Abstract
Background:
Impaired autoregulation capacity implies that changes in cerebral perfusion follow changes in blood pressure; however, no analytical method has explored such a signal causality relationship in infants. We sought to develop a method to assess cerebral autoregulation from a mechanistic point of view and explored the predictive capacity of the method to classify infants at risk for adverse outcomes.
Methods:
The partial directed coherence (PDC) method, which considers synchronicity and directionality of signal dependence across frequencies, was used to analyze the relationship between spontaneous changes in mean arterial pressure (MAP) and the cerebral tissue oxygenation index (TOI). PDCMAP>>TOI indicated that changes in TOI were induced by MAP changes, and PDCTOI>>MAP indicated the opposite.
Results:
The PDCMAP>>TOI and PDCTOI>>MAP values differed. PDCMAP>>TOI adjusted by gestational age predicted low superior vena cava flow (≤41 ml/kg per min), with an area under the receiver operating characteristic curve of 0.72 (95% CI: 0.63–0.81; P < 0.001), whereas PDCTOI>>MAP did not. The adjusted pPDCMAP>>TOI (the average value per patient) predicted severe intracranial hemorrhage and mortality.
Conclusion:
PDCMAP>>TOI allows for a noninvasive physiological interpretation of the pressure autoregulation process in neonates. PDCMAP>>TOI is a good classifier for infants at risk of brain hypoperfusion and adverse outcomes.
Similar content being viewed by others
Main
Cerebral blood flow autoregulation is a complex multifactorial process that has not yet been fully characterized in neonates. The concept of impaired autoregulation implies that changes in cerebral blood flow follow changes in perfusion pressure (1); however, no analytical method has yet explored such a signal causality relationship in infants.
We recently described a new time-frequency domain method to analyze the relationship between two biological signals: bivariate autoregressive spectral coherence (BiAR-COH) (2). This method, which assesses the cerebral autoregulation capacity of preterm infants, notably improved our ability to predict the classification of infants at risk of poor perfusion states when compared with other spectral coherence methods (COH) (3,4). We aimed to further assess cerebral autoregulation capacity from a mechanistic point of view. This capacity is relevant to providing pathophysiological support for the interpretation of information provided by monitoring devices currently in use in clinical practice. An association between arterial pressure changes and cerebral blood flow changes does not mean that the former alters the latter.
The partial directed coherence (PDC) method (5) considers the synchronicity and directionality of signal dependence across frequencies. With respect to synchronicity, the PDC method only takes into account the changes in signals that are closely related in time. In terms of directionality, the PDC method identifies the source of changes, i.e., the changes in signal B that are the physical consequence of changes in signal A (6). Accordingly, the PDC method returns all potential combinations between signals: PDCA>>B and PDCB>>A.
The main objective of this study was to examine whether spontaneous changes in perfusion and oxygenation in the brain, as measured by near-infrared spectroscopy (NIRS), are induced by changes in perfusion pressure; i.e., we explored the pressure autoregulation capacity. We analyzed the spectral causality between two biological signals by quantifying the influence of each signal on the other by means of PDC analyses. For this purpose, we used the tissue oxygenation index (TOI) (as a surrogate of cerebral perfusion) and the continuous mean arterial pressure (MAP). Accordingly, we can infer that PDCMAP>>TOI would quantify the changes in TOI that are influenced by blood pressure and that PDCTOI>>MAP quantifies the reverse. In addition, we explored the predictive capacity of the method to classify infants at risk of cerebral hypoperfusion, as defined by low superior vena cava (SVC) flow and adverse neonatal outcomes.
Results
We studied 54 infants who underwent simultaneous invasive blood pressure monitoring, NIRS and peripheral oxygen saturation (SaO2) monitoring during the first 2 d after birth. These infants had early (first 12 h of life) SVC flow assessments (a minimum of two evaluations, 60 min apart) and were included in an interventional placebo-controlled trial of dobutamine if their SVC flow was ≤ 41 ml/k/min at any time during the first 24 h from birth (7). The total cumulative recording time was 9.5 (3.4) hours (4.3–21.5), which resulted in a mean number of 30-min epochs that satisfied the quality criteria for offline analysis of 6 (1–23). The patients’ mean postnatal age at the first epoch suitable for analysis was 6 (3) hours. Details on the clinical features of the study population are described in Table 1 . Briefly, the mean birth weight was 930 (257) g and the mean gestational age 27.0 (1.9) weeks. At the time the autoregulation studies were conducted, 11 infants were being administered cardiovascular medications due to circulatory impairment and 27 were placed on invasive mechanical ventilation. The serial echocardiography assessment showed that 9 of the 54 infants had SVC flow below the cut-off value of 41 ml/kg per min (defined as low SVC flow) (7,8,9) at some point during the study. During the observation period, the SVC flow measurement closest to a given epoch was recorded. Accordingly, while SVC flow was below 41 ml/kg per min, the time interval between the scan and the epoch was less than 31 min (27.1). This interval was longer (277 min (177)) when the SVC flow was consistently above 41 ml/kg per min. Low SVC flow was present in 12% of the analyzed epochs. All the infants showed stable SVC flow well above the cut-off value in the subsequent studies once SVC flow had normalized.
PDC Analyses
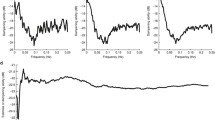
PDCMAP>>TOI and PDCTOI>>MAP analyses yielded different values from each other and were not associated (P < 0.185) ( Figure 1 ).
Two examples of epochs and their corresponding partial directed coherence (PDC) are displayed. The upper panels (a,b) represent tissue oxygenation index (TOI) (black line) and mean arterial pressure (MAP) (gray line) changes. The lower panels (c,d) show the PDC spectral representation, PDCMAP>>TOI (black line) and PDCTOI>>MAP (gray line). The nonshadowed area represents the very low frequency band. The left panels represent MAP influencing TOI (a) and the corresponding PDC values (c). The right panels (b) do not show that influence but instead reflect the opposite, as shown by the high PDCTOI>>MAP values (d). The epoch represented in (a,c) is associated with low SVC flow, whereas the epoch (b,d) is not. The arrows display directionality.
PDCMAP>>TOI predicted low SVC flow (≤41 ml/kg per min), with an area under the receiver operating characteristic (ROC) curve of 0.67 (95% CI: 0.60–0.76; P < 0.001). The prediction capacity of PDCMAP>>TOI adjusted by gestational age (GA) was 0.72 (95% CI: 0.63–0.81; P < 0.001), whereas PDCTOI>>MAP did not predict low SVC flow (P < 0.248) ( Figure 2a ). The highest predictive capacity of PDCMAP>>TOI to classify epochs according to SVC flow was found when the cutoff was set at 31 ml/kg/min (with a ROC of 0.78 (95% CI: 0.68–0.89; P < 0.001). The prediction capacity of PDCMAP>>TOI adjusted by GA was 0.82 (95% CI: 0.72–0.93; P < 0.001) ( Figure 2b ).
The receiver operating characteristic curve shows the performance of PDCMAP>>TOI (black line), PDCMAP>>TOI adjusted by gestational age (gray line) and PDCTOI>>MAP (dashed line) to classify low SVC flow epochs. Panel (a) represents the SVC flow cut-off value of 41 ml/kg/min; and (b) the SVC flow cut-off value of 31 ml/kg/min. MAP, mean arterial pressure; PDC, partial directed coherence; TOI, tissue oxygenation index.
The PDCMAP>>TOI threshold for identifying low SVC flow was 0.554, with a sensitivity and specificity of 0.60 and 0.75, respectively. This threshold defined the PDCMAP>>TOI classifier.
The PDCMAP>>TOI classifier was associated with the percentage of time of MAP below gestational age (GA) minus 5 (MAP<GA-5 mmHg) threshold with a ROC of 0.61 (95% CI: 0.51–0.71; P < 0.037).
To quantify the effect of the autoregulation estimators on patient outcomes, we calculated the average epoch prediction values per patient adjusted by GA, which were defined as pPDCMAP>>TOI and pPDCTOI>>MAP. Early circulatory impairment and the need for cardiovascular support shortly after birth (within the first 24 h) were associated with pPDCMAP>>TOI (OR: 1.362; 95% CI: 1.11–1.67; P < 0.001) ( Figure 3 ) but not with pPDCTOI>>MAP. Severe intracranial hemorrhage (intraventricular grade 3 or periventricular hemorrhagic infarction) was associated with pPDCMAP>>TOI (OR: 4.03; 95% CI: 1.89–8.55; P < 0.003) ( Figure 3 ) but not with pPDCTOI>>MAP. In addition, mortality was associated with pPDCMAP>>TOI (P < 0.010). pPDCMAP>>TOI predicted mortality (OR: 3.13; 95% CI: 1.60–4.45; P < 0.002) ( Figure 3 ), whereas pPDCTOI>>MAP did not.
The pPDCMAP>>TOI classifier for early circulatory impairment and the need for cardiovascular support within 24 h from birth, severe intracranial hemorrhage (intraventricular grade 3 or periventricular hemorrhagic infarction), and death (lined box indicates event present). MAP, mean arterial pressure; TOI, tissue oxygenation index.
Finally, the inclusion of pPDCTOI>>MAP as a covariate of pPDCMAP>>TOI did not improve the predictive capacity of the latter method.
Discussion
This study supports PDCMAP>>TOI as a mechanistic representation of pressure autoregulation capacity in high-risk infants. In this study, we found that the pPDCMAP>>TOI classifier identifies infants at risk of adverse outcomes, such as infants requiring early cardiovascular support due to circulatory impairment, and those who will eventually develop severe intracranial hemorrhage and/or die. Our method also provides snapshots of critical SVC flows and blood pressure levels in relation to autoregulation capacity; which could help improve the strength of other hemodynamic biomarkers that guide treatment decisions.
Autoregulation is defined as the process that keeps blood flow constant, regardless of perfusion pressure (1). Impaired autoregulation therefore implies that a dependence of TOI with respect to MAP is emerging, but not the opposite. This dependence between signals can be quantified statistically. Our method has clearly demonstrated this physiological concept. When used as a covariate, PDCTOI>>MAP does not improve the predictive capacity of PDCMAP>>TOI.
This mechanistic approach is important not only from an academic point of view, but also because the proper interpretation of high-risk patient monitoring is essential. Using SVC flow as an estimate of current brain perfusion, two examples of signal dependence are shown: one represents MAP influencing TOI ( Figure 1a , c ), whereas the other shows MAP not influencing TOI ( Figure 1b , d ). The corresponding PDC values indicate their dependence according to directionality. In the first example ( Figure 1c ), the PDCMAP>>TOI values are high and the epoch is associated with low SVC flow. In the second example ( Figure 1d ), PDCMAP>>TOI values are low and the SVC flow associated with this epoch shows normal values. Although the PDCTOI>>MAP value is high, indicating that TOI changes are influencing MAP changes ( Figure 1b ), this condition is independent of SVC flow status. Classical methods based on frequency domain that are routinely used to assess the signal relationship were inadequate for making distinctions between the examples given above. The examples would have therefore been classified equally as the same phenomenon. However, only the PDCMAP>>TOI is meaningful from a physiological point of view. The advantage of this mechanistic approach to exploring physiological phenomena is that the method has self-validation. The association of this classifier with poorer clinical outcomes further supports the consistency of this pathophysiological information.
PDC, however, did not improve the overall predictive capacity of the BiAR-COH method for adverse outcomes to assess autoregulation in high-risk infants, as was recently reported by our group (2). The BiAR-COH method improved signal dependence interpretation when compared with classical frequency domain methods. This finding was the result of adding the time-domain component (BiAR) to the spectral analyses (COH), which enabled us to discriminate changes that are closely related in time (and are therefore considered dependent) from those that are not time-related and therefore have no mutual dependence. PDC introduces the condition of directionality, such that the system is forced to only consider those events in which changes in perfusion pressure induce changes in cerebral perfusion. To be more precise, the system is only analyzing the pressure autoregulation phenomenon. Thus, other mechanisms that are not strictly involved in the pressure autoregulation process, as it is theoretically known, but that are potentially relevant to the brain oxygen dynamics would be ignored. For example, hypercapnia can increase cerebral blood flow even if the blood pressure is below the lower limit of autoregulation (10). These mechanisms, gathered into the BiAR-COH analyses, can contribute positively to the predictive capacity, although the underlying pathophysiology cannot be inferred. It is of note that the present as well as our previous studies (2,7) used 41 ml/kg/min to define low SVC flow, as generally accepted (8,9). However, according to the results of this study, the SVC flow cutoff value that probably more accurately defines the lower limit of the autoregulatory plateau is 31 ml/kg/min.
We therefore propose PDC to be a valuable complementary analysis to our previous method, the BiAR-COH. The more precise the pathophysiological interpretation, the greater the support for treatment decisions in a clinical setting.
In terms of the study methodology, we preserved the time-domain component (BiAR) as previously reported (2). However, we sought an alternative analytical method (5) to represent the derived time-domain values in the frequency-domain. The selection of epochs to assess autoregulation followed the criteria used in BiAR-COH (2). The PDC method also requires covariance stationarity signals throughout the epoch (11,12), and therefore the mean value of the signal does not change over time, and the autocorrelation function is only time-dependent. These criteria guarantee that the nature of the signal (and its properties) does not change along the epoch, eliminating the influence of the signal itself, and instead focuses on the relationship between signals. The selection of the optimal lags was an automatic process using the Bayesian information criterion (13) and the Akaike information criterion (14), taking the lowest value for each epoch. Thus, operator selection biases were ruled out.
In conclusion, we describe a method that is complementary to BiAR-COH to assess cerebral autoregulation. In addition to its high predictive capacity to classify high-risk infants in terms of neonatal clinical outcomes, the PDCMAP>>TOI method supports that spontaneous changes in perfusion and oxygenation in the brain are induced by changes in perfusion pressure. In other words, the PDCMAP>>TOI provides a mechanistic interpretation of signal dependency and thus a self-validation. This self-validation is relevant because the proper interpretation of the pathophysiology of underlying disease processes is essential to selecting appropriate therapy.
PDCMAP>>TOI assesses only the pressure autoregulation capacity, whereas other components involved in the autoregulation process are not considered. We therefore propose the PDC analysis as a complementary tool to other methods such as BiAR-COH (2).
Methods
Study Procedures
All infants born before 31 wk of gestation who were placed on ventilator support (mean airway pressure ≥4 cm H2O or FiO2 ≥0.3) were prospectively assessed for SVC flow from birth to 96 h. The infants were enrolled in a placebo-controlled trial if the SVC flow was <41 ml/kg per min at any time during the first 24 h after birth (European Union Drug Regulating Authorities Clinical Trials-EudraCT 2009-010901-35; Clinicalgov NCT01605279). Those infants who underwent invasive blood pressure monitoring were considered eligible for the cerebral autoregulation capacity studies. The study protocol was approved by the Ethics Committee for Human Studies at La Paz University Hospital of Madrid (Spain). Written parental informed consent was signed before infants’ enrolment.
The patients’ intravascular pressure (umbilical artery) MAP and SaO2 levels (IntelliVue MP50, Philips, Best, Netherlands) were continuously monitored. TOI was measured with an oximeter (NIRO-200nx oximeter, Hamamatsu Photonics, Fukuoka, Japan), with the sensor placed at the frontal-parietal level. We employed a real-time, simultaneous, time-locked data acquisition system (BioAcSys software, La Paz University Hospital Research Foundation, Madrid, Spain) for the physiological variables and the NIRS-derived TOI. The recording frequency for SaO2, MAP, and TOI was 2 Hz. Signal disruption periods due to infant manipulations or interventions were manually marked.
SVC flow was measured according to the method described by Kluckow and Evans (15). The images were stored and reviewed offline by a single operator who used systematic quality assessment to approve or reject the scans. Standard cranial ultrasound imaging was conducted as soon as possible within the first day, at day 7, day 14, and at term equivalence. Additional studies were scheduled if clinically indicated. The diagnoses were classified according to a previously reported system (16,17).
Data Analyses
An in-house MATLAB toolbox (The MathWorks, Natick, MA) enabled the selection of epochs. Disruption-free zones were automatically segmented into nonoverlapping 30-min epochs if SaO2 levels were stable (less than 5% variation). Each epoch was filtered with a linear high-order low-pass finite-duration impulse response filter at 0.095 Hz and resampled at 0.2 Hz. The mean value of the signal was subtracted.
PDC analyses require the condition of covariance stationarity, which implies that the source of the given signal is constant. Stationarity was tested using the augmented Dickey-Fuller and Kwiatkowski-Phillips-Schmidt-Shin tests (11,12), which were applied separately for the MAP and TOI epoch waveforms.
PDC is a frequency domain representation of the linear relationship between pairs of time series (5). This linear relationship is based on the Granger causality (6), which is defined as the condition in which a time series x(n) is caused by y(x) only if knowledge of the past samples of y(n) helps in predicting the future samples of x.
A frequency-domain interpretation of Granger causality enables us to assess autoregulation across the frequencies that are known to be of most interest in this process. In our case, the Granger causality can be modeled by a bivariate autoregressive process of order p (Equation 1), as follows:

The model order can be found using any method derived from the criteria information theory. In our case, Bayesian information criterion and Akaike information criterion were used (13,14), taking the lowest value (between 3 and 25 samples). Each coefficients’ vector (ax(r)) represents the contribution of lagged samples of each signal to predict the current sample (map(n) and toi(n)). There are as many ax values as model order p. The variables map(n-r) and toi(n-r) represent the lagged samples. The variables wmap(t) and wtoi(t) were used as the prediction error.
Transforming all the coefficients’ vectors to the frequency domain leads to the following equation:

PDC is derived from the following equation (Equation 3):

where the superscript (*) indicates the conjugate transpose.
The variable amap(r) represents the self-contribution of lagged MAP samples in predicting MAP. This concept is expressed in the frequency domain as Âmap(f). The variable amap>>toi(r) represents the contribution of lagged MAP samples in predicting TOI, which is expressed as Âmap>>toi(f) in the frequency domain. The PDCmap>>toi(f) results from the ratio of the signal dependence coefficients and the sum of all contributions (self-contributions and induced contributions).
Each PDC was averaged over the frequency bands of 0.003–0.04 Hz (very low frequency band) to obtain a single PDC value.
Statistics
The data were analyzed using SAS 9.2 statistical software (Cary, NC). The quantitative data are given as means (±SD) and the qualitative data as counts and percentages. ROC curves were used to analyze the predictive capacity of the autoregulation estimators (PDCMAP>>TOI and PDCTOI>>MAP) on clinical outcomes per epoch (adjusted and unadjusted by GA). The autoregulation threshold was calculated using the maximum value of Youden’s index. To quantify the effect of the autoregulation estimators on patient outcome, generalized estimating equations were used to adjust the effect of repeated measures (multiple epochs) in a given patient, adjusted by GA. Accordingly, pPDCMAP>>TOI and pPDCTOI>>MAP were defined as the averaged epoch prediction values per patient.
Statement of Financial Support
The corresponding author acknowledges the financial support of the Spanish Healthcare Research Foundation (Spanish Fondo de Investigación Sanitaria, grant SAS/2481/2009) and the SAMID network (RD08/0072/0018 and RD12/0026/0004).
Disclosure
This study was presented in part at the 2014 Annual Meeting of the European Society for Pediatric Research. The authors have no potential conflicts of interest. The first author who wrote the first draft did not receive an honorarium to produce the manuscript.
References
Lassen NA. Cerebral blood flow and oxygen consumption in man. Physiol Rev 1959;39:183–238.
Riera J, Cabañas F, Serrano JJ, et al. New time-frequency method for cerebral autoregulation in newborns: predictive capacity for clinical outcomes. J Pediatr 2014;165:897–902.e1.
Soul JS, Hammer PE, Tsuji M, et al. Fluctuating pressure-passivity is common in the cerebral circulation of sick premature infants. Pediatr Res 2007;61:467–73.
Wong FY, Leung TS, Austin T, et al. Impaired autoregulation in preterm infants identified by using spatially resolved spectroscopy. Pediatrics 2008;121:e604–11.
Baccalá LA, Sameshima K. Partial directed coherence: a new concept in neural structure determination. Biol Cybern 2001;84:463–74.
Granger CWJ. Investigating causal relations by econometric models and cross-spectral methods. Econometrica 1969;37:424–38.
Bravo MC, López-Ortego P, Sánchez L, et al. Randomized, Placebo-Controlled Trial of Dobutamine for Low Superior Vena Cava Flow in Infants. J Pediatr 2015;167:572–8.e1–2.
Osborn D, Evans N, Kluckow M. Randomized trial of dobutamine versus dopamine in preterm infants with low systemic blood flow. J Pediatr 2002;140:183–91.
Osborn DA, Evans N, Kluckow M. Hemodynamic and antecedent risk factors of early and late periventricular/intraventricular hemorrhage in premature infants. Pediatrics 2003;112(1 Pt 1):33–9.
Häggendahl E, Johanson B. Effects of arterial carbon dioxide tension and oxygen saturation on cerebral blood flow autoregulation in dogs. Acta Physiol Scand 1965;66:Suppl 258:27–53.
Dickey DA, Fuller WA. Distribution of the estimators for autoregressive time series with a unit root. J Am Statist Assoc 1979;74:427–31.
Kwiatkowski D, Philips PCB, Schmidt P, Shin Y. Testing the null hypothesis of stationarity against the alternative of a unit root. J Econometrics 1992;54:159–78.
Schwarz G. Estimating the dimension of a model. Ann Stat 1978;6:461–64.
Akaike H. A new look at the statistical model identification. IEEE T Automat Contr 1974;19:716–23.
Kluckow M, Evans N. Superior vena cava flow in newborn infants: a novel marker of systemic blood flow. Arch Dis Child Fetal Neonatal Ed 2000;82:182–87.
Pellicer A, Valverde E, Elorza MD, et al. Cardiovascular support for low birth weight infants and cerebral hemodynamics: a randomized, blinded, clinical trial. Pediatrics 2005;115:1501–12.
Pellicer A, Bravo MC, Madero R, Salas S, Quero J, Cabañas F. Early systemic hypotension and vasopressor support in low birth weight infants: impact on neurodevelopment. Pediatrics 2009;123:1369–76.
Acknowledgements
The authors would like to thank Maria C Bravo, Laura Sanchez, and Paloma Lopez-Ortego for their contribution to the infants’ monitoring.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Riera, J., Cabañas, F., Serrano, J. et al. New developments in cerebral blood flow autoregulation analysis in preterm infants: a mechanistic approach. Pediatr Res 79, 460–465 (2016). https://doi.org/10.1038/pr.2015.231
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2015.231
- Springer Nature America, Inc.
This article is cited by
-
Near-infrared spectroscopy monitoring of neonatal cerebrovascular reactivity: where are we now?
Pediatric Research (2023)
-
Diffuse correlation spectroscopy blood flow monitoring for intraventricular hemorrhage vulnerability in extremely low gestational age newborns
Scientific Reports (2022)
-
Diagnostic and predictive value of Doppler ultrasound for evaluation of the brain circulation in preterm infants: a systematic review
Pediatric Research (2020)
-
Neonatal cerebrovascular autoregulation
Pediatric Research (2018)