Abstract
Background/Objectives
Alteration of the perinatal nutritional environment is an important risk factor for the development of metabolic diseases in later life. The hormone leptin plays a critical role in growth and development. Previous studies reported that postnatal overnutrition increases leptin secretion during the pre-weaning period. However, a direct link between leptin, neonatal overnutrition, and lifelong metabolic regulation has not been investigated.
Methods
We used the small litter mouse model combined with neonatal leptin antagonist injections to examine whether attenuating leptin during early life improves lifelong metabolic regulation in postnatally overnourished mice.
Results
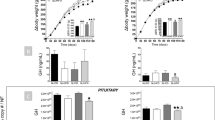
Postnatally overnourished mice displayed rapid weight gain during lactation and remained overweight as adults. These mice also showed increased adiposity and perturbations in glucose homeostasis in adulthood. Neonatal administration of a leptin antagonist normalized fat mass and insulin sensitivity in postnatally overnourished mice. These metabolic improvements were associated with enhanced sensitivity of hypothalamic neurons to leptin.
Conclusions
Early postnatal overnutrition causes metabolic alterations that can be permanently attenuated with the administration of a leptin antagonist during a restricted developmental window.




Similar content being viewed by others
References
Rogers I. The influence of birthweight and intrauterine environment on adiposity and fat distribution in later life. Int J Obes Rel Metabol Disord. 2003;27:755–77.
Cruz ML, Shaibi GQ, Weigensberg MJ, Spruijt-Metz D, Ball GDC, Goran MI. Pediatric obesity and insulin resistance: chronic disease risk and implications for treatment and prevention beyond body weight modification. Ann Rev Nutr. 2005;25:435–68.
Plagemann A. Perinatal nutrition and hormone-dependent programming of food intake. Hormone Res. 2006;65:83–89.
McMillen IC, Adam CL, Muhlhausler BS. Early origins of obesity: programming the appetite regulatory system. J Physiol. 2005;565:9–17.
Taylor PD, Poston L. Developmental programming of obesity in mammals. Exp Physiol. 2007;92:287–98.
Martin-Gronert MS, Ozanne SE. Programming of appetite and type 2 diabetes. Early Hum Dev. 2005;81:981–8.
Bouret S, Levin BE, Ozanne SE. Gene-environment interactions controlling energy and glucose homeostasis and the developmental origins of obesity. Physiol Rev. 2015;95:47–82.
Kennedy GC. The development with age of hypothalamic restraint upon the appetite of the rat. J Endocrinol. 1957;16:9/17.
Widdowson EM, Mc CR. Some effects of accelerating growth. I. General somatic development. Proc R Soc Lond B Biol Sci. 1960;152:188–206.
Knittle JL, Hirsch J. Effect of early nutrition on the development of rat epididymal fat pads: cellularity and metabolism. J Clin Invest. 1968;47:2091–8.
Collden G, Balland E, Parkash J, Caron E, Langlet F, Prevot V, et al. Neonatal overnutrition causes early alterations in the central response to peripheral ghrelin. Molecular Metabolism. 2015;4:15–24.
Kayser BD, Goran MI, Bouret SG. Perinatal overnutrition exacerbates adipose tissue inflammation caused by high-fat feeding in C57BL/6J mice. PLoS One. 2015;10:e0121954.
Davidowa H, Plagemann A. Insulin resistance of hypothalamic arcuate neurons in neonatally overfed rats. Neuroreport. 2007;18:521–4.
Rodrigues AL, De Souza EP, Da Silva SV, Rodrigues DS, Nascimento AB, Barja-Fidalgo C, et al. Low expression of insulin signaling molecules impairs glucose uptake in adipocytes after early overnutrition. J Endocrinol. 2007;195:485–94.
Glavas MM, Kirigiti MA, Xiao XQ, Enriori PJ, Fisher SK, Evans AE, et al. Early overnutrition results in early-onset arcuate leptin resistance and increased sensitivity to high-fat diet. Endocrinology. 2010;151:1598–610.
Yzydorczyk C, Li N, Rigal E, Chehade H, Mosig D, Armengaud JB, et al. Calorie restriction in adulthood reduces hepatic disorders induced by transient postnatal overfeeding in mice. Nutrients. 2019;11:2796.
Ahima R, Prabakaran D, Flier J. Postnatal leptin surge and regulation of circadian rhythm of leptin by feeding. Implications for energy homeostasis and neuroendocrine function. J Clin Invest. 1998;101:1020–7.
Ahima RS, Hileman SM. Postnatal regulation of hypothalamic neuropeptide expression by leptin: implications for energy balance and body weight regulation. Regul Pept. 2000;92:1–7.
Proulx K, Richard D, Walker C-D. Leptin regulates appetite-related neuropeptides in the hypothalamus of developing rats without affecting food intake. Endocrinology. 2002;143:4683–92.
Mistry A, Swick A, Romsos D. Leptin alters metabolic rates before acquisition of its anorectic effect in developing neonatal mice. Am J Physiol. 1999;277:R742–747.
Schmidt I, Fritz A, Scholch C, Schneider D, Simon E, Plagemann A. The effect of leptin treatment on the development of obesity in overfed suckling Wistar rats. Int J Obes Rel Metabol Disord. 2001;25:1168–74.
Bouret SG. Neurodevelopmental actions of leptin. Brain Res. 2010;350:2–9.
Granado M, Fuente-Martín E, García-Cáceres C, Argente J, Chowen JA. Leptin in early life: a key factor for the development of the adult metabolic profile. Obes Facts. 2012;5:138–50.
Briffa JF, McAinch AJ, Romano T, Wlodek ME, Hryciw DH. Leptin in pregnancy and development: a contributor to adulthood disease? Am J Physiol Endocrinol Metabol. 2015;308:E335–E350.
Davidowa H, Plagemann A. Decreased inhibition by leptin of hypothalamic arcuate neurons in neonatally overfed young rats. Neuroreport. 2000;11:2795–8.
Marangon PB, Mecawi AS, Antunes-Rodrigues J, Elias LLK. Perinatal over- and underfeeding affect hypothalamic leptin and ghrelin neuroendocrine responses in adult rats. Physiol Behav. 2020;215:112793.
Coupe B, Ishii Y, Dietrich MO, Komatsu M, Horvath TL, Bouret SG. Loss of autophagy in pro-opiomelanocortin neurons perturbs axon growth and causes metabolic dysregulation. Cell Metabol. 2012;15:247–55.
Habbout A, Li N, Rochette L, Vergely C. Postnatal overfeeding in rodents by litter size reduction induces major short- and long-term pathophysiological consequences. J Nutr. 2013;143:553–62.
Plagemann A, Harder T, Rake A, Melchior K, Rohde W. D√∂rner Gn. Increased number of galanin-neurons in the paraventricular hypothalamic nucleus of neonatally overfed weanling rats. Brain Res. 1999;818:160–3.
Shpilman M, Niv-Spector L, Katz M, Varol C, Solomon G, Ayalon-Soffer M, et al. Development and characterization of high affinity leptins and leptin antagonists. J Biol Chem. 2011;286:4429–42.
Elinav E, Niv-Spector L, Katz M, Price TO, Ali M, Yacobovitz M, Solomon G, Reicher S, Lynch JL, Halpern Z, Banks WA, Gertler A. Pegylated leptin antagonist is a potent orexigenic agent: preparation and mechanism of activity. Endocrinology. 2009;150:3083–91.
Hou M, Liu Y, Zhu L, Sun B, Guo M, Burén J, et al. Neonatal overfeeding induced by small litter rearing causes altered glucocorticoid metabolism in rats. PLoS One. 2011;6:e25726.
López M, Seoane LM, Tovar S, García MC, Nogueiras R, Diéguez C, et al. A possible role of neuropeptide Y, agouti-related protein and leptin receptor isoforms in hypothalamic programming by perinatal feeding in the rat. Diabetologia. 2005;48:140–8.
Ziko I, Sominsky L, Nguyen T-X, Yam K-Y, De Luca S, Korosi A, et al. Hyperleptinemia in neonatally overfed female rats does not dysregulate feeding circuitry. Front Endocrinol. 2017;8:287–287.
Rodrigues AL, de Moura EG, Passos MC, Trevenzoli IH, da Conceição EP, Bonono IT, et al. Postnatal early overfeeding induces hypothalamic higher SOCS3 expression and lower STAT3 activity in adult rats. J Nutr Biochem. 2011;22:109–17.
Kappeler L, De Magalhaes Filho C, Leneuve P, Xu J, Brunel N, Chatziantoniou C, et al. Early postnatal nutrition determines somatotropic function in mice. Endocrinology. 2009;150:314–23.
Boullu-Ciocca S, Dutour A, Guillaume V, Achard V, Oliver C, Grino M. Postnatal diet-induced obesity in rats upregulates systemic and adipose tissue glucocorticoid metabolism during development and in adulthood: its relationship with the metabolic syndrome. Diabetes. 2005;54:197–203.
Cunha AC, Pereira RO, Pereira MJ, Soares Vde M, Martins MR, Teixeira MT, et al. Long-term effects of overfeeding during lactation on insulin secretion-the role of GLUT-2. J Nutr Biochem. 2009;20:435–42.
Plagemann A, Harder T, Rake A, Voits M, Fink H, Rohde W, et al. Perinatal elevation of hypothalamic insulin, acquired malformation of hypothalamic galaninergic neurons, and syndrome x-like alterations in adulthood of neonatally overfed rats. Brain Res. 1999;836:146–55.
Boullu-Ciocca S, Achard V, Tassistro V, Dutour A, Grino M. Postnatal programming of glucocorticoid metabolism in rats modulates high-fat diet-induced regulation of visceral adipose tissue glucocorticoid exposure and sensitivity and adiponectin and proinflammatory adipokines gene expression in adulthood. Diabetes. 2008;57:669–77.
Vickers MH, Gluckman PD, Coveny AH, Hofman PL, Cutfield WS, Gertler A, et al. Neonatal leptin treatment reverses developmental programming. Endocrinology. 2005;146:4211–6.
Vickers MH, Gluckman PD, Coveny AH, Hofman PL, Cutfield WS, Gertler A, et al. The effect of neonatal leptin treatment on postnatal weight gain in male rats is dependent on maternal nutritional status during pregnancy. Endocrinology. 2008;149:1906–13.
Hoggard N, Hunter L, Lea R, Trayhurn P, Mercer J. Ontogeny of the expression of leptin and its receptor in the murine fetus and placenta. Br J Nutr. 2000;83:317–26.
Caron E, Sachot C, Prevot V, Bouret SG. Distribution of leptin-sensitive cells in the postnatal and adult mouse brain. J Comp Neurol. 2010;518:459–76.
Bouret SG, Draper SJ, Bates SH, Kirigiti MA, Chen S, Bjornholm M, et al. Leptin promotes formation of projection pathways from the arcuate nucleus of the hypothalamus through activation of ObRb signaling pathways In Proceedings of the 34nd Annual Meeting The Society For Neuroscience, San Diego, CA. 2004.
Bouret SG, Bates SH, Chen S, Myers MG, Simerly RB. Distinct roles for specific leptin receptor signals in the development of hypothalamic feeding circuits. J Neurosci. 2012;32:1244–52.
Islam M, Sjoholm A, Emilsson V. Fetal pancreatic islets express functional leptin receptors and leptin stimulates proliferation of fetal islet cells. Int J Obes Relat Metab Disord. 2000;24:1246–53.
Croizier S, Prevot V, Bouret Sebastien G. Leptin controls parasympathetic wiring of the pancreas during embryonic life. Cell Rep. 2016;15:36–44.
Attig L, Larcher T, Gertler A, Abdennebi-Najar L, Djiane J. Postnatal leptin is necessary for maturation of numerous organs in newborn rats. Organogenesis. 2011;7:88–94.
Attig L, Brisard D, Larcher T, Mickiewicz M, Guilloteau P, Boukthir S, et al. Postnatal leptin promotes organ maturation and development in IUGR piglets. PLoS One. 2013;8:e64616.
Chen X, Lin J, Hausman DB, Martin RJ, Dean RG, Hausman GJ. Alterations in fetal adipose tissue leptin expression correlate with the development of adipose tissue. Neonatology. 2000;78:41–47.
Aprath-Husmann I, Rohrig K, Gottschling-Zeller H, Skurk T, Scriba D, Birgel M, et al. Effects of leptin on the differentiation and metabolism of human adipocytes. Int J Obesity Rel Metab Disord. 2001;25:1465–70.
Hayashida T, Nakahara K, Mondal MS, Date Y, Nakazato M, Kojima M, et al. Ghrelin in neonatal rats: distribution in stomach and its possible role. J Endocrinol. 2002;173:239–45.
Pinilla L, Barreiro ML, Tena-Sempere M, Aguilar E. Role of ghrelin in the control of growth hormone secretion in prepubertal rats: interactions with excitatory amino acids. Neuroendocrinology. 2003;77:83–90.
Acknowledgements
This work was supported by a research grant from the Inserm (grant 1172, to SGB). We thank the mouse metabolic phenotyping and cellular imaging cores of the UMS2014-US41 for accessing the metabolic cages and confocal microscope, respectively.
Author information
Authors and Affiliations
Contributions
GC and SGB conceived and designed the project. GC performed experiments. SGB, GC, and EC analyzed data. GC and SGB wrote the manuscript. All the authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Colldén, G., Caron, E. & Bouret, S.G. Neonatal leptin antagonism improves metabolic programming of postnatally overnourished mice. Int J Obes 46, 1138–1144 (2022). https://doi.org/10.1038/s41366-022-01093-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-022-01093-4
- Springer Nature Limited
This article is cited by
-
Programming of metabolism by adipokines during development
Nature Reviews Endocrinology (2023)