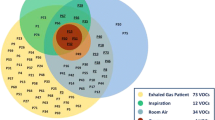
Abstract
Objective
To assess volatile organic compounds (VOCs) in breath samples collected non-invasively from preterm and full-term infants.
Methods
This was a pilot study included preterm and full-term infants who were not intubated or suspected or diagnosed with metabolic or gastrointestinal disorders. The samples were analyzed for VOCs using a selected-ion flow-tube mass spectrometer.
Results
Twenty infants were included; ten preterm and ten full-term infants. Twenty-two VOCs were detected and measurable in all samples. There was a significant difference between preterm and full-term infants for the 2-propanol, acetaldehyde, acetone, acetonitrile, benzene, ethanol, isoprene, pentane, 3-methylhexane, 2-nonene, ethane, triethylamine, and trimethylamine compounds.
Conclusion
It is feasible to measure VOCs in breath samples of preterm and full-term non-intubated infants. Full-term infants express different concentrations than preterm infants. Further studies are needed to examine the utility and reproducibility of measuring VOCs to identify neonatal diseases and predict outcomes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Metabolites are low-molecular-weight compounds that represent the ultimate end products of gene and protein expression. The global collection of metabolites, known as the metabolome, that maintains dynamic homeostasis and helps determine minute-to-minute cellular phenotype [1]. Recent advances in metabolite analysis allow for simultaneous measurement of many metabolites from biologic samples. This broad metabolic analysis can help define unique metabolic patterns and affected pathways present in specific patient groups or disease states [2]. Numerous metabolic processes in the body produce end products which are dissolved in the blood and are excreted by the lungs. Exhaled breath constitutes a complex mixture of hundreds of metabolites, volatile organic compounds (VOCs), that could potentially be used as a safe and noninvasive method of diagnostic and therapeutic monitoring. In adults, human studies showed associations of VOCs with shock, stroke, brain injury, heart failure and liver cirrhosis using breath test for metabolomics screening [3,4,5,6,7,8,9].
In infants, many studies were able to measure VOCs in stool or urine samples [10,11,12,13,14,15]. It is not known if VOCs can be collected accurately and be reflective of body metabolism from breath samples of non-intubated infants, and whether there is a difference between preterm and full-term infants. We hypothesized that VOCs, if feasible to measure, would differ among preterm and full-term infants. If proven feasible, we envision using this technology to predict, and ultimately ameliorate complications of prematurity and other neonatal conditions. Thus, we aimed in this feasibility study to assess VOCs in breath samples collected non-invasively from preterm and full-term infants.
Methods
This pilot study was conducted at the Neonatal Intensive Care Unit (NICU) at the Cleveland Clinic Children’s Hospital. The study was approved by the Cleveland Clinic’s Institute Review Board (IRB). Parental consents were obtained before any subject recruitment.
The study included newborn infants admitted to the NICU. Infants were included if they were born at preterm age (<30 weeks of gestational age (GA)). Infants were included only if they were not intubated nor receiving invasive mechanical ventilation and successfully achieved full enteral feeds for at least 24 h. Infants were excluded if they were intubated and/or supported with invasive mechanical ventilation, receiving parenteral nutrition or not on full feeds, and/or infants with suspected or diagnosed infection, metabolic or gastrointestinal disorders. A reference group of full-term infants (≥37 weeks of GA) were also included. Clinical and demographic data of enrolled infants included GA, postnatal age, race, birth weight, current weight, enteral feeding {formula or breast milk}, respiratory support {none, nasal canula (NC), continuous positive airway pressure (CPAP), high flow nasal canula (HFNC), noninvasive nasal intermittent ventilation (NIMV), or non-invasive-neurally-adjusted ventilatory assist (NIV-NAVA)}, and how much FiO2.
A fifty milliliters of exhaled breath condensate sample were collected from the expiratory side of the breathing tubes for each infant using dissolved gas analysis glass syringe (capacity 50 mL, graduated, 2 mL, tip style, needle-lock Luer, model CADG5157-1EAMKCS3025) (Sigma-Aldrich of Merck KGaA, Darmstadt, Germany). During sample collection investigators did not have a physical contact with the infant. The investigator put on full protective equipment including disposable gowns, gloves, goggles, and masks. All patients were on cardiorespiratory monitors and registered nurse and respiratory therapist bedside during sample collection. After obtaining the sample, the syringe was capped and was put in a non-collapsible box for safe transport for immediate processing and analysis. If the full-term control infant did not require respiratory support, sample was directly obtained from the ambient air inside the incubator.
The samples were analyzed for VOCs using a selected-ion flow-tube mass spectrometer (SIFT-MS), the model used was Voice 200 ultra from SYFT Technologies. The SIFT-MS instrument was run in the selected-ion mode (SIM); this is where the concentration of the VOCs is measured by monitoring the product ion peaks. The compounds monitored were 2-propanol, acetaldehyde, acetone, acetonitrile, acrylonitrile, benzene, carbon disulfide, dimethyl sulfide, ethanol, isoprene, pentane, 1-decene, 1-heptene, 1-nonene, 1-octene, 3-methylhexane, 2-nonene, ammonia, ethane, hydrogen sulfide, triethylamine and trimethylamine. They were selected for their common presence in exhaled breath. All samples were analyzed by the same investigator (DG) who was blinded to the infant’s group or clinical condition.
Statistical analysis was conducted using the Mann-Whitney U (Wilcoxon) test to compare continuous variables. The Independent Sample t-test and Monte Carlo exact test were used to compare the clinical characteristics between the two groups. All analyses were performed using commercially available software (JMP pro version 14.0.0, SAS Institute, Inc, Cary, North Carolina; IBM SPSS Statistics version 21, IBM Corp, Armonk, New York). The level of significance was set at P < 0.05.
Results
The study included twenty infants; ten preterm infants and ten full-term infants. Clinical and demographic data of enrolled infants are presented in Table 1. All preterm infants had respiratory distress and were receiving non-invasive respiratory support at time of enrollment. For the full-term infants’ group, seven infants did not require respiratory support. Preterm infants required higher FiO2 (P = 0.032). Average GA for preterm infants’ group was 25 weeks, while the average GA for the full-term infants’ group was 38 weeks, Table 1. There was no difference between the two groups based on race, 50% of preterm infants and 40% of full-term infants were White. Regarding type of enteral feeding, six preterm infants and 3 full-term infants were receiving breast milk, and the rest were receiving milk formula (P = 0.17).
VOCs were detected in all collected samples (n = 20). Twenty-two VOCs were measurable in all subjects, namely, 2-Propanol, Acetaldehyde, Acetone, Acetonitrile, Acrylonitrile, Benzene, Carbon disulfide, Dimethyl sulfide, Ethanol, Isoprene, Pentane, 1-Decene, 1-Heptene, 1-Nonene, 1-Octene, 3-Methylhexane, 2-Nonene, Ammonia, Ethane, Hydrogen sulfide, Triethylamine, Trimethylamine, Table 2. Overall, there was a significant difference between preterm and full-term infants for the 2-propanol, acetaldehyde, acetone, acetonitrile, benzene, ethanol, isoprene, pentane, 3-methylhexane, 2-nonene, ethane, triethylamine and trimethylamine compounds, Table 2. The highest concentration of VOCs in both groups were observed in 2-Propanol, Acetaldehyde and 1-Heptene. When compared to full-term, preterm infants had higher concentration of one compound, 3-Methylhexane (P = 0.04). Full-term infants had higher concentrations of twelve compounds; 2-propanol, acetaldehyde, acetone, acetonitrile, benzene, ethanol, isoprene, pentane, 2-nonene, ethane, triethylamine and trimethylamine. The rest of the VOCs (nine compounds) did not differ in concentration between the two groups.
Discussion
This pilot study demonstrated that VOCs can be detected and measured non-invasively from breath samples of preterm and full-term infants. In addition, the study showed that full-term infants express different concentrations of VOCs than preterm infants.
Measuring the VOCs from infants’ exhaled breath samples non-invasively is novel. VOCs have been traditionally measured in blood, urine and stool. However, there are limitations related to sampling availability, possibility of cross contamination, and the burden of sample’s processing [10,11,12,13,14,15]. Non-invasive breath sample has several advantages, such as being always available, could be collected at any time, multiple times a day, potential for real-time results, and avoid multiple blood sampling or obtaining urine or stool. Collecting fecal samples from newborn infants has some difficulties due to the lack of regular samples available when infants, especially preterm infants and especially if they are on parenteral nutrition, do not necessarily defecate every day. Therefore, multiple attempts were made to measure VOCs from exhaled breath. Subjects in these attempts were intubated and supported with invasive mechanical ventilation. A single-center study examined tracheal aspirates from intubated preterm infants using a sensor of electronic nose system [16]. However, one of the limitations of this device is the lack of sensitivity; and when simultaneously measuring multiple compounds, detection limits could be challenging [17]. In another study, VOCs were measured in intubated infants using the High-Performance Liquid Chromatography (HPLC) by nano-HPLC coupled to high-resolution Mass Spectroscopy (MS) [18]. Although in adults, multiple studies measured VOCs successfully in breath non-invasively, we could only find a single recent study that measured them in preterm infants non-invasively using the Gas Chromatography–Mass Spectroscopy (GC–MS) device [19]. The current study measured VOCs non-invasively in preterm and full-term infants. This study used the SIFT-MS for analysis. SIFT-MS is relatively newer compared to the GC–MS. SIFT-MS allows real-time absolute quantification of several trace gases simultaneously, even when an abundance of atmospheric gas is present [20] and that might be the reason that, in this study, the device equally detected VOCs in samples of respiratory circuit and of ambient air of the incubator. Of note, GC–MS is relatively expensive and requires highly trained operators [17].
In the current study, we intended to measure VOCs in preterm infants <30 weeks of GA, because this specific preterm population is liable to complications of prematurity and tends to adopt alternative physiological processes and metabolic pathways that might differ significantly from full-term infants. We also included a control group of full-term infants who were ≥37 weeks of GA to validate the detection and measuring processes. There was a significant difference between both groups for certain compounds, specifically, the 2-propanol, acetaldehyde, acetone, acetonitrile, benzene, ethanol, isoprene, pentane, 3-methylhexane, 2-nonene, ethane, triethylamine, and trimethylamine. All these compounds might have potential sources within the body. Isoprene is a marker of cholesterol synthesis, pentane -produced during lipid peroxidation- and ethane increase after tissue injury, ethanol is generated from bacterial metabolism, acetaldehyde is formed after the oxidation of ethanol, methane -produced from gut flora- is an indicator of carbohydrate malabsorption, acetone is produced when the body utilizes fat rather than glucose for energy, 2-propanol is produced from acetone’s reduction, hydrogen sulfide is a by-product of bacterial metabolism in the mouth, benzene and acrylonitrile usually have exogenous sources, and trimethylamine is produced by gut flora [6, 21, 22].
This study has several strengths, it showed the feasibility of detection and measuring VOCs in newborn infants from the exhaled breath using non-invasive method. Furthermore, to our knowledge, this is the first study that showed that full-term infants express different concentrations of VOCs from preterm infants. In addition, the investigator who processed the samples was blinded, therefore there was no operator bias. Nevertheless, given the pilot nature of this study, the sample size was small; that could impose a limitation when comparing groups as the study was not powered for such comparison. However, the study fulfilled its feasibility aim. Although this study was meant to test feasibility of detecting VOCs in non-intubated babies only, we recommend including controls in future studies for comparing infants with vs without breathing tubes. In addition, the following step in future studies would be to ensure reproducibility of this new technique.
In conclusion, it is feasible to measure VOCs in breath samples of non-intubated preterm and full-term infants non-invasively. Full-term infants express different concentrations of VOCs than preterm infants. Further studies are needed to examine the utility of measuring VOCs to identify and monitor neonatal diseases and predict their outcomes.
Data availability
The data that support the findings of this study are available from the corresponding author, MAAF, upon reasonable request.
References
Bujak R, Struck-Lewicka W, Markuszewski MJ, Kaliszan R. Metabolomics for laboratory diagnostics. J Pharm Biomed Anal. 2015;113:108–20.
Begou O, Gika HG, Wilson ID, Theodoridis G. Hyphenated MS-based targeted approaches in metabolomics. Analyst. 2017;142:3079–100.
Slaughter AL, Nunns GR, D’Alessandro A, Banerjee A, Hansen KC, Moore EE, et al. The metabolopathy of tissue injury, hemorrhagic shock, and resuscitation in a rat model. Shock. 2018;49:580–90.
Hu Z, Zhu Z, Cao Y, Wang L, Sun X, Dong J, et al. Rapid and sensitive differentiating ischemic and hemorrhagic strokes by dried blood spot based direct injection mass spectrometry metabolomics analysis. J Clin Lab Anal. 2016;30:823–30.
Posti JP, Dickens AM, Orešič M, Hyötyläinen T, Tenovuo O. Metabolomics profiling as a diagnostic tool in severe traumatic brain injury. Front Neurol. 2017;8:398.
Cikach FS Jr, Dweik RA. Cardiovascular biomarkers in exhaled breath. Prog Cardiovasc Dis. 2012;55:34–43.
Miller-Atkins G, Acevedo-Moreno LA, Grove D, Dweik RA, Tonelli AR, Brown JM, et al. Breath metabolomics provides an accurate and noninvasive approach for screening cirrhosis, primary, and secondary liver tumors. Hepatol Commun. 2020;4:1041–55.
Hunter GW, Xu JC, Biaggi-Labiosa AM, Laskowski D, Dutta PK, Mondal SP, et al. Smart sensor systems for human health breath monitoring applications. J Breath Res. 2011;5:037111.
Dweik RA, Amann A. Exhaled breath analysis: the new frontier in medical testing. J Breath Res. 2008;2:030301.
Fanos V, Caboni P, Corsello G, Stronati M, Gazzolo D, Noto A, et al. Urinary (1)H-NMR and GC-MS metabolomics predicts early and late onset neonatal sepsis. Early Hum Dev. 2014;90:S78–83.
Fanos V, Pintus MC, Lussu M, Atzori L, Noto A, Stronati M, et al. Urinary metabolomics of bronchopulmonary dysplasia (BPD): preliminary data at birth suggest it is a congenital disease. J Matern Fetal Neonatal Med. 2014;27:39–45. Suppl 2
Marincola FC, Dessì A, Pattumelli MG, Corbu S, Ossicini C, Ciccarelli S, et al. (1)H NMR-based urine metabolic profile of IUGR, LGA, and AGA newborns in the first week of life. Clin Chim Acta. 2015;451:28–34.
Correia GD, Wooi Ng K, Wijeyesekera A, Gala-Peralta S, Williams R, MacCarthy-Morrogh S, et al. Metabolic profiling of children undergoing surgery for congenital heart disease. Crit Care Med. 2015;43:1467–76.
El-Metwally D, Chain K, Stefanak MP, Alwis U, Blount BC, LaKind JS, et al. Urinary metabolites of volatile organic compounds of infants in the neonatal intensive care unit. Pediatr Res. 2018;83:1158–64.
Berkhout DJC, Niemarkt HJ, Benninga MA, Budding AE, van Kaam AH, Kramer BW, et al. Development of severe bronchopulmonary dysplasia is associated with alterations in fecal volatile organic compounds. Pediatr Res. 2018;83:412–9.
Rogosch T, Herrmann N, Maier RF, Domann E, Hattesohl A, Koczulla AR, et al. Detection of bloodstream infections and prediction of bronchopulmonary dysplasia in preterm neonates with an electronic nose. J Pediatr. 2014;165:622–4.
Course C, Watkins WJ, Müller CT, Odd D, Kotecha S, Chakraborty M. Volatile organic compounds as disease predictors in newborn infants: a systematic review. J Breath Res. 2021;15:024002.
Kononikhin AS, Starodubtseva NL, Chagovets VV, Ryndin AY, Burov AA, Popov IA, et al. Exhaled breath condensate analysis from intubated newborns by nano-HPLC coupled to high resolution MS. J Chromatogr B Anal Technol Biomed Life Sci. 2017;1047:97–105.
Romijn M, van Kaam AH, Fenn D, Bos LD, van den Akker CHP, Finken MJJ, et al. Exhaled volatile organic compounds for early prediction of bronchopulmonary dysplasia in infants born preterm. J Pediatr. 2023;257:113368.
Smith D, Spanel P. Selected ion flow tube mass spectrometry (SIFT-MS) for on-line trace gas analysis. Mass Spectrom Rev. 2005;24:661–700.
Risby TH, Tittel FK. Current status of midinfrared quantum and interband cascade lasers for clinical breath analysis. Opt Eng. 2010;49:1–14.
Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63.
Acknowledgements
The authors thank Julie Gamary, the NICU’s nursing manager, and all neonatology nurses. Special gratitude to Colleen Kauntz, Brittany Brooks, and all the respiratory therapists at the Cleveland Clinic Main Campus and Cleveland Clinic Fairview for their cooperation. The authors thank the patients and their parents for participation in this study.
Author information
Authors and Affiliations
Contributions
HA conceptualized and designed the study, interpreted the results, and drafted, reviewed, and revised the manuscript. MAAF edited the protocol, got the ethical and IRB approvals, educated the staff, conducted the statistical analysis, interpreted the results, and drafted, reviewed, and submitted the manuscript. MAAF and SA collected the samples. DG ran the biochemical analysis and wrote the methods. MME, JMAS, and RAD helped designing the study, interpreted the analysis, and reviewed, and revised the manuscript. All authors approved the final manuscript for submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was performed following the parents’ informed consent and according to the principles of the Declaration of Helsinki. Approval was obtained from Cleveland Clinic Institutional Review Board.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Farghaly, M.A.A., Abuelazm, S., Elgendy, M.M. et al. Volatile organic compounds in exhaled breath of newborns: a pilot study. J Perinatol (2024). https://doi.org/10.1038/s41372-024-02102-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41372-024-02102-2
- Springer Nature America, Inc.