Abstract
Objectives
To compare serum ferritin and RET-He values among extremely low gestational age neonates ELGANs with other markers of iron-deficient erythropoiesis.
Study Design
This is a secondary analysis of the NICHD Darbepoetin Trial. Study data from placebo recipients who had a serum ferritin, a RET-He, and a mean corpuscular volume (MCV) measurement within a 24-hour period were analyzed for correlation.
Results
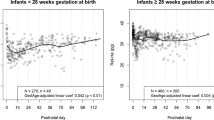
Mixed linear regression models showed no association between ferritin and RET-He at both early (β = 0.0016, p = 0.40) and late (β = −0.0001, p = 0.96) time points. Positive associations were observed between RET-He and MCV at baseline, early, and late time points (p < 0.01, =0.01, <0.001, respectively), while ferritin was not associated with MCV at any time point.
Conclusions
Our study shows that RET-He is better correlated with MCV as a marker of iron-limited erythropoiesis than ferritin. The results suggest that ferritin is limited as a marker of iron sufficiency in premature infants.
Study Identification
FDA IND Number 100138; ClinicalTrials.gov number NCT03169881; NRN ID number NICHD-NRN-0058 (Darbe).




Similar content being viewed by others
Data availability
Inquiries regarding data access can be addressed to the corresponding author.
References
Kling PJ. Iron nutrition, erythrocytes, and erythropoietin in the NICU: Erythropoietic and neuroprotective effects. NeoReviews. 2020;21:e80–8. https://doi.org/10.1542/neo.21-2-e80.
Cusick S, Georgieff M, Rao R. Approaches for reducing the risk of early-life iron deficiency-induced brain dysfunction in children. Nutrients. 2018;10:227 https://doi.org/10.3390/nu10020227.
Geng F, Mai X, Zhan J, Xu L, Zhao Z, Georgieff M, et al. Impact of fetal-neonatal iron deficiency on recognition memory at 2 months of age. J Pediatr. 2015;167:1226–32. https://doi.org/10.1016/j.jpeds.2015.08.035.
Armony-Sivan R, Eidelman AI, Lanir A, Sredni D, Yehuda S. Iron status and neurobehavioral development of premature infants. J Perinatol Off J Calif Perinat Assoc. 2004;24:757–62. https://doi.org/10.1038/sj.jp.7211178.
Amin SB, Orlando M, Wang H. Latent iron deficiency in utero is associated with abnormal auditory neural myelination in ≥ 35 weeks gestational age infants. J Pediatr. 2013;163:1267–71. https://doi.org/10.1016/j.jpeds.2013.06.020.
McArdle HJ, Gambling L, Kennedy C. Iron deficiency during pregnancy: the consequences for placental function and fetal outcome. Proc Nutr Soc. 2014;73:9–15. https://doi.org/10.1017/S0029665113003637.
Baker RD, Greer FR.Committee on Nutrition American Academy of Pediatrics Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0-3 years of age). Pediatrics. 2010;126:1040–50. https://doi.org/10.1542/peds.2010-2576.
Siddappa AM, Rao R, Long JD, Widness JA, Georgieff MK. The assessment of newborn iron stores at birth: a review of the literature and standards for ferritin concentrations. Neonatology. 2007;92:73–82. https://doi.org/10.1159/000100805.
Brugnara C, Schiller B, Moran J. Reticulocyte hemoglobin equivalent (Ret He) and assessment of iron-deficient states. Clin Lab Haematol. 2006;28:303–8. https://doi.org/10.1111/j.1365-2257.2006.00812.x.
Lorenz L, Arand J, Büchner K, Wacker-Gussmann A, Peter A, Poets CF, et al. Reticulocyte haemoglobin content as a marker of iron deficiency. Arch Dis Child Fetal Neonatal Ed. 2015;100:F198–202. https://doi.org/10.1136/archdischild-2014-306076.
Piva E, Brugnara C, Spolaore F, Plebani M. Clinical utility of reticulocyte parameters. Clin Lab Med. 2015;35:133–63. https://doi.org/10.1016/j.cll.2014.10.004.
Löfving A, Domellöf M, Hellström-Westas L, Andersson O. Reference intervals for reticulocyte hemoglobin content in healthy infants. Pediatr Res. 2018;84:657–61. https://doi.org/10.1038/s41390-018-0046-4.
Christensen RD, Henry E, Bennett ST, Yaish HM. Reference intervals for reticulocyte parameters of infants during their first 90 days after birth. J Perinatol Off J Calif Perinat Assoc. 2016;36:61–6. https://doi.org/10.1038/jp.2015.140.
Al-Ghananim RT, Nalbant D, Schmidt RL, Cress GA, Zimmerman MB, Widness JA. Reticulocyte hemoglobin content during the first month of life in critically Ill very low birth weight neonates differs from term infants, children, and adults. J Clin Lab Anal. 2016;30:326–34. https://doi.org/10.1002/jcla.21859.
Lorenz L, Peter A, Arand J, Springer F, Poets CF, Franz AR. Reticulocyte haemoglobin content declines more markedly in preterm than in term infants in the first days after birth. Neonatology. 2017;112:246–50. https://doi.org/10.1159/000477124.
Lorenz L, Peter A, Arand J, Springer F, Poets CF, Franz AR. Reference ranges of reticulocyte haemoglobin content in preterm and term infants: a retrospective analysis. Neonatology. 2017;111:189–94. https://doi.org/10.1159/000450674.
Auerbach M, Staffa SJ, Brugnara C. Using reticulocyte hemoglobin equivalent as a marker for iron deficiency and responsiveness to iron therapy. Mayo Clin Proc. 2021;96:1510–9. https://doi.org/10.1016/j.mayocp.2020.10.042.
Bahr TM, Baer VL, Ohls RK, Christensen TR, Ward DM, Bennett ST, et al. Reconciling markedly discordant values of serum ferritin versus reticulocyte hemoglobin content. J Perinatol Off J Calif Perinat Assoc. 2020. https://doi.org/10.1038/s41372-020-00845-2.
German K, Vu PT, Irvine JD, Juul SE. Trends in reticulocyte hemoglobin equivalent values in critically ill neonates, stratified by gestational age. J Perinatol Off J Calif Perinat Assoc. 2019;39:1268–74. https://doi.org/10.1038/s41372-019-0434-6.
Rysavy MA, Li L, Bell EF, Das A, Hintz SR, Stoll BJ, et al. Between-hospital variation in treatment and outcomes in extremely preterm infants. N Engl J Med. 2015;372:1801–11. https://doi.org/10.1056/NEJMoa1410689.
Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–9. https://doi.org/10.1164/ajrccm.163.7.2011060.
Kim HA, Park S-H, Lee EJ. Iron status in small for gestational age and appropriate for gestational age infants at birth. Korean J Pediatr. 2019;62:102–7. https://doi.org/10.3345/kjp.2018.06653.
Georgieff MK. Iron assessment to protect the developing brain. Am J Clin Nutr. 2017;106:1588S–1593S. https://doi.org/10.3945/ajcn.117.155846.
Camaschella C. Iron-deficiency anemia. N Engl J Med. 2015;372:1832–43. https://doi.org/10.1056/NEJMra1401038.
MacQueen BC, Christensen RD, Ward DM, Bennett ST, O’Brien EA, Sheffield MJ, et al. The iron status at birth of neonates with risk factors for developing iron deficiency: a pilot study. J Perinatol. 2017;37:436–40. https://doi.org/10.1038/jp.2016.234.
Polin RA, Abman SH, Rowitch DH, Benitz WE Fetal and neonatal physiology. 6th ed. Philadelphia: Elsevier, Inc; 2021.
Garcia-Casal MN, Pasricha S-R, Martinez RX, Lopez-Perez L, Peña-Rosas JP. Are current serum and plasma ferritin cut-offs for iron deficiency and overload accurate and reflecting iron status? A systematic review. Arch Med Res. 2018;49:405–17. https://doi.org/10.1016/j.arcmed.2018.12.005.
Delaney KM, Guillet R, Fleming RE, Ru Y, Pressman EK, Vermeylen F, et al. Umbilical cord serum ferritin concentration is inversely associated with umbilical cord hemoglobin in neonates born to adolescents carrying singletons and women carrying multiples. J Nutr. 2019;149:406–15. https://doi.org/10.1093/jn/nxy286.
Bahr TM, Christensen TR, Henry E, Wilkes J, Ohls RK, Bennett ST, et al. Neonatal reference intervals for the complete blood count parameters micror and hypo-he: sensitivity beyond the red cell indices for identifying microcytic and hypochromic disorders. J Pediatr. 2021;239:95–100.e2. https://doi.org/10.1016/j.jpeds.2021.08.002.
Bruegel M, Nagel D, Funk M, Fuhrmann P, Zander J, Teupser D. Comparison of five automated hematology analyzers in a university hospital setting: Abbott Cell-Dyn Sapphire, Beckman Coulter DxH 800, Siemens Advia 2120i, Sysmex XE-5000, and Sysmex XN-2000. Clin Chem Lab Med. 2015;53:1057–71. https://doi.org/10.1515/cclm-2014-0945.
Meintker L, Ringwald J, Rauh M, Krause SW. Comparison of automated differential blood cell counts from abbott sapphire, siemens advia 120, Beckman coulter DxH 800, and Sysmex XE-2100 in normal and pathologic samples. Am J Clin Pathol. 2013;139:641–50. https://doi.org/10.1309/AJCP7D8ECZRXGWCG.
Acknowledgements
The National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the NIH National Heart Lung and Blood Institute (NHLBI) provided grant support for the Neonatal Research Network’s Darbepoetin Trial through cooperative agreements. While NICHD staff had input into the study design, conduct, analysis, and manuscript drafting, the comments and views of the authors do not necessarily represent the views of NICHD, the National Institutes of Health, the Department of Health and Human Services, or the U.S. Government. Participating NRN sites collected data and transmitted it to RTI International, the data coordinating center (DCC) for the network, which stored, managed and analyzed the data for this study. On behalf of the NRN, RTI International had full access to all of the data in the study, and with the NRN Center Principal Investigators, takes responsibility for the integrity of the data and accuracy of the data analysis.We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study.
Funding
Funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (UG1HD087226) and by a grant from the NIH National Heart Lung and Blood Institute (U01HL136318).
Author information
Authors and Affiliations
Consortia
Contributions
TMB: collection and assembly of data, data analysis and interpretation, manuscript writing, editing and final approval of manuscript. ST, ES, AD: collection and assembly of data, editing and final approval of manuscript. data analysis and interpretation, editing and final approval of manuscript. SSB, KRS, CAG, JRL, EFB, ARL, SS, DPC, CR, JF, KZ, MCW: conception and design, data interpretation, manuscript writing, editing and final approval of manuscript. RDC, MCB, RKO: conception and design, collection and assembly of data, manuscript writing, data analysis and interpretation, editing and final approval of manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bahr, T.M., Tan, S., Smith, E. et al. Serum ferritin values in neonates <29 weeks’ gestation are highly variable and do not correlate with reticulocyte hemoglobin content. J Perinatol 43, 1368–1373 (2023). https://doi.org/10.1038/s41372-023-01751-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-023-01751-z
- Springer Nature America, Inc.
This article is cited by
-
Hyperferritinemia among very-low-birthweight infants in Thailand: a prospective cohort study
Journal of Perinatology (2023)