Abstract
Childhood obesity jeopardizes a healthy future for our society’s children as it is associated with increased cardiovascular morbidity and mortality later on in life. Endothelial dysfunction, the first step in the development of atherosclerosis, is already present in obese children and may well represent a targetable risk factor. Technological advancements in recent years have facilitated noninvasive measurements of endothelial homeostasis in children. Thereby this topic ultimately starts to get the attention it deserves. In this paper, we aim to summarize the latest insights on endothelial dysfunction in childhood obesity. We discuss methodological advancements in peripheral endothelial function measurement and newly identified diagnostic markers of vascular homeostasis. Finally, future challenges and perspectives are set forth on how to efficiently tackle the catastrophic rise in cardiovascular morbidity and mortality that will be inflicted on obese children if they are not treated optimally.
Similar content being viewed by others
Introduction
The onset of atherosclerosis, dysfunction of the arterial endothelium, starts early in life and is greatly accelerated in the setting of childhood obesity. Obesity therefore poses a serious health threat to children, increasing the child’s risk of developing clinically overt cardiovascular disease in adult life (1). Although the prevalence of childhood obesity increased dramatically the past three decades, rates now seem to have reached a plateau in most countries. Currently, about 20% of children and adolescents between 12 and 19 y of age in the United States are obese (2).
Endothelial dysfunction is the primum movens in the pathogenesis of atherosclerosis, appearing long before clinical symptoms arise, and might qualify as a surrogate endpoint for cardiovascular disease risk. The endothelium’s primary role is the tight control of the blood vessel diameter. In response to stimuli for increased blood flow demand, endothelial nitric oxide synthase in endothelial cells is activated and produces nitric oxide, which diffuses into the vessel wall to fine-tune vasodilation (3). Although nitric oxide is considered a key regulator of endothelial function, several other factors are involved as well (4,5). The location of the endothelium close to the blood circulation, however, exposes the endothelial cells to many damaging factors. These factors cause harm to endothelial cells leading to endothelial dysfunction, commonly defined as “an imbalance between vasodilating and vasoconstricting substances produced by (or acting on) endothelial cells” (6). Importantly, damage to endothelial cells might also upset vascular smooth cell function in obese children (7,8,9). Novel developments in the field have encouraged researchers to examine alterations in vascular endothelial function not only in adults but also in children and adolescents. Endothelial dysfunction was demonstrated in the major conduit arteries of obese children and is referred to as macrovascular endothelial dysfunction (10). Although endothelial dysfunction is considered a “systemic disorder,” dysfunction of small resistance vessels, microvascular endothelial dysfunction, precedes the development of macrovascular endothelial dysfunction (11).
This growing interest in endothelial homeostasis introduced novel laboratory methods for investigating the endothelium at a cellular level. Endothelial micro particles (EMP) (12,13) as well as endothelial progenitor cells (EPC) (14) and circulating angiogenic cells (CAC) (15) emerged as markers of respectively endothelial damage and repair. All have been implicated in the process of childhood obesity–related endothelial dysfunction (16,17). Therefore, the combined assessment of these cell-based markers and endothelium vasodilatory function may represent a promising option to optimize the risk stratification and the primary care management of obese children.
In this review, we aim to summarize the latest insights on endothelial dysfunction in obese children. We discuss the methodological advancements in endothelial function measurement and new biomarkers of vascular homeostasis. Finally, we explore future challenges and perspectives for the treatment of childhood obesity.
New Factors Influencing Childhood Obesity–Related Endothelial Dysfunction
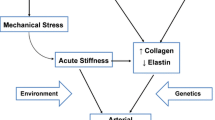
In obese children, multiple cardiovascular risk factors are present, which all negatively affect endothelial function (18). Endothelial dysfunction summarizes the cumulative burden of these risk facts and might therefore represent an excellent surrogate marker for early cardiovascular disease ( Figure 1 ). Risk factors in obese children include not only classical cardiovascular risk factors such as hypertension (19) but also newly discovered cytokines and signaling molecules including micro RNA (miRNAs). The impact of these factors has been demonstrated in clinical and fundamental studies, and below we discuss the latest data in this field.
Determinants of obesity-related endothelial dysfunction in children. On the left, cardiovascular risk factors which can influence endothelial function in obese children are summarized. On the right, noninvasive techniques to assess macrovascular (FMD) and microvascular endothelial dysfunction (Endo-PAT) are shown.
Hypertension during adolescence (20) can lead to severe vascular endothelial dysfunction in adult life (21). In prepubertal children, obesity is strongly associated with hypertension. Counterintuitive, however, obese prepubertal children demonstrate a better functional capacity of their endothelium than the normal-weight normotensive counterparts (22). Accordingly, young obese children apparently develop an early vascular adaptive response to increased blood flow demands. Radtke et al. (23) recently provided evidence in support of this theory. They performed a cold pressure test to measure the change in blood pressure in response to stress in children without known cardiovascular risk factors. Children with a positive test, and thus at increased risk of hypertension, showed greater endothelial capacity. The concept that young obese children are able to elicit an adaptive response of their endothelium to stress may also explain why 6 mo of exercise training does not improve endothelium-dependent flow-mediated dilation of the brachial artery in this population (24), whereas multiple previous studies have demonstrated positive effects of training on endothelial status in (post-) pubertal children (25). In summary, the effects of hypertension on endothelial dysfunction are complex and are influenced by pubertal development.
Dyslipidemia is another potent factor that is involved in impaired endothelial function in obese children. Elevated LDL cholesterol is often seen in obese adults but is rarely observed in obese children (19). Therefore, the focus of attention has recently shifted toward HDL cholesterol. HDL cholesterol is associated with a reduction in cardiovascular risk in adult populations (26). Unexpectedly, pharmacological attempts to raise HDL cholesterol levels in adults did not establish a risk reduction in coronary events (27). HDL, however, is a complex lipoprotein containing more than 1,000 lipids and 70 proteins (28). This multifaceted composition may underlie the diversity of HDL’s actions, including its anti-oxidative and anti-inflammatory properties. Hereof, Matsuo et al. (29) showed that the function of HDL is impaired in obese children. In particular, HDL in the young obese is less capable of stimulating endothelial nitric oxide synthase activity and thus endothelial function (30). Six months of exercise training facilitated a tendency toward the improvement of HDL function, for which the authors speculated that the training was not intensive enough to achieve statistical significance (29).
Recent progresses in the field further indicate that HDL is a major carrier of miRNAs (31). miRNA’s are small (20–25 nucleotides) noncoding RNA molecules that regulate the expression of protein-coding genes and are emerging as new biomarkers and therapeutic targets. Uncovering miRNAs that specifically relate to first signs of endothelial maladaptation may allow an earlier identification of obese children who are at increased cardiovascular risk (31). Moreover, in adults exercise can thrive HDL to induce a more proangiogenic miRNA profile in endothelial cells (32). Whether the latter is also true for children remains to be proven.
Both low cardiorespiratory fitness (33) and physical inactivity (34) are independent predictors of cardiovascular morbidity and mortality. While cardiorespiratory fitness can be objectively assessed by ergo spirometry, physical activity is recorded by using questionnaires and/or accelerometers (35). In young children (5 to 10 y old), physical activity strongly correlates with endothelial function (36). Surprisingly, no correlation of physical (in)activity or cardiorespiratory fitness with endothelial function is observed in adolescents (mean age of 14.5 y) (37). Although adolescents demonstrate high cardiorespiratory fitness in the study of Radtke et al., only 16% of them actually adhered to the recommended 60 min of physical activity per day. Therefore, it could be possible that these adolescents did not perform enough physical activity to keep their endothelial function optimal, while cardiorespiratory fitness was preserved. Vice versa, in young recreationally active men (25 ± 2 y), as little as 5 d of diminished physical activity negatively impacted macrovascular endothelial function in the study by Boyle et al. (38). Furthermore, whereas a high intensity exercise bout increases endothelial function in normal-weight young adults, this response is completely blunted in obese participants (39). However, the differentiation between cardiorespiratory fitness and physical activity still remains complex, and more research will be necessary to investigate their differential effects on childhood obesity–related endothelial dysfunction.
Adipocytes secrete a vast array of cytokines called adipokines and, as such, adipose tissue meets the criteria of an endocrine organ (40). In childhood obesity, hypertrophic adipose tissue is invaded by macrophages, resulting in the upregulation of adipocyte adhesion molecules. This process leads to the diapedesis of monocytes and initiates a vicious circle of adipogenesis and inflammation (41). Several adipokines have a direct effect on endothelial function, including leptin and adiponectin (42,43). Chemerin, a novel adipokine at the crossroad between inflammation and obesity, may possibly influence the vascular endothelium as well. In obese children, increased circulating levels of chemerin have been observed (44), closely correlating with the degree of endothelial dysfunction. Also, addition of chemerin to cultured endothelial cells upregulated the expression of adhesion molecules for white blood cells. Additionally, both childhood obesity and inflammation lead to oxidative stress (45,46,47), since they induce the generation of reactive oxygen species and lower the anti-oxidant capacity.
As in adults, sleep apnea in obese children is highly prevalent (48) and impairs endothelial function (49). The link between sleep apnea, childhood obesity, and inflammation was recently investigated by measuring circulating levels of pentraxin-3, a relatively new biomarker of cardiovascular risk (50). Plasma pentraxin-3 levels correlate positively with BMI and with the severity of obstructive sleep apnea syndrome in obese children. In addition, Kim et al. demonstrated that pentraxin-3 correlates with the number of circulating EMP in obese children (51). More research on pentraxin-3 as a new biomarker in the setting of childhood obesity could be of interest.
Insulin resistance is common among obese children and adolescents. Reciprocal relationships between insulin resistance and endothelial dysfunction cause a vicious cycle, leading to metabolic, renal, and cardiovascular diseases (52). Insulin is a potent vasodilator (53). In case of insulin resistance, however, endothelial cells are selectively resistant for these vasodilatory actions. Insulin will stimulate the production of endothelin-1, a potent vasoconstrictor (54). In healthy individuals, exogenous endothelin-1 led to reduced peripheral insulin sensitivity (55). In summary, insulin resistance may lead to endothelial dysfunction and vice versa.
Psychological psychosocial distress is highly prevalent in obese children (56). In addition, scores for anger, depression, and anxiety are negatively correlated with endothelial function in healthy children (57). Mechanisms, however, are most likely of postnatal origin, since a recent large population study found no correlation between maternal stress during pregnancy and endothelial function in children at the age of 10 to 12 y (58). It remains to be examined, however, whether psychological traits impose an increase in the cardiovascular risk of obese children.
To conclude, the rapidly increasing prevalence of childhood obesity has clearly awakened the scientific community. Still, to prevent a catastrophic increase in the prevalence of cardiovascular disease, we need to step up and improve our understanding of the mechanisms underlying childhood obesity-related endothelial dysfunction. Novel noninvasive methods for the evaluation of endothelial function can now be easily applied in children, thus setting the scene for allowing thorough clinical and translational studies. In the following paragraphs, we discuss these technological advancements in methodology.
Technological Advancements in Obesity-Associated Endothelial Function Measurement
More than 20 y ago, a first noninvasive method, called flow-mediated dilation (FMD), was introduced to measure endothelial function (59). More recently, peripheral arterial tonometry (Endo-PAT) was developed to overcome the user-dependent disadvantage of FMD (60). For Endo-PAT, a pneumatic probe is placed on both index fingers. Then, similar to the FMD method, a sphygmomanometer is insufflated to supra-systolic pressures forcing a transient occlusion of the brachial artery. The shear stress-induced dilation of the small resistance vessels will cause pressure differences, which are registered and expressed as pulse wave amplitudes. The software provided with the Endo-PAT system will then automatically calculate the reactive hyperemia index (RHI) as the ratio of the pulse wave amplitude starting 90 s after occlusion, for 60 s, divided by the baseline pulse wave amplitude. Though, in contrast to FMD, assessment of endothelium-dependent vasodilation is not possible.
In recent past, Chen et al. and Radtke et al. demonstrated that the time needed to reach maximal dilation (i.e., peak response) with the Endo-PAT device is more variable in children and adolescents than in adults (23,61). The Endo-PAT algorithm might not correctly account for physiological adaptations in childhood and puberty (62,63). Therefore, if RHI is used in children, the true peak dilation could be missed. To overcome this hurdle, we recently proposed to use peak response instead of the automatically calculated RHI in children (64,65). Although Endo-PAT was initially set forth as an alternative technique for FMD, a large population-based study recently pointed out that micro- (measured with Endo-PAT) and macrovascular (detected with FMD) endothelial dysfunctions can develop independently of each other (66). Microvascular endothelial dysfunction would precede endothelial dysfunction at the macrovascular level in obese children (12). To conclude, the analysis of Endo-PAT testing in children requires caution, and it seems more correct to use peak response instead of RHI. Further, the uncoupling of macrovascular from microvascular endothelial dysfunction urges the assessment of both Endo-PAT and FMD measures in obese children. Cellular markers of endothelial damage and repair may add further to our knowledge of the vascular endothelium in childhood obesity. These will be discussed in the following paragraphs.
Cell-Derived Markers of Endothelial Damage and Repair in Obese Children
The location of the endothelium close to the circulation exposes the endothelial cells to damaging factors such as lipids and inflammatory proteins. Upon this activation or following apoptosis, endothelial cells will shed small (100 nm–1 µm) particles of the cell membrane into the circulation, called EMP (67). These microparticles are covered with surface antigens from the parental endothelial cell, making quantification possible using specific flow cytometry markers. Interestingly, EMP also carry RNA (including microRNA), DNA, and proteins and can therefore act as delivery vehicles to target cells (68). In the largest study to date, involving 844 adults without a history of cardiovascular disease, EMP counts strongly correlate with cardio-metabolic risk factors, in particular with dyslipidemia (69). Indeed in obese adults, numbers of EMP are increased and also negatively correlated with macrovascular endothelial function (70). Likewise, obese children have higher blood EMP than their normal-weight counterparts (71). We recently demonstrated that CD31+/CD42b- EMP are important indicators of microvascular endothelial health in obese children (65). The relation between EMP and endothelial function, however, might well be bidirectional (72). Evidence from in vitro by Agouni et al. (73) already demonstrated that EMP from adults with the metabolic syndrome have reduced endothelial nitric oxide production and directly influence the endothelium-dependent vasorelaxation. Limits for blood sampling volumes in children, however, have so far hampered research on EMP functioning in pediatric populations.
Next to EMP as markers of endothelial damage, EPC emerged as markers of endothelial repair. The theory that lost endothelial cells are solely replaced by neighboring endothelial cells has now been largely refuted. After endothelial injury, EPC are mobilized from the bone marrow into the circulation under the influence of several chemotactic factors (74). EPC migrate to and incorporate into sites of damaged endothelium (75). Obesity is a prominent predictor of low EPC mobilization, and weight loss is associated with increased circulating EPC and improved macrovascular endothelial function (76). Remarkably, Jung et al. (16) reported an elevated level of circulating EPC in obese children. Methodological issues, raised by the lack of assay standardization and phenotypic definition, could have accounted for this rather unexpected finding. Enumeration of EPC, defined as CD34+/KDR+/CD45dim/- cells, according to recent recommendations (77), shows that the EPC level in obese children is negatively correlated with BMI (65), which is in accordance with data obtained in adults (76). In addition, obese children have less EPC in their bloodstream than normal-weight controls (65). Moreover, in analogy to high EMP, low circulating EPC turns out to be an independent predictor of reduced peak response (65). Increased EMP and reduced EPC blood counts in obese children support the existence of an imbalance between endothelial damage and repair mechanisms in this population ( Figure 2 ).
Imbalance between endothelial damage and repair. The imbalance between endothelial damage (higher counts of circulating EMP) and endothelial repair (lower numbers of EPC and impaired function of CAC) in childhood obesity–related endothelial dysfunction is displayed (b), while the balanced situation in normal-weight children is depicted as well (a).
CAC are the most novel cellular players in vascular regeneration. CAC are the adherent mononuclear cells which emerge after a 4–7 d culture of peripheral blood mononuclear cells in endothelial cell medium using fibronectin-coated plates (77). They contribute to endothelial repair by attracting circulating EPC in the blood and by stimulating their integration into the injured endothelium via the secretion of angiogenic growth factors. Obese adults have reduced and dysfunctional CAC (78). In obese children, CAC are also functionally defective (79), thereby shifting the balance in these children even further towards higher endothelial damage and reduced repair capacity ( Figure 2 ).
Still, in contrast to CAC from obese adults, CAC of obese youngsters are not yet resistant to the proangiogenic effects of leptin. The adipokine leptin, mainly known for its role in regulating human energy homeostasis and appetite (80), has several atherogenic, thrombotic, and angiogenic actions on cardiovascular homeostasis (81). In this regard, leptin enhances the migratory activity of CAC in normal-weight adults (82). In obese adults, leptin resistance of CAC can be defeated by weight loss (83). Interestingly, the migratory ability of CAC in normal-weight children cannot be improved any further upon stimulation with leptin, indicating that in normal-weight children CAC function is likely at its maximum (79).
To conclude, new cellular markers of endothelial damage and repair may allow researchers to gain a greater insight into the endothelial biology of obese children. In vitro work now suggests that these markers do not merely reflect the status of the endothelium but also effectively contribute to the progression or reversion of endothelial dysfunction in pathological conditions. Study results relating to young obese children are discussed in the next paragraphs.
New Insights on Treatment of Endothelial Dysfunction
Endothelial dysfunction is traditionally regarded as a reversible process. Weight loss by reducing caloric intake and/or increasing physical activity tackles multiple cardiovascular risk factors associated with endothelial dysfunction in obese children (84). Besides the indirect effects, weight loss also directly influences the endothelium by stimulation of endothelial nitric oxide synthase (85). In obese children, a combination of diet and exercise training is able to improve macrovascular endothelial function after as little as 6 to 8 wk (86,87) However, 10 mo of supervised diet and exercise is needed to enhance microvascular endothelial function (88). Microvascular endothelial dysfunction therefore seems much harder to tackle than macrovascular endothelial dysfunction. Its longer presence may well explain the discrepancy in time needed to overcome the endothelial malfunctioning and thus reduce the cardiovascular risk.
Along with the improvement of microvascular endothelial function, a differential recruitment of EPC and EMP is observed in obese children. In our recent work, obese children have a peak in the number of blood EPC after 5 mo of diet and exercise, whereas at 10 mo there is a significant drop in circulating EMP. At 10 mo, EPC levels returned to baseline. We hypothesize that the need for high EPC numbers was eliminated since endothelial damage was significantly reduced. In a large cohort of primary school children, 1 y of exercise training on every school day led to a significant increase in the number of EPC (89). However, only 13% of these children were obese or overweight, setting limits for comparison. To our knowledge, the effect of a diet plus exercise treatment on numbers of circulating EMP in obese children had not been described before. Interestingly, microparticles, including EMP, may either be viewed as beneficial or detrimental (73), and further information on EMP in obese children would therefore be of interest.
Future Challenges and Perspectives
The current clinical guidelines to treat childhood obesity merely focus on weight stabilization (and thus reducing BMI by normal growth) in case of moderate obesity and weight reduction in case of severe obesity (90). Children are encouraged to reduce their time spent in sedentary behavior and to increase physical activity up to 60 min per day (91). These recommendations are mainly based on epidemiological studies, in which reduced physical activity was clearly associated with increased incidence of obesity (39). The major goal of obesity therapy in children, however, should be aimed at reducing the long-term risk of cardiovascular morbidity and mortality. For this purpose, longitudinal prospective clinical studies are needed with patient follow up for cardiovascular events to occur. This kind of research would be extremely costly and time consuming. The emerging evidence that endothelial function qualifies as a clinically relevant surrogate endpoint may offer a solution to resolve this issue. We believe that thorough evaluation of the endothelial status in large-scale multicenter trials can create a setting enabling the further optimization of training protocols for obese children. One of these training modalities could be aerobic interval training, since data indicate that this type of training is superior in reversing endothelial dysfunction in children (92). Although the participation of children in clinical studies raises several ethical concerns (93), we hope that researchers will continue the fight against childhood obesity.
Conclusions
In recent years, attention has shifted toward mechanisms of childhood obesity–related endothelial dysfunction after an initial period where all eyes were on obese adults. Determinants of childhood obesity–related endothelial dysfunction, cell based and biochemical, are now increasingly explored. In addition to extensive endothelial activation, dysfunction, and damage, obese children have reduced endogenous vascular repair capacity. Endothelial dysfunction in the smaller resistance vessels also seems harder to beat than large vessel endothelial dysfunction. Obviously, much more research is necessary to fully understand childhood obesity–related micro- and macrovascular endothelial dysfunction in all its facets. We acknowledge that this requires a considerable investment, but the socioeconomic burden of obese children not being treated optimally and growing up to obese adults developing associated comorbidities is even greater. This cost was recently estimated to be none less than $19,000 per person (94). Eventually, studies should not merely be initiated for scientific reasons, but more importantly, to safeguard the cardiovascular future of our society’s obese children.
Statement of Financial Support
No financial support was received for the preparation of this manuscript.
Disclosure
The authors have no financial relationships relevant to this article to disclose.
References
Tirosh A, Shai I, Afek A, et al. Adolescent BMI trajectory and risk of diabetes versus coronary disease. N Engl J Med 2011;364:1315–25.
Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA 2014;311:806–14.
Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 1999;399:601–5.
Flammer AJ, Lüscher TF. Human endothelial dysfunction: EDRFs. Pflugers Arch 2010;459:1005–13.
Virdis A, Ghiadoni L, Taddei S. Human endothelial dysfunction: EDCFs. Pflugers Arch 2010;459:1015–23.
Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation 2007;115:1285–95.
Ciccone MM, Miniello V, Marchioli R, et al. Morphological and functional vascular changes induced by childhood obesity. Eur J Cardiovasc Prev Rehabil 2011;18:831–5.
Peña AS, Wiltshire E, MacKenzie K, et al. Vascular endothelial and smooth muscle function relates to body mass index and glucose in obese and nonobese children. J Clin Endocrinol Metab 2006;91:4467–71.
Aggoun Y, Farpour-Lambert NJ, Marchand LM, Golay E, Maggio AB, Beghetti M. Impaired endothelial and smooth muscle functions and arterial stiffness appear before puberty in obese children and are associated with elevated ambulatory blood pressure. Eur Heart J 2008;29:792–9.
Tounian P, Aggoun Y, Dubern B, et al. Presence of increased stiffness of the common carotid artery and endothelial dysfunction in severely obese children: a prospective study. Lancet 2001;358:1400–4.
Montero D, Walther G, Perez-Martin A, Roche E, Vinet A. Endothelial dysfunction, inflammation, and oxidative stress in obese children and adolescents: markers and effect of lifestyle intervention. Obes Rev 2012;13:441–55.
Montero D, Walther G, Perez-Martin A, et al. Effects of a lifestyle program on vascular reactivity in macro- and microcirculation in severely obese adolescents. J Clin Endocrinol Metab 2014;99:1019–26.
van Ierssel SH, Van Craenenbroeck EM, Conraads VM, et al. Flow cytometric detection of endothelial microparticles (EMP): effects of centrifugation and storage alter with the phenotype studied. Thromb Res 2010;125:332–9.
Van Craenenbroeck EM, Vrints CJ, Haine SE, et al. A maximal exercise bout increases the number of circulating CD34+/KDR+ endothelial progenitor cells in healthy subjects. Relation with lipid profile. J Appl Physiol (1985) 2008;104:1006–13.
Van Craenenbroeck EM, Hoymans VY, Beckers PJ, et al. Exercise training improves function of circulating angiogenic cells in patients with chronic heart failure. Basic Res Cardiol 2010;105:665–76.
Jung C, Fischer N, Fritzenwanger M, et al. Endothelial progenitor cells in adolescents: impact of overweight, age, smoking, sport and cytokines in younger age. Clin Res Cardiol 2009;98:179–88.
Siklar Z, Öçal G, Berberoğlu M, et al. Evaluation of hypercoagulability in obese children with thrombin generation test and microparticle release: effect of metabolic parameters. Clin Appl Thromb Hemost 2011;17:585–9.
Aggoun Y. Obesity, metabolic syndrome, and cardiovascular disease. Pediatr Res 2007;61:653–9.
Bruyndonckx L, Hoymans VY, Van Craenenbroeck AH, et al. Assessment of endothelial dysfunction in childhood obesity and clinical use. Oxid Med Cell Longev 2013;2013:174782.
Falaschetti E, Hingorani AD, Jones A, et al. Adiposity and cardiovascular risk factors in a large contemporary population of pre-pubertal children. Eur Heart J 2010;31:3063–72.
Juonala M, Viikari JS, Rönnemaa T, Helenius H, Taittonen L, Raitakari OT. Elevated blood pressure in adolescent boys predicts endothelial dysfunction: the cardiovascular risk in young Finns study. Hypertension 2006;48:424–30.
Charakida M, Jones A, Falaschetti E, et al. Childhood obesity and vascular phenotypes: a population study. J Am Coll Cardiol 2012;60:2643–50.
Radtke T, Eser P, Kriemler S, Saner H, Wilhelm M. Adolescent blood pressure hyperreactors have a higher reactive hyperemic index at the fingertip. Eur J Appl Physiol 2013;113:2991–3000.
Farpour-Lambert NJ, Aggoun Y, Marchand LM, Martin XE, Herrmann FR, Beghetti M. Physical activity reduces systemic blood pressure and improves early markers of atherosclerosis in pre-pubertal obese children. J Am Coll Cardiol 2009;54:2396–406.
Dias KA, Green DJ, Ingul CB, Pavey TG, Coombes JS. Exercise and vascular function in child obesity: a meta-analysis. Pediatrics 2015;136:e648–59.
Castelli WP, Garrison RJ, Wilson PW, Abbott RD, Kalousdian S, Kannel WB. Incidence of coronary heart disease and lipoprotein cholesterol levels. The Framingham Study. JAMA 1986;256:2835–8.
Barter PJ, Caulfield M, Eriksson M, et al.; ILLUMINATE Investigators. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 2007;357:2109–22.
Landmesser U, von Eckardstein A, Kastelein J, Deanfield J, Lüscher TF. Increasing high-density lipoprotein cholesterol by cholesteryl ester transfer protein-inhibition: a rocky road and lessons learned? The early demise of the dal-HEART programme. Eur Heart J 2012;33:1712–5.
Matsuo Y, Oberbach A, Till H, et al. Impaired HDL function in obese adolescents: impact of lifestyle intervention and bariatric surgery. Obesity (Silver Spring) 2013;21:E687–95.
Müller U, Matsuo Y, Lauber M, et al. Correlation between endothelial function measured by finger plethysmography in children and HDL-mediated eNOS activation – a preliminary study. Metabolism 2013;62:634–7.
Omran A, Elimam D, He F, Peng J, Yin F. Potential role of blood microRNAs as non-invasive biomarkers for early detection of asymptomatic coronary atherosclerosis in obese children with metabolic syndrome. Med Hypotheses 2012;79:889–93.
Riedel S, Radzanowski S, Bowen TS, et al. Exercise training improves high-density lipoprotein-mediated transcription of proangiogenic microRNA in endothelial cells. Eur J Prev Cardiol 2015;22:899–903.
Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 2002;346:793–801.
Högström G, Nordström A, Nordström P. High aerobic fitness in late adolescence is associated with a reduced risk of myocardial infarction later in life: a nationwide cohort study in men. Eur Heart J 2014;35:3133–40.
Van Craenenbroeck EM, Conraads VM. On cars, TVs, and other alibis to globalize sedentarism. Eur Heart J 2012;33:425–7.
Abbott RA, Harkness MA, Davies PS. Correlation of habitual physical activity levels with flow-mediated dilation of the brachial artery in 5-10 year old children. Atherosclerosis 2002;160:233–9.
Radtke T, Kriemler S, Eser P, Saner H, Wilhelm M. Physical activity intensity and surrogate markers for cardiovascular health in adolescents. Eur J Appl Physiol 2013;113:1213–22.
Boyle LJ, Credeur DP, Jenkins NT, et al. Impact of reduced daily physical activity on conduit artery flow-mediated dilation and circulating endothelial microparticles. J Appl Physiol (1985) 2013;115:1519–25.
Goran MI, Treuth MS. Energy expenditure, physical activity, and obesity in children. Pediatr Clin North Am 2001;48:931–53.
Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 2004;89:2548–56.
Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J 2008;29:2959–71.
Park HY, Kwon HM, Lim HJ, et al. Potential role of leptin in angiogenesis: leptin induces endothelial cell proliferation and expression of matrix metalloproteinases in vivo and in vitro. Exp Mol Med 2001;33:95–102.
Torigoe M, Matsui H, Ogawa Y, et al. Impact of the high-molecular-weight form of adiponectin on endothelial function in healthy young men. Clin Endocrinol (Oxf) 2007;67:276–81.
Landgraf K, Friebe D, Ullrich T, et al. Chemerin as a mediator between obesity and vascular inflammation in children. J Clin Endocrinol Metab 2012;97:E556–64.
Araki S, Dobashi K, Yamamoto Y, Asayama K, Kusuhara K. Increased plasma isoprostane is associated with visceral fat, high molecular weight adiponectin, and metabolic complications in obese children. Eur J Pediatr 2010;169:965–70.
Codoñer-Franch P, Valls-Bellés V, Arilla-Codoñer A, Alonso-Iglesias E. Oxidant mechanisms in childhood obesity: the link between inflammation and oxidative stress. Transl Res 2011;158:369–84.
Giannini C, de Giorgis T, Scarinci A, et al. Increased carotid intima-media thickness in pre-pubertal children with constitutional leanness and severe obesity: the speculative role of insulin sensitivity, oxidant status, and chronic inflammation. Eur J Endocrinol 2009;161:73–80.
Verhulst SL, Schrauwen N, Haentjens D, et al. Sleep-disordered breathing in overweight and obese children and adolescents: prevalence, characteristics and the role of fat distribution. Arch Dis Child 2007;92:205–8.
Li AM, Au CT, Chook P, Lam HS, Wing YK. Reduced flow-mediated vasodilation of brachial artery in children with primary snoring. Int J Cardiol 2013;167:2092–6.
Garlanda C, Bottazzi B, Moalli F, et al. Pentraxins and atherosclerosis: the role of PTX3. Curr Pharm Des 2011;17:38–46.
Kim J, Gozal D, Bhattacharjee R, Kheirandish-Gozal L. TREM-1 and pentraxin-3 plasma levels and their association with obstructive sleep apnea, obesity, and endothelial function in children. Sleep 2013;36:923–31.
Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation 2006;113:1888–904.
Steinberg HO, Brechtel G, Johnson A, Fineberg N, Baron AD. Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J Clin Invest 1994;94:1172–9.
Potenza MA, Marasciulo FL, Chieppa DM, et al. Insulin resistance in spontaneously hypertensive rats is associated with endothelial dysfunction characterized by imbalance between NO and ET-1 production. Am J Physiol Heart Circ Physiol 2005;289:H813–22.
Ottosson-Seeberger A, Lundberg JM, Alvestrand A, Ahlborg G. Exogenous endothelin-1 causes peripheral insulin resistance in healthy humans. Acta Physiol Scand 1997;161:211–20.
Latzer Y, Stein D. A review of the psychological and familial perspectives of childhood obesity. J Eat Disord 2013;1:7.
Osika W, Montgomery SM, Dangardt F, et al. Anger, depression and anxiety associated with endothelial function in childhood and adolescence. Arch Dis Child 2011;96:38–43.
van Dijk AE, Dawe K, Deanfield J, et al. The association of maternal prenatal psychosocial stress with vascular function in the child at age 10-11 years: findings from the Avon longitudinal study of parents and children. Eur J Prev Cardiol 2014;21:1097–108.
Celermajer DS, Sorensen KE, Gooch VM, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 1992;340:1111–5.
Kuvin JT, Patel AR, Sliney KA, et al. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J 2003;146:168–74.
Chen Y, Dangardt F, Osika W, Berggren K, Gronowitz E, Friberg P. Age- and sex-related differences in vascular function and vascular response to mental stress. Longitudinal and cross-sectional studies in a cohort of healthy children and adolescents. Atherosclerosis 2012;220:269–74.
Radtke T, Khattab K, Eser P, Kriemler S, Saner H, Wilhelm M. Puberty and microvascular function in healthy children and adolescents. J Pediatr 2012;161:887–91.
Bhangoo A, Sinha S, Rosenbaum M, Shelov S, Ten S. Endothelial function as measured by peripheral arterial tonometry increases during pubertal advancement. Horm Res Paediatr 2011;76:226–33.
Bruyndonckx L, Radtke T, Eser P, et al. Methodological considerations and practical recommendations for the application of peripheral arterial tonometry in children and adolescents. Int J Cardiol 2013;168:3183–90.
Bruyndonckx L, Hoymans VY, Frederix G, et al. Endothelial progenitor cells and endothelial microparticles are independent predictors of endothelial function. J Pediatr 2014;165:300–5.
Hamburg NM, Palmisano J, Larson MG, et al. Relation of brachial and digital measures of vascular function in the community: the Framingham heart study. Hypertension 2011;57:390–6.
Boulanger CM, Amabile N, Tedgui A. Circulating microparticles: a potential prognostic marker for atherosclerotic vascular disease. Hypertension 2006;48:180–6.
Mause SF, Weber C. Microparticles: protagonists of a novel communication network for intercellular information exchange. Circ Res 2010;107:1047–57.
Amabile N, Cheng S, Renard JM, et al. Association of circulating endothelial microparticles with cardiometabolic risk factors in the Framingham Heart Study. Eur Heart J 2014;35:2972–9.
Esposito K, Ciotola M, Schisano B, et al. Endothelial microparticles correlate with endothelial dysfunction in obese women. J Clin Endocrinol Metab 2006;91:3676–9.
Gündüz Z, Dursun İ, Tülpar S, et al. Increased endothelial microparticles in obese and overweight children. J Pediatr Endocrinol Metab 2012;25:1111–7.
Dignat-George F, Boulanger CM. The many faces of endothelial microparticles. Arterioscler Thromb Vasc Biol 2011;31:27–33.
Agouni A, Lagrue-Lak-Hal AH, Ducluzeau PH, et al. Endothelial dysfunction caused by circulating microparticles from patients with metabolic syndrome. Am J Pathol 2008;173:1210–9.
Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997;275:964–7.
Wassmann S, Werner N, Czech T, Nickenig G. Improvement of endothelial function by systemic transfusion of vascular progenitor cells. Circ Res 2006;99:e74–83.
Müller-Ehmsen J, Braun D, Schneider T, et al. Decreased number of circulating progenitor cells in obesity: beneficial effects of weight reduction. Eur Heart J 2008;29:1560–8.
Van Craenenbroeck EM, Van Craenenbroeck AH, van Ierssel S, et al. Quantification of circulating CD34+/KDR+/CD45dim endothelial progenitor cells: analytical considerations. Int J Cardiol 2013;167:1688–95.
MacEneaney OJ, Kushner EJ, Van Guilder GP, Greiner JJ, Stauffer BL, DeSouza CA. Endothelial progenitor cell number and colony-forming capacity in overweight and obese adults. Int J Obes (Lond) 2009;33:219–25.
Bruyndonckx L, Hoymans VY, Frederix G, et al. Circulating angiogenic cells from obese children do not display leptin resistance. Int J Cardiol 2014;174:881–3.
Friedman JM. The function of leptin in nutrition, weight, and physiology. Nutr Rev 2002 60:S1–14; discussion S68–84, 85–7.
Singh M, Bedi US, Singh PP, Arora R, Khosla S. Leptin and the clinical cardiovascular risk. Int J Cardiol 2010;140:266–71.
Schroeter MR, Leifheit M, Sudholt P, et al. Leptin enhances the recruitment of endothelial progenitor cells into neointimal lesions after vascular injury by promoting integrin-mediated adhesion. Circ Res 2008;103:536–44.
Heida NM, Leifheit-Nestler M, Schroeter MR, et al. Leptin enhances the potency of circulating angiogenic cells via src kinase and integrin (alpha)vbeta5: implications for angiogenesis in human obesity. Arterioscler Thromb Vasc Biol 2010;30:200–6.
Meyer AA, Kundt G, Lenschow U, Schuff-Werner P, Kienast W. Improvement of early vascular changes and cardiovascular risk factors in obese children after a six-month exercise program. J Am Coll Cardiol 2006;48:1865–70.
Hambrecht R, Adams V, Erbs S, et al. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation 2003;107:3152–8.
Woo KS, Chook P, Yu CW, et al. Effects of diet and exercise on obesity-related vascular dysfunction in children. Circulation 2004;109:1981–6.
Kelly AS, Wetzsteon RJ, Kaiser DR, Steinberger J, Bank AJ, Dengel DR. Inflammation, insulin, and endothelial function in overweight children and adolescents: the role of exercise. J Pediatr 2004;145:731–6.
Bruyndonckx L, Hoymans VY, De Guchtenaere A, et al. Diet, exercise, and endothelial function in obese adolescents. Pediatrics 2015;135:e653–61.
Walther C, Gaede L, Adams V, et al. Effect of increased exercise in school children on physical fitness and endothelial progenitor cells: a prospective randomized trial. Circulation 2009;120:2251–9.
Spear BA, Barlow SE, Ervin C, et al. Recommendations for treatment of child and adolescent overweight and obesity. Pediatrics 2007;120:Suppl 4:S254–88.
Steinberger J, Daniels SR, Eckel RH, et al.; American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular Nursing; and Council on Nutrition, Physical Activity, and Metabolism. Progress and challenges in metabolic syndrome in children and adolescents: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular Nursing; and Council on Nutrition, Physical Activity, and Metabolism. Circulation 2009;119:628–47.
Tjønna AE, Stølen TO, Bye A, et al. Aerobic interval training reduces cardiovascular risk factors more than a multitreatment approach in overweight adolescents. Clin Sci (Lond) 2009;116:317–26.
Holm JC, Nowicka P, Farpour-Lambert NJ, et al. The ethics of childhood obesity treatment—from the Childhood Obesity Task Force (COTF) of European Association for the Study of Obesity (EASO). Obes Facts 2014;7:274–81.
Finkelstein EA, Graham WC, Malhotra R. Lifetime direct medical costs of childhood obesity. Pediatrics 2014;133:854–62.
Author information
Authors and Affiliations
Corresponding author
PowerPoint slides
Rights and permissions
About this article
Cite this article
Bruyndonckx, L., Hoymans, V., Lemmens, K. et al. Childhood obesity–related endothelial dysfunction: an update on pathophysiological mechanisms and diagnostic advancements. Pediatr Res 79, 831–837 (2016). https://doi.org/10.1038/pr.2016.22
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2016.22
- Springer Nature America, Inc.
This article is cited by
-
Ambulatory blood pressure monitoring in children and adolescents post-hematopoietic stem cell transplantation
Pediatric Nephrology (2024)
-
Multiple non-invasive peripheral vascular function parameters with obesity and cardiometabolic risk indicators in school-aged children
BMC Pediatrics (2022)
-
Supportive treatment of vascular dysfunction in pediatric subjects with obesity: the OBELIX study
Nutrition & Diabetes (2022)
-
Effects of bariatric surgery on retinal microvascular architecture in obese patients
International Journal of Obesity (2019)
-
Endothelial function in children with white-coat hypertension
Heart and Vessels (2018)