Abstract
Patients with schizophrenia receiving antipsychotic treatment present lower mortality rates than those who do not. However, the non-adherence rate is high, which can be partially addressed using long-acting injectable (LAI) antipsychotics. The impact of LAI treatments on all-cause mortality compared to oral antipsychotics remains unclear. To fill that gap, a random effects meta-analysis was conducted to analyze the odds ratio (OR) of all-cause, suicidal, and non-suicidal mortality among patients taking LAI antipsychotics compared to oral antipsychotics (PROSPERO:CRD42023391352). Individual and pooled LAI antipsychotics were analyzed against pooled oral antipsychotics. Sensitivity analyses were performed for study design, setting, and industry sponsorship. Meta-regressions were conducted for gender, age, antipsychotic dose, and race. Seventeen articles, total sample 12,042 patients (N = 5795 oral, N = 6247 LAI) were included. Lower risk of all-cause mortality for patients receiving LAI antipsychotics vs receiving oral antipsychotics was found (OR = 0.79; 95%CI = 0.66–0.95). Statistical significance was maintained when only studies comparing the same LAI and oral antipsychotic were included (OR = 0.79; 95%CI = 0.66–0.95; p = <0.01), as well as for non-suicidal mortality (OR = 0.77: 95%CI = 0.63–0.94; p = 0.01), but not for suicidal mortality (OR = 0.86; 95%CI = 0.59–1.26; p = 0.44). Mortality reduction was more pronounced for LAI antipsychotics in first-episode psychosis (FEP) (OR = 0.79; 95%CI = 0.66–0.96) compared to chronic psychosis. No individual LAI reported statistically significant differences against all pooled oral antipsychotics. LAI antipsychotics are associated with a lower risk of all-cause and non-suicidal mortality in individuals with schizophrenia compared to oral antipsychotics. Better adherence to the medication and health services may explain this difference. Whenever possible, the use of LAIs should be considered from the FEP.
Similar content being viewed by others
Introduction
Background
Schizophrenia is a chronic and debilitating disorder characterized by positive, negative, and cognitive symptoms [1]. Individuals with schizophrenia experience a reduction in life expectancy of 15–20 years compared to the general population [2], with cardiovascular disease being the leading cause of death among this population [3]. This premature mortality is present even from the initial stages of the disorder [4], and has been consistently associated with modifiable factors, including poorer lifestyle conditions [5], limited access to healthcare services [6], and a high rate of comorbid disorders, both psychiatric [7, 8] and physical [9, 10].
While there has been some controversy on the potential increase in cardiovascular deaths caused by long-term use of antipsychotics [11, 12], the protective effect of antipsychotic treatment has now been well-established, with a lower mortality rate in patients who received any antipsychotic treatment compared to those who did not [13, 14]. Furthermore, consistently, long-term treatment with antipsychotic medication dramatically reduces the risk of relapse in multiple-episode and first-episode psychosis (FEP) [15,16,17], along with the associated risk behaviours and stress increase. However, a significant percentage of patients discontinue their oral antipsychotic medication, with non-adherence rates from 30% [18] to up 77% [19, 20].
Long-acting injectable antipsychotics (LAI-AP), including first and second-generation antipsychotics, were introduced in the 1960s to overcome non-adherence in psychotic disorders. They have been shown to improve adherence, provide more stable antipsychotic blood levels and reduce relapses, all-cause hospitalizations and emergency department visits [21, 22]. However, potential adverse effects are still important when starting long-term treatment with LAI-APs. Such medications are often initiated in large single doses, and there is no possibility of discontinuing them rapidly should a serious adverse effect appear. Moreover, some rare adverse events, such as post-injection syndromes [23], are produced exclusively by certain LAI-APs.
A meta-analysis of randomized controlled trials (RCTs) comparing LAI-AP and oral-AP pairs of the same antipsychotic in schizophrenia reported a lack of significant differences among the rate of most reported adverse events, including extrapyramidal side effects, weight gain, and sexual and reproductive functioning, among others [24]. Whether LAI antipsychotics can reduce more serious and infrequent adverse effects, including all-cause, suicidal and non-suicidal mortality, has yet to be addressed.
Objectives
To fill these gaps of knowledge, a systematic review and meta-analysis were performed to try to answer the following research questions:
-
1.
Do people with schizophrenia receiving LAI antipsychotics present lower all-cause, suicidal, and non-suicidal mortality than those receiving oral antipsychotics?
-
2.
Is the difference in the all-cause mortality moderated by (a) the drug antipsychotic, age, sex, race, or type of sample (first-episode psychosis vs chronic schizophrenia), or (b) methodological factors, including the type of study (RCT vs cohort), length of follow-up, industry sponsorship, study quality, or publication bias?
Methods
This study protocol was registered on PROSPERO (registration number: CRD42023391352). The study was conducted in accordance with 'Preferred Reporting Items for Systematic Reviews and Meta-Analyses' (PRISMA) [25] (Supplementary Table S1) and 'Meta-Analyses of Observational Studies in Epidemiology' (MOOSE) checklist [26] (Supplementary Table S2), following 'EQUATOR Reporting Guidelines' [27].
Search strategy and selection criteria
A systematic literature search was carried out by two independent researchers (C.A. and B.P.). Web of Science database (Clarivate Analytics) was searched, incorporating the Web of Science Core Collection, the BIOSIS Citation Index, the KCI-Korean Journal Database, MEDLINE®, the Russian Science Citation Index, and the SciELO Citation Index as well as Cochrane Central Register of Reviews, and Ovid/PsycINFO databases, from inception until January 12th, 2023.
The following keywords were used: '(Antipsychotic OR neuroleptic OR aripiprazole OR bromperidol OR clopenthixol OR flupenthixol OR fluphenazine OR fluspirilene OR haloperidol OR iloperidone OR olanzapine OR paliperidone OR penfluridol OR perphenazine OR pipothiazine OR risperidone OR zuclopenthixol) AND (enanthate OR decanoate OR long-acting injection OR lai OR microsphere OR once monthly OR palmitate OR pamoate)'.
Articles identified were first screened as abstracts, and after excluding those that did not meet the inclusion criteria, the full texts of the remaining articles were assessed for eligibility and inclusion. Inclusion criteria for the systematic review and meta-analyses were: (a) individual studies with original data, (b) comparing patient groups receiving any long-action injectable antipsychotic with patient groups receiving any oral antipsychotic, being followed during the same amount of time, (c) patients meeting criteria for a schizophrenia spectrum disorder, according to DSM [28,29,30] or ICD [31, 32] criteria, (d) including all-cause mortality data (e.g. number of deaths in each study group), (e) nonoverlapping samples (overlap was determined by looking at the inclusion dates, type of population and country in which the study was carried out), and (f) written in English or Spanish language. Exclusion criteria were (a) reviews, clinical cases, study protocols, conferential proceedings, letters, and commentaries, and (b) studies including patients receiving both oral and long-action injectable antipsychotics.
Data extraction
Three researchers (M.P., L.A., V.P.) independently extracted data from all the included studies. The three databases were then cross-checked, and discrepancies were resolved by a senior researcher (A.C.). A summary of selected variables included: first author and year of publication, country and city, sample size, age (mean ± standard deviation [SD]), sex (% female), LAI antipsychotic, setting (inpatient vs outpatient), type of study (cohort vs randomized controlled trial vs others), industry sponsorship of the study, number of deceases in each group and cause of death if specified, medical comorbidities, quality assessment (see below), and key findings.
Risk of bias (quality) assessment
Risk of bias was assessed using Newcastle–Ottawa Scale for cross-sectional and cohort studies [33] (Supplementary Table S3).
Strategy for data synthesis and statistics
First, we provided a systematic synthesis of the findings from the included studies.
Second, we performed meta-analyses on all-cause mortality comparing LAI and oral antipsychotics. All LAI antipsychotics were pooled for a single analysis, and subgroup meta-analyses were subsequently conducted for each LAI antipsychotic where data allowed for it. Oral antipsychotics were pooled in all the performed analyses, as available data did not allow for analyzing each antipsychotic. We also conducted a secondary analysis to meta-analyze only those studies comparing LAI antipsychotics with the same oral antipsychotic. The odds ratio (OR) with a 95% confidence interval (CI) was calculated using the number of deaths and sample sizes for each sample [34], without adjusting by any variable. An OR greater than 1 indicates that the LAI antipsychotic group have a higher risk of death than the oral antipsychotic group. The Mantel-Haenszel correction accounted for death as a rare, dichotomous event in this analysis [35]. Heterogeneity among studies was assessed using the Q statistic, with the proportion of the total variability in odds ratio estimates evaluated using the I2 index, classifying the heterogeneity as low (I2 = 25%), medium (I2 = 50%), and high (I2 = 75%) [36].
Next, we followed the same statistical procedure to separately meta-analyze the mortality due to suicide and the mortality due to any other cause for those articles that provided specific data in this regard. Additionally, we conducted a meta-analysis of all-cause mortality in those studies that offered a comparison between LAI and oral formulations of the same antipsychotic.
Meta-regressions were performed to determine the effect of the (a) age, (b) mean antipsychotic dose (calculated as their chlorpromazine equivalent dose), (c) % of females, (d) % of white race, and (e) follow-up length on the outcomes of interest where more than 7 articles were available. Sensitivity analyses were performed to determinate the differences depending on (a) design (cohort vs RCT), (b) type of sample (FEP versus chronic versus other), (c) drug (risperidone/paliperidone versus aripiprazole versus others), and (d) whether the study was industry-sponsored or not. As heterogeneity was expected to be high, the random-effects model was used. Publication bias was assessed by visually inspecting funnel plots and performing Egger’s test.
All analyses were conducted within Comprehensive Meta-Analysis Version 4 [37] and R 1.4.1106 [38]. The significance level was set at a p < 0.05, two-sided.
Results
Sample characteristics
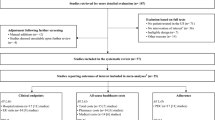
The literature search yielded 3077 non-duplicated citations through electronic database, which were screened for eligibility; 48 articles were assessed in full text, and 28 were excluded, mainly due to their design or lack of all-cause mortality data (n = 15), sample overlap (n = 5) or sample including affective psychosis patients (n = 3). The final systematic review and meta-analysis database included 20 studies, as seen in the PRISMA Flow Diagram (Fig. 1) [39]. Three articles meeting the inclusion criteria had to be excluded from the meta-analysis since they reported 0 deaths in the LAI-AP and oral AP groups and thus a reliable OR could not be calculated [40,41,42].
Data were extracted for a total sample size of 12042 patients, including 5795 receiving any oral antipsychotic and 6247 receiving any LAI antipsychotic from over 75 countries across all continents. The median study follow-up was 100.0 (range = 20–729.4) weeks. Fifteen studies were RCT, and two were cohort studies. There were 18 studies involving second generation LAI-AP (risperidone = 8 [43,44,45,46,47,48,49,50], paliperidone = 4 [51,52,53,54], aripiprazole = 3 [55,56,57], olanzapine = 1 [58]) and 1 study including multiple LAI-APs [59]. 12 studies were sponsored by the industry, 13 included patients with chronic schizophrenia, 2 patients with a FEP, and 2 did not specify the phase of the psychotic illness. The mean age and SD of the sample was 38.3 ± 6.1 and ranged from 18 to 78 years. The mean proportion of females in the included studies was 41.5%. 51.5% were white. Study quality scores ranged from 6 to 9 in the NOS scale. The overall mean and SD quality score of the included studies was 7.2 ± 0.8. (Table 1).
All-cause mortality odds ratio
Altogether, 537 deaths were reported, 295 in the oral-AP group and 242 in the LAI-AP group. OR for all-cause mortality was 0.79 overall (95% CI = 0.66–0.95, k = 17, p = 0.01), implying a statistically significant lower risk of all-cause death for patients receiving LAI-AP. The OR was not altered after applying the Mantel-Haenszel correction (Fig. 2). When the analyses were repeated after removing the article with the most weight on the overall results [59], although the trend was maintained, the results were not powered enough to achieve statistical significance (OR = 0.66, 95% CI = 0.43–1.04, p = 0.056).
Heterogeneity was non-significant with all the studies included in the analysis (Q = 5.57, I2 < 0.01%, p = 0.992), which means estimated effects differ across studies due to random sampling error [60]. Publication bias was not identified neither by visual inspection of the funnel plot (Fig. S1) nor by Egger’s test (p = 0.81).
Meta-regressions showed no significant effect of age, follow-up length, % of females, % of white race, mean LAI antipsychotic dose in chlorpromazine equivalents or quality (NOS score) (Table 2). When stratified by subgroups, studies not sponsored by the industry (OR = 0.79; 95% CI 0.66 – 0.95) and FEP samples (OR = 0.79; 95% CI 0.66 – 0.96) showed lower mortality when treated with LAI antipsychotics than with oral antipsychotics. No significant differences in all-cause mortality were found for any drug LAI antipsychotic; due to the number of articles included for each LAI antipsychotic the analyses were only powered to ascertain the effect on mortality of risperidone/paliperidone and aripiprazole (Table 3).
To verify that the difference between LAI and oral AP groups was due to the formulation (rather than differences between the active principals administered to each patient group), an additional meta-analysis was conducted, including only those studies where each LAI-AP was compared to the same oral-AP. 8 articles reporting on 8,169 patients [45, 51, 52, 55, 56, 58, 59, 61] (4173 in the LAI-AP group and 3,996 in the oral-AP group) were included. OR for all-cause mortality was also 0.79 (95% CI = 0.66–0.95; k = 8; p = <0.01), thus confirming statistically significant lower mortality for the LAI formulation when the antipsychotic agent was the same for both study groups (Fig. S2). Heterogeneity was non-significant (Q = 1.92, I2 < 0.01%, p = 0.964) and publication bias was not identified neither by visual inspection of the funnel plot (Fig. S3) nor by Egger’s test (p = 0.06). Meta-regressions showed no significant effect of % of females, % of white race, or quality score (Table S5).
Suicidal and non-suicidal mortality odds ratio
13 articles provided stratified data for suicidal and non-suicidal deaths [43,44,45,46, 48,49,50, 54,55,56, 58, 61, 62], including a total sample of 10,952 patients (5651 receiving LAI antipsychotics and 5301 receiving oral AP). However, in 5 of them reported 0 deaths in the LAI-AP and oral AP groups for both causes and had to be excluded from the meta-analyses.
110 deaths due to suicide were reported, 52 in the LAI-AP group and 58 in the oral group. OR for suicidal mortality was 0.86 (95% CI = 0.59–1.26; k = 8; p = 0.44), implying no statistically significant differences between the two groups (Fig. S4). Heterogeneity was non-significant (Q = 2.41, I2 < 0.01%, p = 0.934) and publication bias was not identified neither by visual inspection of the funnel plot (Fig. S5) nor by Egger’s test (p = 0.75). Meta-regressions showed no significant effect of % of females or quality score (Table S6). Not enough data was found to examine the effect of mean age, follow-up length, antipsychotic or % of white race.
As for non-suicidal mortality, 421 deaths were reported, 185 in the LAI-AP group and 236 in the oral group. OR for mortality due to causes other than suicide was 0.77 (95% CI = 0.63–0.94; k = 13; p = 0.01), indicating lower mortality in the LAI-AP group (Fig. S6). Heterogeneity was non-significant (Q = 3.57, I2 < 0.01%, p = 0.990) and publication bias was not identified neither by visual inspection of the funnel plot (Fig. S7) nor by Egger’s test (p = 0.06). Again, meta-regressions did not find significant effect of mean age, follow-up length, % of females, % of white race, or quality score (Table S7).
Discussion
This systematic review and meta-analysis have identified, for the first time on a large scale and at a global level, a lower risk of all-cause mortality in schizophrenia patients receiving LAI-APs than in those receiving oral antipsychotics, with an OR of 0.79 (95% CI 0.66–0.95. This was more pronounced among first-episode psychosis patients than in chronic samples and in non-industry sponsored studies. When meta-analyzing only those studies where each LAI was compared to the same oral antipsychotic, the statistical significance was maintained, which indicates these results were due to the formulation, rather than potential differences between the antipsychotics administered to each study group.
The meta-analysis results seem to be largely driven by a large observational study [59]. However, after removing it from the analyses, the trend is maintained, and a lack of statistical power can easily explain its lack of statistical significance. Mortality, whether from suicidal or non-suicidal causes, requires large samples and lengthy follow-ups to study with precision. This can be a potential explanation for the fact that two previous meta-analyses did not identify significant differences in the mortality risk associated with LAI-APs compared with oral-APs [24, 63]. In both cases, those meta-analyses only included RCTs, which may not be the best way to study mortality due to shorter follow-up periods and extremely exigent inclusion criteria. Patients enroled in RCTs may significantly differ from the general patient population in important ways, such as comorbidities, engagement with health care providers and adherence, substance use or risk of suicide, and may not reflect real-world practice and outcomes [64, 65]. Moreover, mortality is a very infrequent event that relatively short follow-up periods fail to report adequately. Our work, besides including other study types like population cohorts, also includes a far greater sample of patients receiving both oral and long-acting injectable antipsychotics.
The results of our study are consistent with other large observational cohort studies in literature. Tang and Taipale identified in large national cohorts from Taiwan and Sweden, respectively, a significant lower risk of all-cause mortality in those patients receiving LAI-APs than in those receiving oral-APs [66, 67]. Although the aforementioned studies were not included in our systematic review due to not meeting our inclusion criteria, their findings are consistent with ours. Furthermore, the Taipale study shows that antipsychotic use was associated with a 50% lower risk when compared with no use [66], with the lowest risk of death being observed with second-generation LAIs. First-episode psychosis might be a particularly vulnerable stage of the illness, where nonadherence is particularly high [68] and relapses are associated with a worse disease course and prognosis [69], including an increase in suicidality [70]. Therefore, it is not surprising that the results of our meta-analysis show even greater differences for FEP populations.
According to some studies [71,72,73,74], LAIs may improve treatment adherence, obtaining lower relapse and hospitalization rates (treatment discontinuation being the leading cause for relapse [75]). There is some controversy in this regard, with the recent randomized trial by Winter van Rossum et al. finding no significant differences in all-cause discontinuation between LAI-AP and oral AP. However, this conclusion is not supported by the totality of the data from their study, in which they found significant differences favouring LAIs in the discontinuation rate for very relevant reasons (including, among others, suicide attempts and all-cause death) [76].
Another important finding of our study is that, when separately analyzing suicidal and non-suicidal mortality, LAI antipsychotics only showed a statistically significant effect in reducing the latter, with an OR of 0.77 (95% CI = 0.63–0.94). Several reasons may explain the lower non-suicidal mortality risk among patients treated with LAI antipsychotics, including both the treatment of psychiatric symptoms and a stronger engagement to health services. Patients treated with LAIs periodically go to the health centre for treatment administration, allowing the possible diagnosis of other treatable comorbidities and early detection of the non-adherence [77]. Furthermore, individuals who maintain a sustained antipsychotic adherence present healthier lifestyle choices, including greater help-seeking behaviours and overall treatment adherence, including cardiometabolic medications [78]. Interestingly, in a recent article by Lieslehto et al. [79], general medical comorbidities were linked to primary nonadherence to antipsychotic treatment (which was also more pronounced for the oral antipsychotics), which could suggest an accumulation of poor lifestyle choices (including obesity or tobacco smoking) in a subgroup of a nonadherent patients, that could also lead to higher mortality.
On the other hand, no significant differences in suicidal mortality were found between LAI and oral antipsychotic-treated patients. Some authors suggest a decrease in the risk of consumed suicide in patients treated with LAI-AP [59]. It might be possible that our lack of statistical power could explain this contradiction. It is also important to note that, in clinical practice, patients receiving LAI-AP differ from those receiving oral antipsychotics, presenting higher symptom severity and a greater rate of previous hospitalizations [80], increased substance use [81], and higher rates of self-harming behaviours [82]. These differences are relevant regarding comorbidity and mortality and cannot always be effectively controlled in observational studies. Therefore, we believe that the superiority of LAIs in terms of mortality could be even more robust if these confounding factors could be controlled. Well-powered studies are needed to unravel which specific causes of death could be prevented or delayed by these medications.
Finally, it is important to notice that this study does not aim to explore the differences between specific antipsychotic drugs. Not all antipsychotics have LAI formulations available, and among those who have, important differences in terms of mortality have been found between first- and second-generation LAI antipsychotics [66]. The present work mostly includes data from atypical antipsychotics, which should be considered when extending these conclusions to all antipsychotic drugs.
Strengths and limitations
To the authors’ knowledge, this is the largest meta-analysis analyzing mortality risk in samples where schizophrenia patients receiving LAI-APs are compared to those receiving oral-APs. The study includes a large study sample from more than 75 countries on all continents. Unlike other previous meta-analyses, this one includes data from randomized controlled trials and observational studies with populations more representative of clinical practice. All of this supports the generalization of its findings.
Nevertheless, some limitations need to be considered. There were not enough data to perform meta-regressions to assess the effect of the combination of antipsychotics and other psychotropic drugs on all-cause mortality. Concomitant benzodiazepine use, for instance, has been previously linked with higher natural cause mortality in schizophrenia patients [83], whereas the combination of antipsychotics and antidepressants has been associated with lower mortality risk in the same group [51]. Finally, while the inclusion of RCT in our analyses increases the generalization of our findings, most RCTs present relatively short-term follow-up periods, which might not be ideal when studying rare events such as mortality. Some do not include mortality data, and when they do, they rarely mention the specific cause of death. This further complicates the establishment of relationships between medications and specific outcomes.
Conclusion
Long-acting injectable antipsychotics are associated with significantly lower all-cause and non-suicidal mortality risk in adult patients with schizophrenia, especially at the early stages of illness. Therefore, when possible and clinically indicated on an individual patient basis, its use should be preferred over oral antipsychotics.
References
Tang C-H, Ramcharran D, Yang C-WW, Chang C-C, Chuang P-Y, Qiu H, et al. A nationwide study of the risk of all-cause, sudden death, and cardiovascular mortality among antipsychotic-treated patients with schizophrenia in Taiwan. Schizophr Res. 2021;237:9–19.
Huang Y, Zhao N. Mental health burden for the public affected by the COVID-19 outbreak in China: Who will be the high-risk group? Psychol Health Med 2021;26:23–34.
Gaebel W, Schreiner A, Bergmans P, Arce R, Rouillon F, Cordes J, et al. Relapse prevention in schizophrenia and schizoaffective disorder with risperidone long-acting injectable vs quetiapine: results of a long-term, open-label, randomized clinical trial. Neuropsychopharmacology. 2011;36:548.
Xiao L, Zhao Q, Li A, Sun J, Wu B, Wang L, et al. Efficacy and safety of aripiprazole once-monthly versus oral aripiprazole in Chinese patients with acute schizophrenia: a multicenter, randomized, double-blind, non-inferiority study. Psychopharmacology. 2022;239:243–51.
Ishigooka J, Nakamura J, Fujii Y, Iwata N, Kishimoto T, Iyo M, et al. Efficacy and safety of aripiprazole once-monthly in Asian patients with schizophrenia: a multicenter, randomized, double-blind, non-inferiority study versus oral aripiprazole. Schizophr Res. 2015;161:421–8.
Correll CU, Solmi M, Croatto G, Schneider LK, Rohani‐Montez SC, Fairley L, et al. Mortality in people with schizophrenia: a systematic review and meta‐analysis of relative risk and aggravating or attenuating factors. World Psychiatry. 2022;21:248–71.
Macfadden W, Ma Y-W, Thomas Haskins J, Bossie CA, Alphs L. A prospective study comparing the long-term effectiveness of injectable risperidone long-acting therapy and oral aripiprazole in patients with schizophrenia. Psychiatry. 2010;7:23–31.
Lian L, Kim DD, Procyshyn RM, Cázares D, Honer WG, Barr AM. Long-acting injectable antipsychotics for early psychosis: A comprehensive systematic review. PloS ONE. 2022;17:e0267808.
Detke HC, Weiden PJ, Llorca P-M, Choukour M, Watson SB, Brunner E, et al. Comparison of olanzapine long-acting injection and oral olanzapine: a 2-year, randomized, open-label study in outpatients with schizophrenia. J Clin Psychopharmacol. 2014;34:426–34.
Keenan A, Lin D, Shepherd J, Bailey H, Benson C, Meakin S. Patient-psychiatrist discordance and drivers of prescribing long-acting injectable antipsychotics for schizophrenia management in the real-world: a point-in-time survey. BMC Psychiatry. 2022;22:187.
Kane JM, Detke HC, Naber D, Sethuraman G, Lin DY, Bergstrom RF, et al. Olanzapine long-acting injection: a 24-week, randomized, double-blind trial of maintenance treatment in patients with schizophrenia. Am J Psychiatry. 2010;167:181–9.
Keks NA, Ingham M, Khan A, Karcher K. Long-acting injectable risperidone v. olanzapine tablets for schizophrenia or schizoaffective disorder: randomised, controlled, open-label study. Br J Psychiatry. 2007;191:131–9.
Kishi T, Matsunaga S, Iwata N. Mortality risk associated with long-acting injectable antipsychotics: a systematic review and meta-analyses of randomized controlled, trials. Schizophr Bull. 2016;42:1438–45.
Wei Y, Yan VKC, Kang W, Wong ICK, Castle DJ, Gao L, et al. Association of long-acting injectable antipsychotics and oral antipsychotics with disease relapse, health care use, and adverse events among people with schizophrenia. JAMA Netw Open. 2022;5:e2224163.
Altman D. Practical statistics for medical research. London: Chapman & Hall; 1991.
Malla A, Chue P, Jordan G, Stip E, Koczerginski D, Milliken H, et al. An exploratory, open-label, randomized trial comparing risperidone long-acting injectable with oral antipsychotic medication in the treatment of early psychosis. Clin Schizophr Relat Psychoses. 2016;9:198–208.
Owen MJ, Sawa A, Mortensen PB. Schizophrenia. Lancet. 2016;338:86–97.
Altman DG, Simera I, Hoey J, Moher D, Schulz K. EQUATOR: reporting guidelines for health research. Lancet. 2008;371:1149–50.
Huang C-Y, Fang S-C, Shao Y-HJ. Comparison of long-acting injectable antipsychotics with oral antipsychotics and suicide and all-cause mortality in patients with newly diagnosed schizophrenia. JAMA Netw Open. 2021;4:e218810.
Haukka J, Tiihonen J, Härkänen T, Lönnqvist J. Association between medication and risk of suicide, attempted suicide and death in nationwide cohort of suicidal patients with schizophrenia. Pharmacoepidemiol Drug Saf. 2008;17:686–96.
Emsley R, Chiliza B, Asmal L. The evidence for illness progression after relapse in schizophrenia. Schizophr Res. 2013;148:117–21.
Tenback D, Pijl B, Smeets H, Van OSJ, Van Harten P. All-cause mortality and medication risk factors in schizophrenia: a prospective cohort study. J Clin Psychopharmacol. 2012;32:31–35.
Hunt GE, Large MM, Cleary M, Lai HMX, Saunders JB. Prevalence of comorbid substance use in schizophrenia spectrum disorders in community and clinical settings, 1990–2017: systematic review and meta-analysis. Drug Alcohol Depend. 2018;191:234–58.
Sicotte R, Iyer SN, Kiepura B, Abdel-Baki A. A systematic review of longitudinal studies of suicidal thoughts and behaviors in first-episode psychosis: course and associated factors. Soc Psychiatry Psychiatr Epidemiol. 2021;56:2117–54.
Nordentoft M, Wahlbeck K, Hällgren J, Westman J, Ösby U, Alinaghizadeh H, et al. Excess mortality, causes of death and life expectancy in 270,770 patients with recent onset of mental disorders in Denmark, Finland and Sweden. PloS one. 2013;8:e55176.
Misawa F, Kishimoto T, Hagi K, Kane JM, Correll CU. Safety and tolerability of long-acting injectable versus oral antipsychotics: A meta-analysis of randomized controlled studies comparing the same antipsychotics. Schizophr Res. 2016;176:220–30.
Yaegashi H, Kirino S, Remington G, Misawa F, Takeuchi H. Adherence to oral antipsychotics measured by electronic adherence monitoring in schizophrenia: a systematic review and meta-analysis. CNS Drugs. 2020;34:579–98.
Shi L, Ascher-Svanum H, Zhu B, Faries D, Montgomery W, Marder SR. Characteristics and use patterns of patients taking first-generation depot antipsychotics or oral antipsychotics for schizophrenia. Psychiatr Serv. 2007;58:482–8.
Li W, Yang Y, An F-R, Zhang L, Ungvari GS, Jackson T, et al. Prevalence of comorbid depression in schizophrenia: A meta-analysis of observational studies. J Affect Disord. 2020;273:524–31.
Diagnostic and statistical manual of mental disorders. Washington, DC: American Psychiatric Association Publishing; 2022.
Diagnostic and statistical manual of mental disorders: DSM-IV-TR. Washington, DC: American Psychiatric Association; 2000.
Catalan A, García L, Sanchez‐Alonso S, Gil P, Díaz‐Marsá M, Olivares JM, et al. Early intervention services, patterns of prescription and rates of discontinuation of antipsychotic treatment in first‐episode psychosis. Early Interv Psychiatry. 2021;15:1584–94.
Hickling LM, Kouvaras S, Nterian Z, Perez-Iglesias R. Non-adherence to antipsychotic medication in first-episode psychosis patients. Psychiatry Res. 2018;264:151–4.
Coles AS, Knezevic D, George TP, Correll CU, Kane JM, Castle D. Long-acting injectable antipsychotic treatment in schizophrenia and co-occurring substance use disorders: a systematic review. Front Psychiatry. 2021;12:808002.
Stubbs B, Koyanagi A, Veronese N, Vancampfort D, Solmi M, Gaughran F, et al. Physical multimorbidity and psychosis: comprehensive cross sectional analysis including 242,952 people across 48 low- and middle-income countries. BMC Med. 2016;14:189.
Winter-van Rossum I, Weiser M, Galderisi S, Leucht S, Bitter I, Glenthoj B, et al. Efficacy of oral versus long-acting antipsychotic treatment in patients with early-phase schizophrenia in Europe and Israel: a large-scale, open-label, randomised trial (EULAST). 2023. https://doi.org/10.1016/S2215-0366(23)00005-6.
Lipsey MW, Wilson DB. Practical meta-analysis. vol. 49. Thousand Oaks: Sage; 2009.
Torniainen M, Mittendorfer-Rutz E, Tanskanen A, Björkenstam C, Suvisaari J, Alexanderson K, et al. Antipsychotic treatment and mortality in schizophrenia. Schizophr Bull. 2015;41:656–63.
Ratliff JC, Palmese LB, Reutenauer EL, Liskov E, Grilo CM, Tek C. The effect of dietary and physical activity pattern on metabolic profile in individuals with schizophrenia: a cross-sectional study. Compr Psychiatry. 2012;53:1028–33.
Lawrence D, Kisely S. Inequalities in healthcare provision for people with severe mental illness. J Psychopharmacol. 2010;24:61–68.
García S, Martínez-Cengotitabengoa M, López-Zurbano S, Zorrilla I, López P, Vieta E, et al. Adherence to antipsychotic medication in bipolar disorder and schizophrenic patients: a systematic review. J Clin Psychopharmacol. 2016;36:355–71.
PRISMA. PRISMA Flow Diagram. 2021. http://prisma-statement.org/prismastatement/flowdiagram.aspx 2021.
Computing RF for S. R: A language and environment for statistical computing. Vienna, Austria; 2021.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. 2009;339:e78–336.
Kishimoto T, Nitta M, Borenstein M, Kane JM, Correll CU. Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image, studies. J Clin Psychiatry. 2013;74:957–65.
Ioannidis JPA, Patsopoulos NA, Evangelou E. Uncertainty in heterogeneity estimates in meta-analyses. BMJ. 2007;335:914–6.
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in, Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12.
Diagnostic and statistical manual of mental disorders: DSM-5. Washington, DC: American Psychiatric Publishing; 2013.
Deslandes PN, Dwivedi M, Sewell RDE. Five-year patient outcomes with risperidone long-acting injection or oral aripiprazole. Ther Adv Psychopharmacol. 2015;5:151–7.
Kennedy-Martin T, Curtis S, Faries D, Robinson S, Johnston J. A literature review on the representativeness of randomized controlled trial samples and implications for the external validity of trial results. Trials. 2015;16:495.
Schreiner A, Aadamsoo K, Altamura AC, Franco M, Gorwood P, Neznanov NG, et al. Paliperidone palmitate versus oral antipsychotics in recently diagnosed schizophrenia. Schizophr Res. 2015;169:393–9.
Xiao X, Zhu X, Fu S, Hu Y, Li X, Xiao J. Psychological impact of healthcare workers in China during COVID-19 pneumonia epidemic: A multi-center cross-sectional survey investigation. J Affect Disord. 2020;274:405–10.
Taipale H, Tanskanen A, Mehtälä J, Vattulainen P, Correll CU, Tiihonen J. 20‐year follow‐up study of physical morbidity and mortality in relationship to antipsychotic treatment in a nationwide cohort of 62,250 patients with schizophrenia (FIN20). World Psychiatry. 2020;19:61–68.
Lieslehto J, Tiihonen J, Lähteenvuo M, Tanskanen A, Taipale H. Primary nonadherence to antipsychotic treatment among persons with schizophrenia. Schizophr Bull. 2022;48:655–63.
Fang S-C, Huang C-Y, Shao Y-HJ. Long-term outcomes of early use of long-acting injectable antipsychotics in schizophrenia. Environ Monit Assess. 2022;195:83.
de Arce Cordón R, Eding E, Marques-Teixeira J, Milanova V, Rancans E, Schreiner A. Descriptive analyses of the aripiprazole arm in the risperidone long-acting injectable versus quetiapine relapse prevention trial (ConstaTRE). Eur Arch Psychiatry Clin Neurosci. 2012;262:139–49.
Fleischhacker WW, Sanchez R, Perry PP, Jin N, Peters-Strickland T, Johnson BR, et al. Aripiprazole once-monthly for treatment of schizophrenia: double-blind, randomised, non-inferiority study. Br J Psychiatry. 2014;205:135–44.
Porcelli S, Bianchini O, De Girolamo G, Aguglia E, Crea L, Serretti A. Clinical factors related to schizophrenia relapse. Int J Psychiatry Clin Pract. 2016;20:54–69.
Chue P, Eerdekens M, Augustyns I, Lachaux B, Molčan P, Eriksson L, et al. Comparative efficacy and safety of long-acting risperidone and risperidone oral tablets. Eur Neuropsychopharmacol. 2005;15:111–7.
Tiihonen J, Haukka J, Taylor M, Haddad PM, Patel MX, Korhonen P. A nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophrenia. Am J Psychiatry. 2011;168:603–9.
Monti S, Grosso V, Todoerti M, Caporali R. Randomized controlled trials and real-world data: differences and similarities to untangle literature data. Rheumatology. 2018;57:vii54–vii58.
Rosenheck RA, Krystal JH, Lew R, Barnett PG, Fiore L, Valley D, et al. Long-acting risperidone and oral antipsychotics in unstable schizophrenia. N. Engl J Med. 2011;364:842–51.
Ostuzzi G, Bertolini F, Tedeschi F, Vita G, Brambilla P, Fabro L, et al. Oral and long‐acting antipsychotics for relapse prevention in schizophrenia‐spectrum disorders: a network meta‐analysis of 92 randomized trials including 22,645 participants. World Psychiatry. 2022;21:295–307.
HBM. Hedges, L. Higgins, & J Rothstein. Comprehensive meta-analysis. vol. 4. Biostat, Englewood, NJ; 2022.
Correll CU, Solmi M, Veronese N, Bortolato B, Rosson S, Santonastaso P, et al. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: a large‐scale meta‐analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry. 2017;16:163–80.
Arango C, Bombín I, González-Salvador T, García-Cabeza I, Bobes J. Randomised clinical trial comparing oral versus depot formulations of zuclopenthixol in patients with schizophrenia and previous violence. Eur Psychiatry. 2006;21:34–40.
Alphs L, Mao L, Lynn Starr H, Benson C. A pragmatic analysis comparing once-monthly paliperidone palmitate versus daily oral antipsychotic treatment in patients with schizophrenia. Schizophr Res. 2016;170:259–64.
Taipale H, Tanskanen A, Correll CU, Tiihonen J. Real-world effectiveness of antipsychotic doses for relapse prevention in patients with first-episode schizophrenia in Finland: a nationwide, register-based cohort study. Lancet. 2022;9:271–9.
Hong J, Novick D, Brugnoli R. Changes in adherence and treatment costs following initiation of oral or depot typical antipsychotics among previously non-adherent patients with schizophrenia. Hum Psychopharmacol. 2013;28:438–46.
Seebaluck J, Downes MA, Brown J, Harris K, Isoardi KZ, Chan BS. Case series profile of olanzapine post‐injection delirium/sedation syndrome. Br J Clin Pharmacol. 2023;89:903–7.
Solmi M, Tiihonen J, Lähteenvuo M, Tanskanen A, Correll CU, Taipale H. Antipsychotics use is associated with greater adherence to cardiometabolic medications in patients with schizophrenia: results from a nationwide, within-subject design study. Schizophr Bull. 2022;48:166–75.
Vancampfort D, Stubbs B, Mitchell AJ, Hert MD, Wampers M, Ward PB, et al. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta‐analysis. World Psychiatry. 2015;14:339–47.
Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomized studies in meta-analyses. University of Ottawa, Ontario, Canada. 2012.
World Health Organization (WHO). International Classification of Diseases, Eleventh Revision (ICD-11). 2021.
Buckley PF, Schooler NR, Goff DC, Hsiao J, Kopelowicz A, Lauriello J, et al. Comparison of SGA oral medications and a long-acting injectable SGA: the PROACTIVE study. Schizophr Bull. 2015;41:449–59.
Hert MD, Detraux J, Winkel R, Yu W, Correll CU. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev. 2011;8:114–26.
Fidler V, Auvinen A-P. The Mantel-Haenszel procedure revisited: models and generalizations. PloS ONE. 2013;8:e58327.
Smith DJ, Langan J, McLean G, Guthrie B, Mercer SW. Schizophrenia is associated with excess multiple physical-health comorbidities but low levels of recorded cardiovascular disease in primary care: cross-sectional study. BMJ Open. 2013;3:e002808.
Taipale H, Mittendorfer-Rutz E, Alexanderson K, Majak M, Mehtälä J, Hoti F, et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res. 2018;197:274–80.
Emsley R, Chiliza B, Asmal L, Mashile M, Fusar-Poli P. Long-acting injectable antipsychotics in early psychosis: a literature review. Early Interv Psychiatry. 2013;7:247–54.
The ICD-10 classification of mental and behavioural disorders. Geneva: WHO; 1993.
Kim E, Correll CU, Mao L, Starr HL, Alphs L. Once-monthly paliperidone palmitate compared with conventional and atypical daily oral antipsychotic treatment in patients with schizophrenia. CNS Spectr. 2016;21:466–77.
Alphs L, Brown B, Turkoz I, Baker P, Fu D-J, Nuechterlein KH. The Disease Recovery Evaluation and Modification (DREaM) study: effectiveness of paliperidone palmitate versus oral antipsychotics in patients with recent-onset schizophrenia or schizophreniform disorder. Schizophr Res. 2022;243:86–97.
Funding
This research received funding from the Biobizkaia Health Research Institute for publication fees.
Author information
Authors and Affiliations
Contributions
CA (Conceptualization and protocol writing, original draft, methodology, systematic search); GSP (Conceptualization, original draft, methodology); MP (Systematic search, data extraction, writing—review and editing); VPR (Systematic search, data extraction, writing—review and editing); AB (Statistics, writing—review and editing); LA (Systematic search, data extraction); ICS (Statistics, writing—review and editing); NA (Statistics, figure design); PFP (Conceptualization and protocol writing, supervision, writing—review and editing); IZ (Methodology, writing—review and editing); AGP (Methodology, writing—review and editing); MAGT (Conceptualization and protocol writing, supervision, writing—review and editing); AC (Conceptualization and protocol writing, supervision, writing—review and editing)
Corresponding author
Ethics declarations
Competing interests
CA received personal fees or grants from Janssen Cilag and Neuraxpharm outside the current work. GSP has received honoraria from Janssen Cilag, Lundbeck and Angelini outside the current work. PFP has received grant support from Lundbeck and honoraria fees from Angelini, Menarini, and Lundbeck outside the current work. AGP has received grants and served as consultant, advisor or CME speaker for the following entities: Jannsen-Cilag, Lundbeck, Otsuka, Pfizer, Sanofi-Aventis, Exeltis, the Spanish Ministry of Science and Innovation (CIBERSAM), the Ministry of Science (Carlos III Institute), and the Basque Government. AC received personal fees or grants from Lundbeck, ROVI, and Janssen Cilag outside the current work. The rest of the authors reported no biomedical financial interests or potential conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Aymerich, C., Salazar de Pablo, G., Pacho, M. et al. All-cause mortality risk in long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis. Mol Psychiatry (2024). https://doi.org/10.1038/s41380-024-02694-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41380-024-02694-3
- Springer Nature Limited