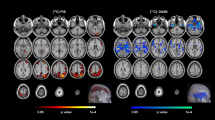
Abstract
The dorsal raphe nucleus (DRN) is one of the earliest targets of Alzheimer’s disease-related tau pathology and is a major source of brain serotonin. We used [18F]Fluoro-m-tyrosine ([18F]FMT) PET imaging to measure serotonin synthesis capacity in the DRN in 111 healthy adults (18–85 years-old). Similar to reports in catecholamine systems, we found elevated serotonin synthesis capacity in older adults relative to young. To establish the structural and functional context within which serotonin synthesis capacity is elevated in aging, we examined relationships among DRN [18F]FMT net tracer influx (Ki) and longitudinal changes in cortical thickness using magnetic resonance imaging, longitudinal changes in self-reported depression symptoms, and AD-related tau and β-amyloid (Aβ) pathology using cross-sectional [18F]Flortaucipir and [11C]Pittsburgh compound-B PET respectively. Together, our findings point to elevated DRN [18F]FMT Ki as a marker of poorer aging trajectories. Older adults with highest serotonin synthesis capacity showed greatest temporal lobe cortical atrophy. Cortical atrophy was associated with increasing depression symptoms over time, and these effects appeared to be strongest in individuals with highest serotonin synthesis capacity. We did not find direct relationships between serotonin synthesis capacity and AD-related pathology. Exploratory analyses revealed nuanced effects of sex within the older adult group. Older adult females showed the highest DRN synthesis capacity and exhibited the strongest relationships between entorhinal cortex tau pathology and increasing depression symptoms. Together these findings reveal PET measurement of the serotonin system to be a promising marker of aging trajectories relevant to both AD and affective changes in older age.



Similar content being viewed by others
References
Andrés-Benito P, Fernández-Dueñas V, Carmona M, Escobar LA, Torrejón-Escribano B, Aso E, et al. Locus coeruleus at asymptomatic early and middle Braak stages of neurofibrillary tangle pathology. Neuropathol Appl Neurobiol. 2017;43:373–92.
Parvizi J, Van Hoesen GW, Damasio A. The selective vulnerability of brainstem nuclei to Alzheimer’s disease. Ann Neurol. 2001;49:53–66.
Rüb U, Stratmann K, Heinsen H, Del Turco D, Seidel K, den Dunnen W, et al. The Brainstem Tau Cytoskeletal Pathology of Alzheimer’s Disease: A Brief Historical Overview and Description of its Anatomical Distribution Pattern, Evolutional Features, Pathogenetic and Clinical Relevance. Curr Alzheimer Res. 2016;13:1178–97.
Šimić G, Leko MB, Wray S, Harrington C, Delalle I, Jovanov-Milošević N, et al. Monoaminergic Neuropathology in Alzheimer’s disease. Prog Neurobiol. 2017;151:101–38.
Stratmann K, Heinsen H, Korf H, Del Turco D, Ghebremedhin E, Seidel K, et al. Precortical Phase of Alzheimer’s Disease (AD)‐Related Tau Cytoskeletal Pathology. Brain Pathol. 2015;26:371–86.
Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the Pathologic Process in Alzheimer Disease: Age Categories From 1 to 100 years. J Neuropathol Exp Neurol. 2011;70:960–9.
Grinberg LT, Rüb U, Ferretti REL, Nitrini R, Farfel JM, Polichiso L, et al. The dorsal raphe nucleus shows phospho-tau neurofibrillary changes before the transentorhinal region in Alzheimer’s disease. A precocious onset? Neuropathol Appl Neurobiol. 2009;35:406–16.
Ehrenberg AJ, Nguy AK, Theofilas P, Dunlop S, Suemoto CK, Di Lorenzo Alho AT, et al. Quantifying the accretion of hyperphosphorylated tau in the locus coeruleus and dorsal raphe nucleus: the pathological building blocks of early Alzheimer’s disease. Neuropathol Appl Neurobiol. 2017;43:393–408.
Aletrino MA, Vogels OJM, Van Domburg PHMF, Ten Donkelaar HJ. Cell loss in the nucleus raphes dorsalis in alzheimer’s disease. Neurobiol Aging. 1992;13:461–8.
Gottfries CG, Bartfai T, Carlsson A, Eckernäs S, Svennerholm L. Multiple biochemical deficits in both gray and white matter of Alzheimer brains. Prog Neuropsychopharmacol Biol Psychiatry. 1986;10:405–13.
Palmer AM, Wilcock GK, Esiri MM, Francis PT, Bowen DM. Monoaminergic innervation of the frontal and temporal lobes in Alzheimer’s disease. Brain Res. 1987;401:231–8.
Storga D, Vrecko K, Birkmayer JGD, Reibnegger G. Monoaminergic neurotransmitters, their precursors and metabolites in brains of Alzheimer patients. Neurosci Lett. 1996;203:29–32.
Geyer MA, Puerto A, Dawsey WJ, Knapp S, Bullard WP, Mandell AJ. Histologic and enzymatic studies of the mesolimbic and mesostriatal serotonergic pathways. Brain Res. 1976;106:241–56.
Diniz BS, Butters MA, Albert SM, Dew MA, Reynolds CF. Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry. 2013;202:329–35.
Meltzer MDC. Serotonin in Aging, Late-Life Depression, and Alzheimer’s Disease: The Emerging Role of Functional Imaging. Neuropsychopharmacology. 1998;18:407–30.
Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D. Depression and Risk for Alzheimer Disease. Arch Gen Psychiatry. 2006;63:530–8.
Han FF, Wang HX, Wu JJ, Yao W, Hao CF, Pei JJ. Depressive symptoms and cognitive impairment: A 10-year follow-up study from the Survey of Health, Ageing and Retirement in Europe. Eur Psychiatry. 2021;64:e55.
Krell-Roesch J, Syrjanen JA, Rakusa M, Vemuri P, Machulda MM, Kremers WK, et al. Association of Cortical and Subcortical β-Amyloid With Standardized Measures of Depressive and Anxiety Symptoms in Adults Without Dementia. J Neuropsychiatry Clin Neurosci. 2021;33:64–71.
Gurera JW, Isaacowitz DM. Emotion regulation and emotion perception in aging: A perspective on age-related differences and similarities. In: Emotion and cognition. San Diego, CA, US: Elsevier Academic Press; 2019. p. 329–51. (Progress in brain research).
Cotter DL, Walters SM, Fonseca C, Wolf A, Cobigo Y, Fox EC, et al. Aging and Positive Mood: Longitudinal Neurobiological and Cognitive Correlates. Am J Geriatr Psychiatry Off J Am Assoc Geriatr Psychiatry. 2020;28:946–56.
Jorm AF. Does old age reduce the risk of anxiety and depression? A review of epidemiological studies across the adult life span. Psychol Med. 2000;30:11–22.
Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–91.
Berry AS, Shah VD, Baker SL, Vogel JW, O’Neil JP, Janabi M, et al. Aging Affects Dopaminergic Neural Mechanisms of Cognitive Flexibility. J Neurosci Off J Soc Neurosci. 2016;36:12559–69.
Ciampa CJ, Parent JH, Lapoint MR, Swinnerton KN, Taylor MM, Tennant VR, et al. Elevated Dopamine Synthesis as a Mechanism of Cognitive Resilience in Aging. Cereb Cortex. 2022;32:2762–72.
Parent JH, Ciampa CJ, Harrison TM, Adams JN, Zhuang K, Betts MJ, et al. Locus coeruleus catecholamines link neuroticism and vulnerability to tau pathology in aging. NeuroImage. 2022;263:119658.
Ciampa CJ, Parent JH, Harrison TM, Fain RM, Betts MJ, Maass A, et al. Associations among locus coeruleus catecholamines, tau pathology, and memory in aging. Neuropsychopharmacology. 2022;47:1106–13.
Berry AS, Shah VD, Jagust WJ. The Influence of Dopamine on Cognitive Flexibility Is Mediated by Functional Connectivity in Young but Not Older Adults. J Cogn Neurosci. 2018;30:1330–44.
VanBrocklin HF, Blagoev M, Hoepping A, O’Neil JP, Klose M, Schubiger PA, et al. A new precursor for the preparation of 6-[18F]Fluoro-l-m-tyrosine ([18F]FMT): efficient synthesis and comparison of radiolabeling. Appl Radiat Isot. 2004;61:1289–94.
Boyes BE, Cumming P, Martin WRW, McGeer EG. Determination of plasma [18F]−6-fluorodopa during positron emission tomography: Elimination and metabolism in carbidopa treated subjects. Life Sci. 1986;39:2243–52.
Firnau G, Sood S, Chirakal R, Nahmias C, Garnett ES. Metabolites of 6-[18F]fluoro-L-dopa in human blood. J Nucl Med Off Publ Soc Nucl Med. 1988;29:363–9.
Hoffman JM, Melega WP, Hawk TC, Grafton SC, Luxen A, Mahoney DK, et al. The effects of carbidopa administration on 6-[18F]fluoro-L-dopa kinetics in positron emission tomography. J Nucl Med Off Publ Soc Nucl Med. 1992;33:1472–7.
Melega WP, Hoffman JM, Luxen A, Nissenson CH, Phelps ME, Barrio JR. The effects of carbidopa on the metabolism of 6-[18F]fluoro-L-dopa in rats, monkeys and humans. Life Sci. 1990;47:149–57.
Patlak CS, Blasberg RG. Graphical Evaluation of Blood-to-Brain Transfer Constants from Multiple-Time Uptake Data. Generalizations. J Cereb Blood Flow Metab. 1985;5:584–90.
Brown WD, DeJesus OT, Pyzalski RW, Malischke L, Roberts AD, Shelton SE, et al. Localization of trapping of 6-[18F]fluoro-L-m-tyrosine, an aromatic L-amino acid decarboxylase tracer for PET. Synapse. 1999;34:111–23.
Ito H, Ota M, Ikoma Y, Seki C, Yasuno F, Takano A. et al. Quantitative analysis of dopamine synthesis in human brain using positron emission tomography with L-[β-11C]DOPA. Nucl Med Commun. 2006;27:723–31.
Ito H, Shidahara M, Takano H, Takahashi H, Nozaki S, Suhara T. Mapping of central dopamine synthesis in man, using positron emission tomography with l-[β-11C]DOPA. Ann Nucl Med. 2007;21:355–60.
Doppler CEJ, Kinnerup MB, Brune C, Farrher E, Betts M, Fedorova TD, et al. Regional locus coeruleus degeneration is uncoupled from noradrenergic terminal loss in Parkinson’s disease. Brain. 2021;144:2732–44.
Kranz GS, Hahn A, Savli M, Lanzenberger R. Challenges in the differentiation of midbrain raphe nuclei in neuroimaging research. Proc Natl Acad Sci. 2012;109:E2000.
Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci. 2000;97:11050–5.
Reuter M, Fischl B. Avoiding asymmetry-induced bias in longitudinal image processing. NeuroImage. 2011;57:19–21.
Reuter M, Rosas HD, Fischl B. Highly accurate inverse consistent registration: a robust approach. NeuroImage. 2010;53:1181–96.
Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. NeuroImage. 2012;61:1402–18.
Schöll M, Lockhart SN, Schonhaut DR, O’Neil JP, Janabi M, Ossenkoppele R, et al. PET Imaging of Tau Deposition in the Aging Human Brain. Neuron. 2016;89:971–82.
Price JC, Klunk WE, Lopresti BJ, Lu X, Hoge JA, Ziolko SK, et al. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2005;25:1528–47.
Villeneuve S, Rabinovici G, Cohn-Sheehy B, Madison C, Ayakta N, Ghosh P, et al. Existing Pittsburgh Compound-B positron emission tomography thresholds are too high: statistical and pathological evaluation. Brain. 2015;138:2020–33.
Baker SL, Maass A, Jagust WJ. Considerations and code for partial volume correcting [18F]-AV-1451 tau PET data. Data Brief. 2017;15:648–57.
Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl). 1991;82:239–59.
Maass A, Lockhart SN, Harrison TM, Bell RK, Mellinger T, Swinnerton K, et al. Entorhinal Tau Pathology, Episodic Memory Decline, and Neurodegeneration in Aging. J Neurosci. 2018;38:530–43.
Harrison TM, Maass A, Adams JN, Du R, Baker SL, Jagust WJ. Tau deposition is associated with functional isolation of the hippocampus in aging. Nat Commun. 2019;10:4900.
Arenaza-Urquijo EM, Vemuri P. Resistance vs resilience to Alzheimer disease. Neurology. 2018;90:695–703.
Stern Y, Arenaza-Urquijo EM, Bartrés-Faz D, Belleville S, Cantilon M, Chetelat G, et al. Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement J Alzheimers Assoc. 2020;16:1305–11.
Hayes, AF. Introduction to Mediation, Moderation, and Conditional Process Analysis. 3rd edition. New York: The Guilford Press; 2022.
Maechler M, Rousseeuw P, Croux C, Todorov V, Ruckstuhl A, Salibian-Barrera M, et al. robustbase: Basic Robust Statistics. R package version 0.99-0, http://robustbase.r-forge.r-project.org/. 2023
Tsuiki K, Mück-Šeler D, Diksic M. Autoradiographic evaluation of the influence of hypothalamic 5,7-dihydroxytryptamine lesion on brain serotonin synthesis. Biochem Pharmacol. 1995;49:633–42.
Dejesus OT, Endres CJ, Shelton SE, Nickles RJ, Holden JE. Noninvasive assessment of aromatic L-amino acid decarboxylase activity in aging rhesus monkey brain in vivo. Synapse. 2001;39:58–63.
Eberling JL, Roberts JA, Taylor SE, VanBrocklin HF, O’Neil JP, Nordahl TE. No effect of age and estrogen on aromatic L- amino acid decarboxylase activity in rhesus monkey brain. Neurobiol Aging. 2002;23:479–83.
Moore RY, Whone AL, Brooks DJ. Extrastriatal monoamine neuron function in Parkinson’s disease: An 18F-dopa PET study. Neurobiol Dis. 2008;29:381–90.
Pavese N, Rivero-Bosch M, Lewis SJ, Whone AL, Brooks DJ. Progression of monoaminergic dysfunction in Parkinson’s disease: A longitudinal 18F-dopa PET study. NeuroImage. 2011;56:1463–8.
Fritschy JM, Grzanna R. Restoration of ascending noradrenergic projections by residual locus coeruleus neurons: compensatory response to neurotoxin-induced cell death in the adult rat brain. J Comp Neurol. 1992;321:421–41.
Acheson AL, Zigmond MJ. Short and long-term changes in tyrosine hydroxylase activity in rat brain after subtotal destruction of central noradrenergic neurons. J Neurosci. 1981;1:493–504.
Lapchak PA, Jenden DJ, Heft F. Compensatory elevation of acetylcholine synthesis in vivo by cholinergic neurons surviving partial lesions of the septohippocampal pathway. J Neurosci. 1991;11:2821–8.
DeKosky ST, Ikonomovic MD, Styren SD, Beckett L, Wisniewski S, Bennett DA, et al. Upregulation of choline acetyltransferase activity in hippocampus and frontal cortex of elderly subjects with mild cognitive impairment. Ann Neurol. 2002;51:145–55.
Rosa-Neto P, Benkelfat C, Sakai Y, Leyton M, Morais JA, Diksic M. Brain regional α-[11C]methyl-L-tryptophan trapping, used as an index of 5-HT synthesis, in healthy adults: absence of an age effect. Eur J Nucl Med Mol Imaging. 2007;34:1254–64.
DeJesus OT. Positron-labeled DOPA analogs to image dopamine terminals. Drug Dev Res. 2003;59:249–60.
Nahmias C, Wahl L, Chirakal R, Firnau G, Garnett ES. A probe for intracerebral aromatic amino-acid decarboxylase activity: Distribution and kinetics of [18F]6-fluoro-L-m-tyrosine in the human brain. Mov Disord. 1995;10:298–304.
DeJesus OT, Endres CJ, Shelton SE, Nickles RJ, Holden JE. Evaluation of fluorinated m-tyrosine analogs as PET imaging agents of dopamine nerve terminals: comparison with 6-fluoroDOPA. J Nucl Med Off Publ Soc Nucl Med. 1997;38:630–6.
Brown WD, DeJesus OT, Pyzalski RW, Malischke L, Roberts AD, Shelton SE, et al. Localization of trapping of 6-[(18)F]fluoro-L-m-tyrosine, an aromatic L-amino acid decarboxylase tracer for PET. Synap N Y N. 1999;34:111–23.
Fiandaca MS, Varenika V, Eberling J, McKnight T, Bringas J, Pivirotto P, et al. Real-time MR imaging of adeno-associated viral vector delivery to the primate brain. NeuroImage. 2009;47:T27–35.
DeJesus OT, Flores LG, Murali D, Converse AK, Bartlett RM, Barnhart TE, et al. Aromatic l-amino acid decarboxylase turnover in vivo in rhesus macaque striatum: A microPET study. Brain Res. 2005;1054:55–60.
Duchemin AM, Neff NH, Hadjiconstantinou M. Aromatic l-amino acid decarboxylase phosphorylation and activation by PKGIαin vitro. J Neurochem. 2010;114:542–52.
Duchemin AM, Berry MD, Neff NH, Hadjiconstantinou M. Phosphorylation and activation of brain aromatic L-amino acid decarboxylase by cyclic AMP-dependent protein kinase. J Neurochem. 2000;75:725–31.
Neff NH, Wemlinger TA, Duchemin AM, Hadjiconstantinou M. Clozapine Modulates Aromatic l-Amino Acid Decarboxylase Activity in Mouse Striatum. J Pharmacol Exp Ther. 2006;317:480–7.
Li XM, Juorio AV, Boulton AA. Induction of aromatic L-amino acid decarboxylase mRNA by interleukin-1 beta and prostaglandin E2 in PC12 cells. Neurochem Res. 1994;19:591–5.
Snyder SH, Axelrod J, Wurtman RJ. Effect of Gonadal Hormones on Aromatic Amino Acid Decarboxylase in the Rat Uterus. Endocrinology. 1966;78:1135–8.
Hadjiconstantinou M, Neff NH. Enhancing Aromatic L-amino Acid Decarboxylase Activity: Implications for L-DOPA Treatment in Parkinson’s Disease. CNS Neurosci Ther. 2008;14:340–51.
Beliveau V, Svarer C, Frokjaer VG, Knudsen GM, Greve DN, Fisher PM. Functional connectivity of the dorsal and median raphe nuclei at rest. NeuroImage. 2015;116:187–95.
Karrer TM, McLaughlin CL, Guaglianone CP, Samanez-Larkin GR. Reduced serotonin receptors and transporters in normal aging adults: a meta-analysis of PET and SPECT imaging studies. Neurobiol Aging. 2019;80:1–10.
Møller M, Jakobsen S, Gjedde A. Parametric and regional maps of free serotonin 5HT1A receptor sites in human brain as function of age in healthy humans. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2007;32:1707–14.
Grinberg LT, Rueb U, Heinsen H. Brainstem: Neglected Locus in Neurodegenerative Diseases. Front Neurol. 2011;2:42.
Jacobs HIL, Becker JA, Kwong K, Engels-Domínguez N, Prokopiou PC, Papp KV, et al. In vivo and neuropathology data support locus coeruleus integrity as indicator of Alzheimer’s disease pathology and cognitive decline. Sci Transl Med. 2021;13:eabj2511.
Dulawa SC, Janowsky DS. Cholinergic Regulation of Mood: From Basic and Clinical Studies to Emerging Therapeutics. Mol Psychiatry. 2019;24:694–709.
Dunlop BW, Nemeroff CB. The Role of Dopamine in the Pathophysiology of Depression. Arch Gen Psychiatry. 2007;64:327–37.
Frey BN, Skelin I, Sakai Y, Nishikawa M, Diksic M. Gender differences in α-[11C]MTrp brain trapping, an index of serotonin synthesis, in medication-free individuals with major depressive disorder: A positron emission tomography study. Psychiatry Res Neuroimaging. 2010;183:157–66.
Sakai Y, Nishikawa M, Leyton M, Benkelfat C, Young SN, Diksic M. Cortical trapping of α-[11C]methyl-l-tryptophan, an index of serotonin synthesis, is lower in females than males. NeuroImage. 2006;33:815–24.
Nishizawa S, Benkelfat C, Young SN, Leyton M, Mzengeza S, de Montigny C, et al. Differences between males and females in rates of serotonin synthesis in human brain. Proc Natl Acad Sci. 1997;94:5308–13.
Jovanovic H, Lundberg J, Karlsson P, Cerin Å, Saijo T, Varrone A, et al. Sex differences in the serotonin 1A receptor and serotonin transporter binding in the human brain measured by PET. NeuroImage. 2008;39:1408–19.
Colaianna M, Tucci P, Zotti M, Morgese MG, Schiavone S, Govoni S, et al. Soluble beta amyloid(1-42): a critical player in producing behavioural and biochemical changes evoking depressive-related state? Br J Pharmacol. 2010;159:1704–15.
Sheline YI, West T, Yarasheski K, Swarm R, Jasielec MS, Fisher JR, et al. An antidepressant decreases CSF Aβ production in healthy individuals and in transgenic AD mice. Sci Transl Med. 2014;6:236re4.
Cirrito JR, Disabato BM, Restivo JL, Verges DK, Goebel WD, Sathyan A, et al. Serotonin signaling is associated with lower amyloid-β levels and plaques in transgenic mice and humans. Proc Natl Acad Sci. 2011;108:14968–73.
Matchett BJ, Grinberg LT, Theofilas P, Murray ME. The mechanistic link between selective vulnerability of the locus coeruleus and neurodegeneration in Alzheimer’s disease. Acta Neuropathol (Berl). 2021;141:631–50.
Berry AS, Harrison TM. New perspectives on the basal forebrain cholinergic system in Alzheimer’s disease. Neurosci Biobehav Rev. 2023;150:105192.
Acknowledgements
This research was supported by the following grants: National Institutes of Health grant AG058748 (ASB), AG072328 (ASB), AG034570 (WJJ), AG062542 (WJJ), AG044292 (WJJ), DA034685 (MD), and Alzheimer’s Association Award AARF-17–530186 (ASB). Avid Radiopharmaceuticals enabled the use of the Flortaucipir tracer but did not provide direct funding and were not involved in data analysis or interpretation. MR data were collected at the Henry H. Wheeler, Jr. Brain Imaging Center, which receives support from the National Science Foundation through their Major Research Instrumentation Program, award number BCS-0821855. We thank Michael Sommerauer for generously sharing the raphe region of interest.
Author information
Authors and Affiliations
Contributions
ASB, WJJ, MD: Conceptualization, Methodology, Validation, Resources, Supervision. ASB, WJJ: Investigation, Data Curation, and Project Administration. ASB, TM, CJC, JHP, MRL: Formal Analysis. ASB and TM: Writing – Original Draft. ASB, TM, WJJ, MD, CJC, JHP, MRL: Writing – Review and Editing. TM: Visualization. ASB, WJJ, MD: Funding Acquisition.
Corresponding author
Ethics declarations
Competing interests
Dr. Jagust has served as a consultant for Biogen and Bioclinica and holds equity interest in Optoceutics. There are no other financial disclosures.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Markova, T.Z., Ciampa, C.J., Parent, J.H. et al. Poorer aging trajectories are associated with elevated serotonin synthesis capacity. Mol Psychiatry 28, 4390–4398 (2023). https://doi.org/10.1038/s41380-023-02177-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-023-02177-x
- Springer Nature Limited