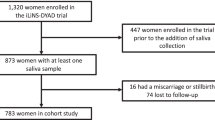
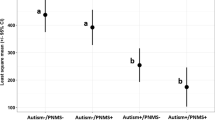
Abstract
Objective
To determine whether prenatal sex hormones from maternal saliva are associated with birth-weight-for-gestational age.
Study design
We measured salivary progesterone, testosterone, estradiol, dehydroepiandrosterone (DHEA), and cortisone in 504 pregnant women in a Mexico City cohort. We performed linear and modified Poisson regression to examine associations of log-transformed hormones with birth-weight-for-gestational age z-scores and the risk of small-for-gestational age (SGA) and large-for-gestational age (LGA) adjusting for maternal age, sex, BMI, parity, smoking, education, and socioeconomic status.
Results
In total, 15% of infants were SGA and 2% were LGA. Each interquartile range increment in testosterone/estradiol ratio was associated with a 0.12 decrement in birth-weight-for-gestational age z-score (95% CI: −0.27 to −0.02) and a 50% higher risk of SGA versus appropriate-for-gestational age (AGA) (95% CI: 1.13–1.99).
Conclusion
Higher salivary testosterone/estradiol ratios may affect fetal growth, and identifying the predictors of hormone levels may be important to optimizing fetal growth.

Similar content being viewed by others
References
Pallotto EK, Kilbride HW. Perinatal outcome and later implications of intrauterine growth restriction. Clin Obstet Gynecol. 2006;49:257–69.
Larroque B, Bertrais S, Czernichow P, Léger J. School difficulties in 20-year-olds who were born small for gestational age at term in a regional cohort study. Pediatrics. 2001;108:111–5.
Strauss RS. Adult functional outcome of those born small for gestational age: twenty-six-year follow-up of the 1970 British Birth Cohort. JAMA. 2000;283:625–32.
Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115:e290–296.
Clausson B, Cnattingius S, Axelsson O. Preterm and term births of small for gestational age infants: a population-based study of risk factors among nulliparous women. Br J Obstet Gynaecol. 1998;105:1011–7.
Jolly MC, Sebire NJ, Harris JP, Regan L, Robinson S. Risk factors for macrosomia and its clinical consequences: a study of 350,311 pregnancies. Eur J Obstet Gynecol Reprod Biol. 2003;111:9–14.
Surkan PJ, Hsieh C-C, Johansson ALV, Dickman PW, Cnattingius S. Reasons for increasing trends in large for gestational age births. Obstet Gynecol. 2004;104:720–6.
Fowden A, Forhead A. Endocrine mechanisms of intrauterine programming. Reproduction. 2004;127:515–26.
Fowden AL, Forhead AJ, Sferruzzi-Perri AN, Burton GJ, Vaughan OR. Review: endocrine regulation of placental phenotype. Placenta. 2015;36(Suppl 1):S50–59.
Hampson E, Phillips SD, Soares CN, Steiner M. Steroid concentrations in antepartum and postpartum saliva: normative values in women and correlations with serum. Biol Sex Differ. 2013;4:7.
Darne J, McGarrigle HH, Lachelin GC. Saliva oestriol, oestradiol, oestrone and progesterone levels in pregnancy: spontaneous labour at term is preceded by a rise in the saliva oestriol:progesterone ratio. Br J Obstet Gynaecol. 1987;94:227–35.
Hakim C, Padmanabhan V, Vyas AK. Gestational hyperandrogenism in developmental programming. Endocrinology. 2017;158:199–212.
Makieva S, Saunders PT, Norman JE. Androgens in pregnancy: roles in parturition. Hum Reprod Update. 2014;20:542–59.
Manikkam M, Crespi EJ, Doop DD, Herkimer C, Lee JS, Yu S, et al. Fetal programming: prenatal testosterone excess leads to fetal growth retardation and postnatal catch-up growth in sheep. Endocrinology. 2004;145:790–8.
Wolf CJ, Hotchkiss A, Ostby JS, LeBlanc GA, Gray JLE. Effects of prenatal testosterone propionate on the sexual development of male and female rats: a dose-response study. Toxicol Sci. 2002;65:71–86.
Herman RA, Jones B, Mann DR, Wallen K. Timing of prenatal androgen exposure: anatomical and endocrine effects on juvenile male and female rhesus monkeys. Horm Behav. 2000;38:52–66.
Carlsen SM, Jacobsen G, Romundstad P. Maternal testosterone levels during pregnancy are associated with offspring size at birth. Eur J Endocrinol. 2006;155:365–70.
Voegtline KM, Costigan KA, Kivlighan KT, Henderson JL, DiPietro JA. Sex-specific associations of maternal prenatal testosterone levels with birth weight and weight gain in infancy. J Dev Orig health Dis. 2013;4:280–4.
Nordman H, Voutilainen R, Antikainen L, Jaaskelainen J. Prepubertal children born large for gestational age have lower serum DHEAS concentrations than those with a lower birth weight. Pedia Res. 2017;82:285–9.
Hartwig IR, Pincus MK, Diemert A, Hecher K, Arck PC. Sex-specific effect of first-trimester maternal progesterone on birthweight. Hum Reprod. 2013;28:77–86.
Mucci LA, Lagiou P, Tamimi RM, Hsieh C-C, Adami H-O, Trichopoulos D. Pregnancy estriol, estradiol, progesterone and prolactin in relation to birth weight and other birth size variables (United States). Cancer Causes Control. 2003;14:311–8.
Lagiou P, Samoli E, Hsieh CC, Lagiou A, Xu B, Yu GP, et al. Maternal and cord blood hormones in relation to birth size. Eur J Epidemiol. 2014;29:343–51.
Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pedia. 2013;13:59.
Braun JM, Wright RJ, Just AC, Power MC, Tamayo YOM, Schnaas L, et al. Relationships between lead biomarkers and diurnal salivary cortisol indices in pregnant women from Mexico City: a cross-sectional study. Environ health : a Glob access Sci source. 2014;13:50.
Burris HH, Braun JM, Byun HM, Tarantini L, Mercado A, Wright RJ, et al. Association between birth weight and DNA methylation of IGF2, glucocorticoid receptor and repetitive elements LINE-1 and Alu. Epigenomics. 2013;5:271–81.
Wilcox RR. Estimating measure of location and scale. In: Wilcox RR, editor. Introduction to robust estimation and hypothesis testing. Waltham, Elsevier Inc; 2012, p. 43–101.
Capurro H, Konichezky S, Fonseca D, Caldeyro-Barcia R. A simplified method for diagnosis of gestational age in the newborn infant. J Pedia. 1978;93:120–2.
Sanders AP, Burris HH, Just AC, Motta V, Svensson K, Mercado-Garcia A, et al. microRNA expression in the cervix during pregnancy is associated with length of gestation. Epigenetics. 2015;10:221–8.
Institute of Medicine National Research Council Committee to Reexamine IOMPWG. The National Academies Collection: reports funded by National Institutes of Health. In: Rasmussen KM, Yaktine AL, editors. Weight gain during pregnancy: reexamining the guidelines. Washington (DC), National Academies Press (US) National Academy of Sciences; Washington (DC), 2009, p. 241–62.
Carrasco A. The Amai system of classifying households by socio-economic level: the experience of Mexico and its comparison with Brazil and Argentina. ESOMAR, Latin American Conference, Sao Paolo, May 2002.
Ward C, Lewis S, Coleman T. Prevalence of maternal smoking and environmental tobacco smoke exposure during pregnancy and impact on birth weight: retrospective study using Millennium Cohort. BMC Public Health. 2007;7:81.
Sir-Petermann T, Hitchsfeld C, Maliqueo M, Codner E, Echiburu B, Gazitua R, et al. Birth weight in offspring of mothers with polycystic ovarian syndrome. Hum Reprod. 2005;20:2122–6.
Salamalekis E, Bakas P, Vitoratos N, Eleptheriadis M, Creatsas G. Androgen levels in the third trimester of pregnancy in patients with preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2006;126:16–9.
McCowan L, Horgan RP. Risk factors for small for gestational age infants. Best Pract Res Clin Obstet Gynaecol. 2009;23:779–93.
Perez-Sepulveda A, Monteiro LJ, Dobierzewska A, Espana-Perrot PP, Venegas-Araneda P, Guzman-Rojas AM, et al. Placental aromatase is deficient in placental ischemia and preeclampsia. PLoS One. 2015;10:e0139682.
Sir-Petermann T, Maliqueo M, Angel B, Lara HE, Perez-Bravo F, Recabarren SE. Maternal serum androgens in pregnant women with polycystic ovarian syndrome: possible implications in prenatal androgenization. Hum Reprod. 2002;17:2573–9.
Sathishkumar K, Elkins R, Chinnathambi V, Gao H, Hankins GD, Yallampalli C. Prenatal testosterone-induced fetal growth restriction is associated with down-regulation of rat placental amino acid transport. Reprod Biol Endocrinol: RBE. 2011;9:110.
Sun M, Maliqueo M, Benrick A, Johansson J, Shao R, Hou L, et al. Maternal androgen excess reduces placental and fetal weights, increases placental steroidogenesis, and leads to long-term health effects in their female offspring. Am J Physiol Endocrinol Metab. 2012;303:E1373–1385.
Craig ZR, Wang W, Flaws JA. Endocrine-disrupting chemicals in ovarian function: effects on steroidogenesis, metabolism and nuclear receptor signaling. Reproduction. 2011;142:633–46.
Deyssenroth MA, Gennings C, Liu SH, Peng S, Hao K, Lambertini L, et al. Intrauterine multi-metal exposure is associated with reduced fetal growth through modulation of the placental gene network. Environ Int. 2018;120:373–81.
Everson TM, Kappil M, Hao K, Jackson BP, Punshon T, Karagas MR, et al. Maternal exposure to selenium and cadmium, fetal growth, and placental expression of steroidogenic and apoptotic genes. Environ Res. 2017;158:233–44.
Chen L, Chen X, Chen X, Hu Z, Li X, Su Y, et al. Ziram inhibits aromatase activity in human placenta and JEG-3 cell line. Steroids. 2017;128:114–9.
Veiga-Lopez A, Kannan K, Liao C, Ye W, Domino SE, Padmanabhan V. Gender-specific effects on gestational length and birth weight by early pregnancy BPA exposure. J Clin Endocrinol Metab. 2015;100:E1394–1403.
He S, Allen JC Jr., Malhotra R, Ostbye T, Tan TC. Association of maternal serum progesterone in early pregnancy with low birth weight and other adverse pregnancy outcomes. J Matern-fetal neonatal Med : Off J Eur Assoc Perinat Med, Fed Asia Ocean Perinat Soc, Int Soc Perinat Obstet. 2016;29:1999–2004.
Meuwese CL, Euser AM, Ballieux BE, van Vliet HA, Finken MJ, Walther FJ, et al. Growth-restricted preterm newborns are predisposed to functional adrenal hyperandrogenism in adult life. Eur J Endocrinol. 2010;163:681–9.
Rode L, Langhoff-Roos J, Andersson C, Dinesen J, Hammerum MS, Mohapeloa H, et al. Systematic review of progesterone for the prevention of preterm birth in singleton pregnancies. Acta Obstet Gynecol Scand. 2009;88:1180–9.
Vanston C, Watson NV, Zava DT. Sex steroid monitoring during pregnancy using a saliva test. Seattle: Nurse Practitioners in Women’s Health 11th Annual Premiere Women’s Health Conference, Seattle, October 15–18, 2008.
Potischman N, Troisi R, Thadhani R, Hoover RN, Dodd K, Davis WW, et al. Pregnancy hormone concentrations across ethnic groups: implications for later cancer risk. Cancer Epidemiol, Biomark Prev: a Publ Am Assoc Cancer Res, cosponsored Am Soc Prev Oncol. 2005;14:1514–20.
Sferruzzi-Perri AN, Sandovici I, Constancia M, Fowden AL. Placental phenotype and the insulin-like growth factors: resource allocation to fetal growth. J Physiol. 2017;595:5057–93.
Acknowledgements
This work was supported by NIH grants R01 ES014930, R01 ES026033, R01 ES021357, R24 ES028522, R01 ES013744, R01 ES023450, P30 ES023515, K99ES027508, K23ES024803, and K23ES022242. Co-investigators and staff at the INSP were also supported and received partial funding from the National Institute of Public Health/Ministry of Health of Mexico. We acknowledge Dr. Clemens Kirschbaum for performing the steroid hormone panel on the saliva samples. We acknowledge the American British Cowdray Medical Center for providing research facilities, which made it possible to conduct the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Svensson, K., Just, A.C., Fleisch, A.F. et al. Prenatal salivary sex hormone levels and birth-weight-for-gestational age. J Perinatol 39, 941–948 (2019). https://doi.org/10.1038/s41372-019-0385-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-019-0385-y
- Springer Nature America, Inc.
This article is cited by
-
Associations of salivary aldosterone levels during pregnancy with maternal blood pressure and birth weight-for-gestational age in a Mexico City birth cohort
Journal of Perinatology (2024)
-
Association between prenatal androgens and cord blood androgens, a path analysis
Scientific Reports (2023)
-
Association between foetal sex and adverse neonatal outcomes in women with gestational diabetes
Archives of Gynecology and Obstetrics (2023)