Abstract
Objective
We analyze phototherapy rates after implementation of a Hyperbilirubinemia Clinical Pathway (HCP), which placed full-term ABOi DAT negative newborns on the low risk phototherapy nomogram, rather than medium risk, as previously done.
Study design
A chart review was performed for ABOi newborns born ≥36 weeks gestation between January 2020 and October 2021. Primary outcome measures were rates of phototherapy across pre- and post-intervention groups and among DAT negative newborns.
Results
There was an increased proportion of newborns assigned to the low risk curve after the intervention. There were no significant differences in phototherapy rates among the intervention groups, although there was a non-significant decrease in phototherapy rates among DAT negative newborns after the intervention. There were no increases in adverse outcomes.
Conclusions
Providers adhered to the guidelines after implementation of the HCP. ABOi DAT negative newborns can be viewed as low risk for hyperbilirubinemia requiring phototherapy.
Similar content being viewed by others
Introduction
Neonatal hyperbilirubinemia is defined as a total serum bilirubin above 5 mg/dL (86 umol/L) during the newborn period [1], with 60% of newborns experiencing this condition [2]. Complications, such as kernicterus, are extremely rare [2], and universal bilirubin screening among hospitals has decreased the prevalence of severe hyperbilirubinemia and potential complications [3].
Hemolytic disease of the newborn (HDN), a pathologic etiology of neonatal hyperbilirubinemia, is frequently due to blood antigen incompatibility between the pregnant person and fetus (e.g., ABO or Rh). ABO incompatibility (ABOi) occurs in ~20% of pregnancies, with only about 1% of these newborns developing HDN [2]. This low rate may be due to decreased anti-ABO antibody transport across the placenta due to these antibodies being predominantly IgM class [4]. It may also be due to decreased development of A and B antigens on fetal red blood cells [4]. Parameters used to identify and diagnose HDN vary, but the diagnosis is suggested with rapidly progressive hyperbilirubinemia and/or neonatal anemia [4, 5]. A proposed method of detecting those who are ABOi and at increased risk of HDN has been the direct antiglobulin (Coomb’s) test (DAT), a screening immunoassay used to determine presence of maternal antibodies on the neonate’s red blood cells [6]. Data on the utility of DAT screening varies, which may lead to overuse of phototherapy for patients at lower risk of HDN [6,7,8,9].
Despite the guidelines via the American Academy of Pediatrics (AAP) in 2004 and 2009 [2, 10], there has been wide variation in hospital practice related to hyperbilirubinemia [11]. Hospital wide clinical practice guidelines for hyperbilirubinemia have been shown to improve outcomes related to unnecessary phototherapy and hospital costs [12, 13]. The 2004 AAP guidelines presented risk factors associated with increased neurotoxicity risk related to hyperbilirubinemia [2], which was further clarified in the 2009 update [10]. Isoimmune hemolytic disease is considered a neurotoxicity risk factor, but as previously stated, definitions may vary, and not all ABOi neonates exhibit hemolytic disease [2, 10]. In 2022, the AAP published another update to prior guidelines for the management and prevention of hyperbilirubinemia in the newborn [14]. In this update, the authors clearly define parameters that may indicate hemolysis, including a rapid rate (>0.3 mg/dL/h) of increase in the serum or transcutaneous bilirubin (TcB) in the first 24 h or >0.2 mg/dL/h thereafter [14]. They also propose that the DAT helps identify infants at risk for hyperbilirubinemia attributable to hemolysis and identify a positive direct antiglobulin test as a neurotoxicity risk factor [14]. Infants that are DAT-negative may be managed with usual care per these updated guidelines [14].
In our hospital, a large community-based academic hospital in the northeastern United States, a Hyperbilirubinemia Clinical Pathway (HCP) was implemented by physicians in the Newborn Nursery in January 2021, aimed at standardizing hyperbilirubinemia care among ABOi newborns. Specifically within this guideline, in attempt to decrease unnecessary phototherapy, full-term newborns that were ABOi and DAT negative were placed on the low risk curve (LRC) on the phototherapy nomogram [2, 10, 15] given likely lower risk of HDN [16]. We performed a retrospective single-center cohort study for ABO-incompatible newborns and compared rates of phototherapy before and after implementation of this guideline in the newborn nursery. We then compared rates of phototherapy among DAT negative newborns.
Methods
Context
Within our community hospital, there are ~2800–2900 newborns each year with phototherapy rates approximating 4% among all newborns. Prior to the implementation of our HCP, our department’s practice was based on the AAP guidelines [2, 10] as well as guidelines from surrounding hospitals. Providers were placing all newborns with ABOi, regardless of DAT status, on the medium risk curve (MRC) on the phototherapy nomogram. Of note, in our hospital, we obtain blood type and DAT status on all newborns born to mothers with blood type O or Rh-negative status. The new clinical guideline, started in January 2021 (Supplementary Fig. 1), implemented the following interventions: newborns that were ABOi and DAT negative will be placed on the LRC (previously placed on MRC) on the phototherapy nomogram if full term (≥38 weeks gestational age) [2, 10, 15], newborns that undergo phototherapy will be assessed immediately for need for supplementation, phototherapy will be discontinued when the total bilirubin is at least 2–3 mg/dL below the light level [17,18,19], and rebound bilirubin level will be obtained only if the newborn is deemed at high risk for continued phototherapy or exhibiting a poor response to phototherapy [20].
Study design
We performed a retrospective chart review for newborns between January 2020 and October 2021. The study population included newborns in the nursery that had ABOi. Patients who were transferred or immediately admitted to the NICU within 24 h of life, unless for severe hyperbilirubinemia, were excluded, given difference in hyperbilirubinemia guidelines. Patients who are born <36 weeks gestation are admitted directly to the NICU within our hospital, and were excluded. Patients were identified via EPIC queries for maternal blood type, extracting data for mothers with blood type O. Infant blood type was then manually verified and a manual chart review was then performed to extract data if ABOi was identified. Risk curve assignment, assigned by providers for each patient on the phototherapy nomogram, was obtained by review of admission and progress notes within the electronic medical record. Patient data was then de-identified and recorded in Excel. This study was determined to not be human subjects research and exempt by our Institutional Review Board under the policies and procedures of the Human Research Protection Program.
Measures
Patient demographic and baseline information included: sex, gestational age at birth, delivery type, birth weight, and infant blood type, including DAT status. The DAT is routinely performed in cord blood samples for all ABOi newborns at our hospital. DAT status was obtained via review of infant laboratory results.
The primary outcomes were: (1) risk curve assignments on the phototherapy nomogram used in the 2004 AAP guidelines [2] and (2) rates of phototherapy across intervention groups and among DAT negative newborns. If risk curve assignments were changed by providers during hospitalization due to high risk bilirubins and concern for isoimmune hemolytic disease, the final assignment was recorded.
Secondary outcome measures included length of stay, 7-day re-admission rate related to hyperbilirubinemia, nadir weight decrease during hospitalization, feeding method at discharge, number of bilirubin laboratory tests, bilirubin at 12 and 24 h of life. Bilirubin levels were initially measured as TcB at 12 and 24 h of life and confirmed with serum bilirubin (TsB) level if above light level, which is standard practice within our hospital for all ABOi newborns. If both TcB and TsB were reported at 12 or 24 h of life, TsB was used in analysis. The length of stay was calculated as the difference between admission electronic time stamp and discharge time stamp from the hospital.
Statistical analysis
Patient baseline/demographic and treatment characteristics distributions were summarized using counts and frequencies for categorical data and median (min–max) for continuous data. Differences in patient characteristics were assessed using the Fisher’s Exact test (categorical) or the Mann-Whitney-Wilcoxon test (continuous) where appropriate. All p values are based on a two-sided hypothesis with significance testing at a 0.05 level. R version 4.0 was used for all analyses.
Results
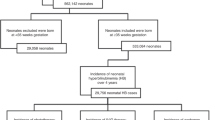
In our initial EPIC query, 573 newborns were found to have ABOi at 36 weeks gestational age or above between January 2020 and October 2021 (Fig. 1). Of these, 31 newborns were excluded due to immediate admission or transfer to the NICU within 24 h of life for reasons other than hyperbilirubinemia. Our final sample size included 542 total ABOi newborns born during our analyzed time period. Of these, 279 newborns were pre-guideline implementation (January 2020–December 2020) and 263 newborns were post-guideline implementation (January 2021–October 2021). There were no significant differences among baseline characteristics between all newborns across intervention groups, except for birth weight (pre-intervention median: 3365 g; post-intervention median 3285 g; p = 0.043) (Table 1). There were no significant differences among baseline characteristics among ABOi DAT negative newborns across intervention groups (Table 2).
Primary outcomes
A significantly higher proportion of newborns were assigned to the LRC on the phototherapy nomogram after the HCP implementation (pre/post-intervention non-LRC: 91.4% vs 35.4% respectively, p < 0.0001; pre/post-intervention LRC: 8.6% vs 64.6% respectively, p < 0.0001) (Table 1). There were also a significantly higher proportion of DAT negative newborns assigned to the LRC after the HCP implementation (pre/post intervention non-LRC: 89.9% vs 28.2%, respectively, p < 0.0001; pre/post-intervention LRC: 10.1% vs 71.8%, respectively, p < 0.0001) (Table 2). There were no significant differences among the intervention groups for rates of phototherapy (24.0 % vs 19.4%, respectively; p = 0.212), including among DAT negative newborns (pre/post-intervention: 22.3% vs 15.8%; p = 0.08) (Fig. 2).
Secondary outcomes
There were no significant differences among secondary outcomes between intervention groups for all newborns (Supplementary Table 1), as well as for DAT negative newborns (Supplementary Table 2). Within the entire pre-intervention group there were 10 newborns with missing bilirubin levels either 12 or 24 h of life. Within the entire post-intervention group, there were 11 newborns with missing bilirubin levels at either 12 or 24 h of life. These newborns were excluded from calculations involving bilirubin levels.
Discussion
After implementation of the HCP, providers generally adhered to the new guidelines as shown by the significant increase in LRC assignment on the phototherapy nomogram in the post-intervention group, for all ABOi newborns and for ABOi DAT negative newborns (Tables 1 and 2, respectively). With the increased LRC assignment among ABOi DAT negative newborns, there was a decreased rate of phototherapy after the intervention that did not reach significance (Fig. 2). There were reassuringly no changes in length of stay and re-admission rates for phototherapy among DAT negative newborns after the intervention (Supplementary Table 2). This supports prior findings that ABOi DAT negative newborns are likely at a low risk of HDN due to isoimmune hemolytic disease [16, 21]. Moreover, although risk curve assignment depends on gestational age (e.g., newborns with gestational age <38 weeks and no risk factors are placed on the MRC), there were no significant changes between the pre- and post-intervention groups among DAT negative newborns in regards to gestational age.
While our overall rates of phototherapy among ABOi DAT negative newborns were higher than baseline rates of phototherapy in all newborns at our hospital (~4% and 5.8% during our pre- and post-intervention period, respectively), there was no increased incidence of adverse outcomes among our patient population after placing these newborns on the LRC. Interestingly, there are highly variable rates of phototherapy among ABOi DAT negative newborns in the literature [22,23,24]. Our phototherapy rates among ABOi DAT negative newborns may be elevated due to subthreshold phototherapy initiation among providers [25], providers not adhering to the HCP, or other causes of hemolysis [16] (e.g., G6PD deficiency, in which testing may be negative during acute hemolytic episode) [26]. For example, in the post-intervention group, there were 6 ABOi DAT negative newborns that were incorrectly placed on the MRC and received phototherapy. Moreover, there were 7 ABOi DAT negative newborns with low risk 12 and 24 h of life bilirubins that received phototherapy. While this would still not account for higher than baseline rates, our study indicates the safety of treating ABOi DAT negative newborns as low risk for hyperbilirubinemia requiring phototherapy without an increase in adverse events.
With the release of the new hyperbilirubinemia guidelines by the AAP in 2022 [10], close attention to neurotoxicity risk factors is crucial. Our study supports the guidance put forth by the AAP in that ABOi DAT negative newborns may be viewed as low risk and managed with routine care. If hemolysis is suspected due to phototherapy initiation within the first 24 h of life or rapid rate of increase of bilirubin, only then should the patient be treated as having hemolytic disease and considered higher risk. Providers should then undertake further work up.
There are several limitations to our study. Our study is a single-center cohort study, which limits generalizability. We also excluded newborns that were initially admitted to the NICU for reasons other than hyperbilirubinemia, including <36 weeks gestational age. These newborns may have needed phototherapy during their hospitalization and would not be captured. While a decrease in phototherapy was observed among pre- and post-intervention ABOi DAT negative newborns, our study may have been underpowered to detect a statistically significant difference. Lastly, the time period when this study took place was during the COVID-19 pandemic, which may have impacted factors not accounted for in our analyses.
Our study shows that newborns with ABOi and a negative DAT can be safely viewed as low risk without any subsequent increase in rates of phototherapy, re-admission rates, or lengths of stay. Our findings support the new AAP guidelines recently released stating that if DAT is obtained and negative, these patients can be managed with routine care. Larger follow up studies are needed and should address further identification of risk factors contributing to hyperbilirubinemia requiring phototherapy.
Data availability
The dataset generated and analyzed during the study are available from the corresponding author on reasonable request.
References
Porter ML, Dennis BL. Hyperbilirubinemia in the term newborn. Am Fam Phys. 2002;65:599–606.
Subcommittee on Hyperbilirubinemia. Management of Hyperbilirubinemia in the Newborn Infant 35 or More Weeks of Gestation. Pediatrics. 2004;114:297–316.
Kuzniewicz MW, Escobar GJ, Newman TB. Impact of universal bilirubin screening on severe hyperbilirubinemia and phototherapy use. Pediatrics. 2009;124:1031–9.
Delaney M, Matthews DC. Hemolytic disease of the fetus and newborn: managing the mother, fetus, and newborn. Hematol Am Soc Hematol Educ Program. 2015;2015:146–51.
Murray NA, Roberts IA. Haemolytic disease of the newborn. Arch Dis Child Fetal Neonatal Ed. 2007;92:F83–8.
Valsami S, Politou M, Boutsikou Τ, Boutsikou T, Briana D, Paptesta M, et al. Importance of Direct Antiglobulin Test (DAT) in Cord Blood: Causes of DAT (+) in a Cohort Study. Pediatr Neonatol. 2015;56:256–60.
Dinesh D. Review of positive direct antiglobulin tests found on cord blood sampling. J Paediatr Child Health. 2005;41:504–7.
Keir A, Agpalo M, Lieberman L, Callum J. How to use: the direct antiglobulin test in newborns. Arch Dis Child Educ Pr Ed. 2015;100:198–203.
Özgönenel B, Kukreja G, O’Malley B, Bluth MH. Neonatal BO Incompatibility Is Associated With a Positive Cord Blood Direct Antiglobulin Test in Infants of Black Ethnicity. J Pediatr Hematol Oncol. 2015;37:e453–7.
Maisels MJ, Bhutani VK, Bogen D, Newman TB, Stark AR, Watchko JF, et al. Hyperbilirubinemia in the Newborn Infant ≥35 Weeks’ Gestation: An Update With Clarifications. Pediatrics. 2009;124:1193–8.
DePorre AG, Hall M, Puls HT, Daly A, Gay JC, Bettenhausen JL, et al. Variation in Care and Clinical Outcomes Among Infants Hospitalized With Hyperbilirubinemia. Hosp Pediatr. 2020;10:844–50.
Romero HM, Ringer C, Leu MG, Beardsley E, Kelly K, Fesinmeyer MD, et al. Neonatal jaundice: improved quality and cost savings after implementation of a standard pathway. Pediatrics. 2018;141:e20161472.
Preloger E, Wedoff M, Lemke JT, Pan A, Nelson A. Decreasing Laboratory Testing for Neonatal Jaundice Through Revision of a Clinical Practice Pathway. Hosp Pediatr. 2022;12:e67–e72.
Kemper AR, Newman TB, Slaughter JL, Maisels MJ, Watchko JF, Downs SM, et al. Clinical Practice Guideline Revision: Management of Hyperbilirubinemia in the Newborn Infant 35 or More Weeks of Gestation. Pediatrics. 2022;150:e2022058859.
Bhutani VK, Gourley GR, Adler S, Kreamer B, Dalin C, Johnson LH. Noninvasive measurement of total serum bilirubin in a multiracial predischarge newborn population to assess the risk of severe hyperbilirubinemia. Pediatrics 2000;106:E17 https://doi.org/10.1542/peds.106.2.e17.
Herschel M, Karrison T, Wen M, Caldarelli L, Baron B. Isoimmunization Is Unlikely to Be the Cause of Hemolysis in ABO-Incompatible but Direct Antiglobulin Test-Negative Neonates. Pediatrics. 2002;110:127–30.
Bhutani VK, Stark AR, Lazzeroni LC, Poland R, Gourley GR, Kazmierczak S, et al. Predischarge screening for severe neonatal hyperbilirubinemia identifies infants who need phototherapy. J Pediatr. 2013;162:477–82.
Keren R, Luan X, Friedman S, Saddlemire S, Cnaan A, Bhutani VK. A comparison of alternative risk-assessment strategies for predicting significant neonatal hyperbilirubinemia in term and near- term infants. Pediatrics. 2008;121:e170–e179.
Kuzniewicz MW, Park J, Niki H, Walsh EM, McCulloch CE, Newman TB. Predicting the Need for Phototherapy After Discharge. Pediatrics. 2021;147:e2020019778.
Chang PW, Kuzniewicz MW, McCulloch CE, Newman TB. A Clinical Prediction Rule for Rebound Hyperbilirubinemia Following Inpatient Phototherapy. Pediatrics. 2017;139:e20162896.
Schutzman DL, Sekhon R, Hundalani S. Hour-specific bilirubin nomogram in infants with ABO incompatibility and direct Coombs-positive results. Arch Pediatr Adolesc Med. 2010;164:1158–64.
Peeters B, Geerts I, Van Mullem M, Micalessi I, Saegeman V, Moerman J. Post-test probability for neonatal hyperbilirubinemia based on umbilical cord blood bilirubin, direct antiglobulin test, and ABO compatibility results. Eur J Pediatr. 2016;175:651–7.
Alshammari S, Alqashami A, Alhumud S, Aladadh M, Alsaif S, Ali K. Neonatal ABO incompatibility, influence of blood group, and coomb’s test on outcome. J Clin Neonatol. 2022;11:212–8.
Ozolek JA, Watchko JF, Mimouni F. Prevalence and lack of clinical significance of blood group incompatibility in mothers with blood type A or B. J Pediatr. 1994;125:87–91.
Wickremasinghe AC, Kuzniewicz MW, McCulloch CE, Newman TB. Efficacy of Subthreshold Newborn Phototherapy During the Birth Hospitalization in Preventing Readmission for Phototherapy. JAMA Pediatrics. 2018;172:378–85.
Frank JE. Diagnosis and management of G6PD deficiency. Am Fam Phys. 2005;72:1277–82.
Acknowledgements
The authors thank Elizabeth Woodard, NP, Ginny Combs, MSN, Cheryl Slater, MSN and Jennifer Pfau, MD, for their assistance with implementation of the Hyperbilirubinemia Clinical Pathway at Boston Medical Center.
Funding
This work was supported by a Fred Lovejoy Resident Research and Education Award and the Urban Health and Advocacy Track Grant.
Author information
Authors and Affiliations
Contributions
Dr JMG conceptualized and designed the study, collected data, drafted the paper and revised the paper. Dr EMA and Mr ST collected data, and reviewed and revised the paper. Mr BMB designed the data collection instruments, carried out analyses, and reviewed and revised the paper. Dr BS designed the study, and reviewed and revised the paper. Dr TG conceptualized and designed the study, coordinated and supervised data collection, and critically reviewed the paper. All authors approved the final paper as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gabbay, J.M., Agneta, E.M., Turkington, S. et al. Rates of phototherapy among ABO-incompatible newborns with a negative direct antiglobulin test. J Perinatol 43, 1357–1362 (2023). https://doi.org/10.1038/s41372-023-01650-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-023-01650-3
- Springer Nature America, Inc.