Abstract
Background:
We explored the relationship between tympanic membrane displacement (TMD) measurements, a tool to monitor intracranial pressure noninvasively, and clinical features and death in children with acute coma in Kilifi, Kenya.
Methods:
Between November 2007 and September 2009, we made serial TMD measurements and clinical observations on children with acute coma (Blantyre coma score (BCS) ≤ 2) on the pediatric high dependency unit of Kilifi District Hospital, and on well children presenting to the hospital’s outpatient department for routine follow-up. We examined middle ear function using tympanometry and measured cardiac pulse (CPA) and respiratory pulse pressure amplitudes (RPA) using the TMD analyzer.
Results:
We recruited 75 children (32 (43%) females; median age 3.3 (IQR: 2.0, 4.3) years). Twenty-one (28%) children died. Higher TMD measurements predicted death. Adjusting for diagnosis, every 50 nl rise in both semirecumbent and recumbent CPA was associated with increased odds of death associated with intracranial herniation (OR: 1.61, 95% confidence interval (CI): 1.07, 2.41; P = 0.02 and OR: 1.35, 95% CI: 1.10, 1.66; P ≤ 0.01 respectively).
Conclusion:
Raised TMD pulse pressure measurements are associated with death and may be useful in detecting and monitoring risk of intracranial herniation and intracranial pressure in childhood coma.
Similar content being viewed by others
Main
Acute coma is a common severe neurological presentation of infectious diseases in children. In sub-Saharan Africa, it is most frequently caused by cerebral malaria (CM), acute bacterial meningitis (ABM), and viral encephalitides. These diseases are associated with high mortality and neurocognitive sequelae among survivors. Predictors of poor outcome include deep coma, recurrent seizures, shock, and raised intracranial pressure (ICP). Raised ICP is common in all these encephalopathies. It impairs cerebral perfusion, leading to ischemic brain injury, and may result in death due to intracranial herniation. Indeed, catastrophic intracranial herniation is well described in association with a variety of encephalopathic illnesses, and is a real risk in the acute setting, including well-resourced setups.
Intensive monitoring of ICP may help to identify patients at risk of herniation and improve outcome. However, tools for monitoring ICP are invasive and require technical proficiency to ensure safety and accuracy, and cannot therefore be placed promptly in the acutely unconscious patient at risk of herniation. Their use is complicated by probe displacement, hemorrhage, and infection, which are particularly pertinent limitations in resource poor sub-Saharan Africa (1). Noninvasive tools could circumvent these limitations, provide insights into the pathophysiology of these encephalopathies, and allow for development of appropriate interventions to improve outcome.
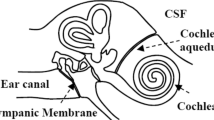
Intra-aural pressure measurements using the Tympanic Membrane Displacement (TMD) analyzer may be of value in noninvasive monitoring of ICP dynamics, and in identifying patients at greatest risk of intracranial herniation. The TMD analyzer utilizes the communication between the subarachnoid space and the inner ear through the cochlear aqueduct which allows for transmission of ICP dynamics to the perilymphatic space (2). The resultant change in perilymphatic pressure dynamics causes alteration in the kinematics of the middle ear ossicles causing volume displacements of the tympanic membrane (3,4). These nanolitre (10−9 l) displacements can be detected by the TMD analyzer through an air displacement sensor probe sealed onto the external auditory meatus. Previous studies have suggested significant correlation between baseline TMD measurements, derived through stimulation of the stapedial reflex, and direct ICP measurements, albeit with concerns over significant intersubject variability (5). Normal variations in ICP dynamics caused by systemic pressure changes due to the cardiac and respiratory cycles can also be perceived by the TMD analyzer (Supplementary Figure S1 online). Intracranial pathology may increase these pulsatile variations due to reduced intracranial compliance secondary to raised ICP. Thus, TMD analyzer measurement of these variations (cardiac pulse amplitudes (CPA) and respiratory pulse amplitudes (RPA)) may provide an indirect measure of changes in ICP dynamics. These TMD pulse pressure measurements have not been well studied.
In this study, we sought to explore the relationship between TMD cardiac and respiratory pulse amplitude measurements and clinical features of raised ICP, and death, in children presenting with acute nontraumatic coma in a rural district hospital in Kilifi, Kenya.
Results
Admission Clinical Characteristics and Outcome
We recruited 75 children out of an eligible 113 at the high dependency unit ( Figure 1 ). There were no differences in the median age, sex, depth of coma at admission, diagnosis, and mortality, between those who were recruited and those who were not (Supplementary Table S1 online). The median age was 3.3 (IQR: 2.0–4.3) years, and 32 (43%) were female. Forty children (53%) had CM, 23 (31%) unknown encephalopathy, 8 (11%) ABM, and 4 (5%) had sepsis. The median duration of coma at the time of admission was 3.5 (IQR 1–6) hours. Children recruited at the outpatient department (OPD) were of median age 5.6 (IQR: 4.2, 7.7) years.
Study flow chart for children in the high dependency unit.
No child received an osmotic agent as part of initial resuscitation. At admission, only irregular respiration was independently associated with death (OR: 4.9 (95% confidence interval (CI): 1.1, 22.3) ( Table 1 ). The median duration of resuscitation from the time of admission to the start of study observations was 2.9 (IQR: 1.5–6.2) hours. Twenty-one (28%) children died; 15 (71%) of the deaths were immediately preceded by clinical syndromes suggestive of intracranial herniation (median admission observation lead time of 5 (IQR: 2, 10) hours). Seventeen (80%) of the children who died had clinical features of raised ICP during their admission compared to 14 (26%) among those who survived (P < 0.001).
Nine (43%) children died within the first 24 h of admission. Of the 54 survivors, 9 (17%) woke up within the first 12 h of admission and 22 (41%) were fully conscious at 24 h. Children with ABM or bacteremia had greater risk of death compared to children with CM (OR: 9.4 (95% CI: 1.82, 48.98; P < 0.01). Children with abnormal middle ear function as assessed through tympanometry on admission were more likely to die compared to those with normal tympanometry (OR: 16.3; 95% CI: 1.7, 158.5; P < 0.01) (6).
TMD Measurement, Raised ICP and Death
Admission TMD measurements were successful in 63 (84%) children in the semirecumbent position and in 60 (80%) in the recumbent position. There was good agreement between the two raters (κ = 0.70, P <0.01). CorrespondingTMD measurements were greater for children with clinical features of raised ICP compared to maximal measurements for those without ( Table 2 ). Children with clinical features of raised ICP had greater semirecumbent CPA measurements than normal outpatient children although this difference was not as remarkable as that between high dependency unit children with and without clinical features of raised ICP. By itself, no clinical feature considered as part of the criteria for raised ICP or progressive herniation presented significantly different TMD measurements between those with the feature and those without.
Except for RPA measurements in the recumbent position, children who died had significantly higher admission TMD measurements compared to those who survived ( Table 3 ).
Adjusting for diagnosis, every 50 nl rise in both semirecumbent and recumbent CPA was associated with increased odds of death associated with intracranial herniation (OR: 1.61, 95% CI: 1.07, 2.41; P = 0.02 and OR: 1.35, 95% CI: 1.10, 1.66; P ≤ 0.01 respectively). Similarly, 50 nl increases in semirecumbent and recumbent RPA, although not statistically significant, suggested a 13% increase in odds of death (OR: 1.13, 95% CI: 0.98, 1.31; P = 0.10 and OR: 1.09, 95% CI: 0.94, 1.25; P = 0.25 respectively). Children whose death was preceded by features of intracranial herniation had significantly higher semirecumbent and recumbent CPA measurements compared to those who did not have these features as assessed using the Kruskal-Wallis test; (semirecumbent median 243 (IQR: 158, 373) nl vs. 132 (IQR: 123, 167) nl; P = 0.03 and recumbent median CPA 347 (IQR: 171, 521) nl vs. 157 (IQR: 136, 203) nl; P = 0.02) ( Figures 2 and 3 ). These differences were not apparent for RPA measurements.
Tympanic membrane displacement (TMD) measurements vs. outcome in association with herniation. CPA, cardiac pulse amplitude; RPA, respiratory pulse amplitude. Median TMD measurement measurements were greater among children whose death was preceded by clinical features suggestive of herniation, compared to those whose deaths were not associated with clinical signs of herniation, and those who survived.
Serial measurements of cardiac pulse amplitude (CPA) in semirecumbent (a) and recumbent postures (b). Children who had syndromes of herniation preceding their deaths appeared to have the highest measurements. Key Filled circle, Survived intact; Filled triangle, survived with sequelae; Filled box, Died without herniation; Filled inverted triangle, Herniated and Died.
Discussion
One of the greatest priorities in research on the management of children with encephalopathic illnesses in sub-Saharan Africa is that of developing simple and inexpensive tools for diagnosis and monitoring the effect of treatment. In children with acute coma, such tools could be used to detect important risk factors for poor neurological outcomes, potentially advance understanding of the pathophysiology of nontraumatic encephalopathies and facilitate the development of effective life-saving interventions. Raised ICP is a common complication of childhood nontraumatic encephalopathies in sub-Saharan Africa and is associated with poor outcome. The need to develop noninvasive tools for monitoring ICP dynamics is one such priority.
In this study, we explored the relationship between TMD pulse amplitude measurements and outcome of children presenting to our hospital with acute nontraumatic coma. The TMD pulse amplitude measurements are likely a measure of the variations in ICP dynamics due to the cardiac (CPA) and respiratory (RPA) cycles through a window between the intracranial compartment and the inner ear. We demonstrated that children who died had greater semirecumbent CPA and RPA, and recumbent CPA, than those who survived. Further, among those who died, children whose deaths were preceded by features of intracranial herniation, had higher CPA measurements than those who did not have these features. Children with clinical features of raised ICP had higher TMD measurements than those without. However, the difference in TMD measurements between children with raised ICP and normal OPD children was not remarkable. Although the OPD children did not present the optimal group to derive data from, their information raises the possibility of preexisting abnormalities in intracranial pressure dynamics, perhaps in relation to middle ear disease, in the general pediatric population, predisposing them to adverse outcomes in case of a neurological insult (6).
Systemic variations in pressure and flow cause fluctuations in ICP and intracranial fluid flow. Thus, arterial blood pressure variations due to the cardiac cycle cause concurrent variations in ICP and fluid flow. To a lesser extent, respiratory and vasomotor induced oscillations also affect ICP and flow. This pressure pulsatility can be modified by altered intracranial compliance, defined by an exponential pressure and volume relationship in the cranium (1). Accordingly, reduced intracranial compliance, perhaps due to increased ICP or intracranial herniation, causes greater pulsatile variations, which is therefore an indirect gauge of ICP dynamics. We think that the TMD analyzer provide a measure of these pulsatile variations through the ear canal.
Intracranial pulsatility can also be affected by restrictions in the venous or cerebrospinal fluid (CSF) outflow pathways as observed in Arnold-Chiari malformation. Theoretically, chronic anemias, rickets, and other skeletal dysplasias may similarly modify the cranio-cerebral anatomical configuration and result in altered ICP pulsatility. Thus, the relationship between ICP and pressure pulsatility, whether measured by the TMD analyzer or any other method, may not always be consistent. Indeed, a finding of high pulsatile pressure variations among adult patients with subarachnoid hemorrhage was demonstrated in only 60% of observed instances of raised ICP (7). In our data, differences in TMD measurements between those who died and those who survived were greater for the semirecumbent state. Nonetheless, high pulsatile pressure variations as measured by the TMD analyzer represent increased risk of death, either as a result of raised ICP or intracranial herniation.
Our study sample size was small, hence the wide confidence intervals in some the results; a larger study could help confirm our findings. Since this study was conducted in a resource poor environment, the management of the comatose children was not optimal; we were not able to ventilate the patients, monitor ICP, undertake cooling, or perform imaging studies. Thus, the amount of information that we could derive from our study was limited. However, our setting is very similar to the situation for a child arriving in casualty anywhere in the World; there is a significant risk of death from herniation before an ICP monitor can be inserted. These data suggest that TMD may be a useful technique for predicting that risk when clinical signs are subtle or unavailable as the priority for the patient is intubation and ventilation requiring sedation and paralysis.
Intra-aural pressure measurements may be useful for detecting and monitoring altered ICP dynamics and risk of intracranial herniation in children with acute coma. Studies incorporating imaging and direct ICP measurements will be useful in elucidating the relationship between the middle ear and ICP dynamics.
Methods
The study was carried out at the pediatric high dependency unit of Kilifi District Hospital on the rural coast of Kenya. The study was approved by the Kenya Medical Research Institute Ethics Review Committee (SSC 1249) and was conducted between November 2007 and September 2009. We made observations on children aged between 6 mo and 13 y presenting in coma (Blantyre Coma Score (BCS) ≤2, persisting for more than 30 min after correction of hypoglycemia or treatment of a seizure). To determine values in awake children, we evaluated children (aged 2–13 y) presenting at the Kilifi District Hospital OPD for consideration for recruitment into a study on moderate malnutrition but found not to be eligible because they were well-nourished. We excluded children who had abnormal middle ear function as determined by tympanometry and those with signs and symptoms of neurological illnesses. Separate data from some of the children recruited into this study have previously been published (8). We recorded clinical signs consistent with herniation of the brain through the tentorium or the foramen magnum (pupil size and reaction to light, oculo-vestibular reflexes, decorticate or decerebrate posturing, respiratory pattern abnormalities) on admission and at 4 hourly intervals until the child regained consciousness or died. FK, who was blinded to the TMD data, determined whether there was clinical evidence of cerebral herniation (9). We excluded children known to have sickle cell disease, epilepsy, or developmental delay, considering the possibility of pre-existent altered ICP dynamics and the likely difficulty in distinguishing the role of these comorbid conditions in the course of the encephalopathy, and determining new neurological sequelae as an outcome.
Standard Care
At admission, we provided emergency care based on standard guidelines for investigation and management (10,11). We performed lumbar puncture when the children were stable and examined the CSF for evidence of infection. All children received first-line parenteral antibiotics (benzyl-penicillin and chloramphenicol) and antimalarial (intravenous quinine or artesunate) therapy until otherwise guided by the results of three initial malaria slide microscopy done 8 h apart, and bacterial culture. We classified children as having CM based on the WHO definition; coma in a child with malaria parasitemia in the absence of evidence for an alternative explanation for cause of illness (12). We considered a diagnosis of ABM when bacteria were isolated on CSF culture, detected on Gram staining or bacterial antigen testing, or when there was a CSF leucocyte count of at least 10 per µl and the CSF to blood glucose ratio was less than 0.67. Children who had blood culture confirmed bacteremia in the absence of any indication of ABM were assumed to have sepsis. We classified children who did not have any history of trauma and no indication of CM, ABM, or bacteremia, as encephalopathy of unknown etiology.
Study Procedure
Upon obtaining consent from the parents or guardians, we examined the children’s ears using an otoscope and a handheld tympanometer (Interacoustics AS, Assens, Denmark). We classified tympanometry measurements as either normal or abnormal using the Liden and Jerger criteria (13). This classification is based on a plot of the compliance of the tympanic membrane and the middle ear pressure. For consistency, we used the right ear for TMD measurements unless tympanometry was abnormal on that side and normal on the left. When possible, we took measurements in both semirecumbent (at an angle of ~45 degrees) and recumbent positions. Beyond screening for the study, normal children from the OPD did not undergo any extra laboratory or radiological procedures. Peak-to-peak amplitudes of the TMD cardiac and respiratory pulses were measured by two raters (S.G. and Emma Digby). The TMD analyzer was calibrated within 50 m of sea level, a similar altitude to our setting.
Statistical Analysis
Data were captured onto a Filemaker Pro 10 version 1database system (FileMaker, Santa Clara, CA) and analyzed using Intercooled STATA software version 11.0 (StataCorp LP, College Station, TX). We explored continuous data for any violations of the normality assumption visually using scatter and histograms plots and formally by applying the Shapiro-Wilk test. For continuous data, we applied the Student’s t-test, Kruskal Wallis, and Mann-Whitney rank sum test, to examine the mean differences in TMD measurements as appropriate. We applied the χ2 and Fisher’s exact tests of associations as appropriate for categorical data. To examine for the association between mortality and TMD measurements, we applied a multivariable logistic regression model adjusting for diagnosis (cerebral malaria, meningitis, sepsis, and unknown encephalopathy). All statistical significance was assessed at the conventional 5% level and where appropriate, results were reported as odds ratio (OR) with 95% CI.
Statement of Financial Support
This study was supported by the Wellcome Trust, UK, through a fellowship awarded to C.N. (070114). S.G. was supported by the Royal Society of Tropical Medicine and Hygiene Centenary Scholarship to analyze this work.
Disclosure
R.M. is a majority shareholder in Marchbanks Measurement Systems Ltd, a non-profit making company and spin-out from Southampton University that manufactures the TMD Analyzer for clinical evaluation purposes. The rest of the authors declare no conflict of interest.
References
Czosnyka M, Pickard JD. Monitoring and interpretation of intracranial pressure. J Neurol Neurosurg Psychiatry 2004;75:813–21.
Traboulsi R, Avan P. Transmission of infrasonic pressure waves from cerebrospinal to intralabyrinthine fluids through the human cochlear aqueduct: Non-invasive measurements with otoacoustic emissions. Hear Res 2007;233:30–9.
Thalen EO, Wit HP, Segenhout JM, Albers FW. Dynamics of inner ear pressure change caused by intracranial pressure manipulation in the guinea pig. Acta Otolaryngol 2001;121:470–6.
Samuel M, Burge DM, Marchbanks RJ. Tympanic membrane displacement testing in regular assessment of intracranial pressure in eight children with shunted hydrocephalus. J Neurosurg 1998;88:983–95.
Reid A, Marchbanks RJ, Burge DM, et al. The relationship between intracranial pressure and tympanic membrane displacement. Br J Audiol 1990;24:123–9.
Gwer S, Chengo E, Newton CR, Kirkham FJ. Unexpected relationship between tympanometry and mortality in children with nontraumatic coma. Pediatrics 2013;132:e713–7.
Eide PK, Rapoport BI, Gormley WB, Madsen JR. A dynamic nonlinear relationship between the static and pulsatile components of intracranial pressure in patients with subarachnoid hemorrhage. J Neurosurg 2010;112:616–25.
Gwer S, Sheward V, Birch A, et al. The tympanic membrane displacement analyser for monitoring intracranial pressure in children. Childs Nerv Syst 2013;29:927–33.
Newton CR, Kirkham FJ, Winstanley PA, et al. Intracranial pressure in African children with cerebral malaria. Lancet 1991;337:573–6.
World Health Organization. Pocket book of hospital care for children - guidelines for the management of common illnesses with limited resources. Geneva: World Health Organization, 2005.
Berkley JA, Mwangi I, Ngetsa CJ, et al. Diagnosis of acute bacterial meningitis in children at a district hospital in sub-Saharan Africa. Lancet 2001;357:1753–7.
World Health Organization. Guidelines for the treatment of malaria. 2nd edn. Geneva: World Health Organization, 2010.
Onusko E. Tympanometry. Am Fam Physician 2004;70:1713–20.
Acknowledgements
Emma Digby helped in analysis of measurements for the purpose of inter-rater comparison. Rachel Odhiambo developed the study database and facilitated data entry. Piet Kager of University of Amsterdam reviewed the manuscript before submission. Henry Athiany of London School of Tropical Medicine and Hygiene provided guidance on some aspects of statistical analyses. This manuscript is published with the permission of the Director of Kenya Medical Research Institute.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Figure S1
(TIFF 9273 kb)
Supplementary Table S1
(DOC 33 kb)
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Gwer, S., Kazungu, M., Chengo, E. et al. Abnormal intra-aural pressure waves associated with death in African children with acute nontraumatic coma. Pediatr Res 78, 38–43 (2015). https://doi.org/10.1038/pr.2015.57
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2015.57
- Springer Nature America, Inc.