okamoto H. Molecular characterization of the subnuclei in rat habenula. J Comp Neurol. 2012;520:4051–66.
Namboodiri VMK, Rodriguez-Romaguera J, Stuber GD. The habenula. Curr Biol. 2016;26:R873–77.
Zahm DS, Root DH. Review of the cytology and connections of the lateral habenula, an avatar of adaptive behaving. Pharmacol Biochem Behav. 2017;162:3–21.
Brinschwitz K, et al. Glutamatergic axons from the lateral habenula mainly terminate on GABAergic neurons of the ventral midbrain. Neuroscience. 2010;168:463–76.
Hu H, Cui Y, Yang Y. Circuits and functions of the lateral habenula in health and in disease. Nat Rev Neurosci. 2020;21:277–95.
Quina LA, et al. Efferent pathways of the mouse lateral habenula: efferent pathways of the mouse lateral habenula. J Comp Neurol. 2015;523:32–60.
Zhou L, et al. Organization of functional long-range circuits controlling the activity of serotonergic neurons in the dorsal raphe nucleus. Cell Rep. 2017;18:3018–32.
Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–5.
Matsumoto M, Hikosaka O. Representation of negative motivational value in the primate lateral habenula. Nat Neurosci. 2009;12:77–84.
Hong S, Jhou TC, Smith M, Saleem KS, Hikosaka O. Negative reward signals from the lateral habenula to dopamine neurons are mediated by rostromedial tegmental nucleus in primates. J Neurosci. 2011;31:11457–71.
Laurent V, Wong FL, Balleine BW. The lateral habenula and its input to the rostromedial tegmental nucleus mediates outcome-specific conditioned inhibition. J Neurosci. 2017;37:10932–42.
Li H, et al. Three rostromedial tegmental afferents drive triply dissociable aspects of punishment learning and aversive valence encoding. Neuron. 2019;104:987–99.e4
Proulx CD, et al. A neural pathway controlling motivation to exert effort. Proc Natl Acad Sci. 2018;115:5792–7.
Stamatakis AM, Stuber GD. Activation of lateral habenula inputs to the ventral midbrain promotes behavioral avoidance. Nat Neurosci. 2012;15:1105–7.
Coffey KR, Marx RG, Vo EK, Nair SG, Neumaier JF. Chemogenetic inhibition of lateral habenula projections to the dorsal raphe nucleus reduces passive coping and perseverative reward-seeking in rats. Neuropsychopharmacology. 2020;45:1115–24.
Lammel S, et al. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212–7.
Li B, et al. Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature. 2011;470:535–9.
Szőnyi A, et al. Median raphe controls acquisition of negative experience in the mouse. Science. 2019;366:eaay8746.
Clerke JA, Congiu M, Mameli M. Neuronal adaptations in the lateral habenula during drug withdrawal: Preclinical evidence for addiction therapy. Neuropharmacology. 2021;192:108617.
Mathis V, Kenny PJ. From controlled to compulsive drug-taking: The role of the habenula in addiction. Neurosci Biobehav Rev. 2019;106:102–11.
Shah A, et al. The lateral habenula and alcohol: Role of glutamate and M-type potassium channels. Pharmacol Biochem Behav. 2017;162:94–102.
Valentinova K, et al. Morphine withdrawal recruits lateral habenula cytokine signaling to reduce synaptic excitation and sociability. Nat Neurosci. 2019;22:1053–6.
Haack AK, et al. Lesions of the lateral habenula increase voluntary ethanol consumption and operant self-administration, block yohimbine-induced reinstatement of ethanol seeking, and attenuate ethanol-induced conditioned taste aversion. PLoS ONE. 2014;9:e92701.
Glover EJ, McDougle MJ, Siegel GS, Jhou TC, Chandler LJ. Role for the rostromedial tegmental nucleus in signaling the aversive properties of alcohol. Alcohol Clin Exp Res. 2016;40:1651–61.
Tandon S, Keefe KA, Taha SA. Excitation of lateral habenula neurons as a neural mechanism underlying ethanol-induced conditioned taste aversion: LHb activity mediates ethanol-induced aversion. J Physiol. 2017;595:1393–412.
Zuo W, et al. Ethanol drives aversive conditioning through dopamine 1 receptor and glutamate receptor-mediated activation of lateral habenula neurons: LHb and alcohol addiction. Addict Biol. 2017;22:103–16.
Sheth C, Furlong TM, Keefe KA, Taha SA. The lateral hypothalamus to lateral habenula projection, but not the ventral pallidum to lateral habenula projection, regulates voluntary ethanol consumption. Behav Brain Res. 2017;328:195–208.
Kang S, et al. Ethanol withdrawal drives anxiety-related behaviors by reducing m-type potassium channel activity in the lateral habenula. Neuropsychopharmacology. 2017;42:1813–24.
Kang S, Li J, Bekker A, Ye J-H. Rescue of glutamate transport in the lateral habenula alleviates depression- and anxiety-like behaviors in ethanol-withdrawn rats. Neuropharmacology. 2018;129:47–56.
Li J, et al. Inhibition of AMPA receptor and CaMKII activity in the lateral habenula reduces depressive-like behavior and alcohol intake in rats. Neuropharmacology. 2017;126:108–20.
Zuo W, et al. Ethanol potentiates both GABAergic and glutamatergic signaling in the lateral habenula. Neuropharmacology. 2017;113:178–87.
Shiwalkar, N, Zuo, W, Bekker A, Ye J-H, The role of the lateral habenula circuitries in alcohol use disorders. In Neuroscience of Alcohol 153-61 (Elsevier, 2019). https://doi.org/10.1016/B978-0-12-813125-1.00016-7.
Glover EJ, Starr EM, Chao Y, Jhou TC, Chandler LJ. Inhibition of the rostromedial tegmental nucleus reverses alcohol withdrawal-induced anxiety-like behavior. Neuropsychopharmacology (2019). https://doi.org/10.1038/s41386-019-0406-8.
Smith RJ, Anderson RI, Haun HL, Mulholland PJ, Griffin WC 3rd, Lopez MF, et al. Dynamic c-Fos changes in mouse brain during acute and protracted withdrawal from chronic intermittent ethanol exposure and relapse drinking. Addict. Biol. 2020;25:e12804. https://doi.org/10.1111/adb.12804.
Brown PL, Shepard PD. Lesions of the fasciculus retroflexus alter footshock-induced cfos expression in the mesopontine rostromedial tegmental area of rats. PLoS ONE. 2013;8:e60678.
Jhou TC, et al. Cocaine drives aversive conditioning via delayed activation of dopamine-responsive habenular and midbrain pathways. J Neurosci. 2013;33:7501–12.
Meye FJ, et al. Shifted pallidal co-release of GABA and glutamate in habenula drives cocaine withdrawal and relapse. Nat Neurosci. 2016;19:1019–24.
Lahti L, et al. Differentiation and molecular heterogeneity of inhibitory and excitatory neurons associated with midbrain dopaminergic nuclei. Development. 129957 (2015). https://doi.org/10.1242/dev.129957.
Smith RJ, Vento PJ, Chao YS, Good CH, Jhou TC. Gene expression and neurochemical characterization of the rostromedial tegmental nucleus (RMTg) in rats and mice. Brain Struct Funct. 2019;224:219–38.
Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The Rostromedial Tegmental Nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 2009;61:786–800.
Funding
This work was supported by funding from the National Institutes on Alcohol Abuse and Alcoholism grants AA019967 and AA027706 (LJC), T32 AA007474 (TBN), and F31 AA029622 (TBN), P50 AA010761 (JJW), and from the National Institute of Drug Abuse grant F31 DA045485 (KMB). The authors have nothing to disclose.
Author information
Authors and Affiliations
Contributions
TBN and LJC designed the experiments and co-wrote the manuscript. TBN performed the experiments, carried out the statistical analysis, and graphed the data. DTV assisted with behavioral experiments. KMB performed slice electrophysiology experiments and analysis with oversight by JJW. BDB assisted with the immunofluorescence experiments.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Nentwig, T.B., Vaughan, D.T., Braunscheidel, K.M. et al. The lateral habenula is not required for ethanol dependence-induced escalation of drinking. Neuropsychopharmacol. 47, 2123–2131 (2022). https://doi.org/10.1038/s41386-022-01357-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-022-01357-7
- Springer Nature Switzerland AG
This article is cited by
-
LPA1 receptors in the lateral habenula regulate negative affective states associated with alcohol withdrawal
Neuropsychopharmacology (2023)
-
Subcortical serotonin 5HT2c receptor-containing neurons sex-specifically regulate binge-like alcohol consumption, social, and arousal behaviors in mice
Nature Communications (2023)