Abstract
Surfaces of cyclo-olefin polymers (COPs) are photoactivated by vacuum ultraviolet (VUV) light and can be bonded with a practical bonding strength at low temperatures below Tg. The VUV irradiation condition was optimized, the maximum interfacial toughness was obtained for 5 min of irradiation and a longer irradiation time resulted in a decrease in toughness. We also found that a high-humidity environment caused the decrease in toughness. We investigated the mechanism of the low-temperature bonding of COP by characterizing the fracture surfaces using high-resolution scanning electron microscopy and imaging the bonded interfaces using energy-filtering transmission electron microscopy and scanning transmission electron microscopy. The fracture surfaces exhibited a large number of nanofibrils, whose features are similar to those we previously observed in the polymer–polymer interfaces that were welded in melt conditions. We conclude that the interfacial failure occurs where the polymer chains connected at the interface by hydrogen bonding are pulled out. Thus, the entanglement is important in the bonding of polymers by surface-activation processes.
Similar content being viewed by others
Introduction
Cyclo-olefin polymer (COP) is a low-cost amorphous polymer with various attractive features such as high transparency, high heat resistance, low water absorption, stable refractive index and low birefringence. Owing to those outstanding properties, COP has been used in many opt-electrical and medical applications. Because of the simple chemical structure as shown in Figure 1, which has no effective polar functional groups for adhesion, surface modification is necessary to achieve the high adhesion of COP.1 There has been extensive work to develop practical methods to modify the COP surface. For this purpose, several oxidative processes, including corona discharge treatment,2, 3, 4, 5 plasma etching,6, 7 ultraviolet irradiation8 and chemical solution etching,9 have been investigated for COP surface modification. Those methods can effectively introduce oxygen-containing chemical moieties onto the COP surfaces. Surface photografting modification in a gas or liquid phase has also attracted widespread attention.9, 10, 11, 12, 13 These surface modification techniques can effectively enhance surface activation by modifying the surface topography, chemical composition and surface energy. However, the mechanism underlying adhesion promotion through such surface modifications has not been fully understood.
Sugimura and co-workers14 investigated COP surface activation by vacuum ultraviolet (VUV) treatment. The wavelength of VUV is shorter than 200 nm, enabling selective and specific chemical modification of solid surfaces to be performed with a small degree of bulk part damage. Because VUV has more photon energy than conventional UV light, it dissociates polymer chemical bonds more effectively than conventional UV irradiation. Sugimura and co-workers14 investigated how VUV treatment modified the COP surface to produce a hydrophilic surface using an excimer lamp as a VUV source at a wavelength of 172 nm. The resulting functional groups generated on the COP surfaces were discussed in terms of the VUV irradiation conditions. Oxygen-containing functional groups such as ether, ketone and carboxyl groups were generated.
The COP surface modification by VUV was found to be effective for the adhesiveless direct bonding of COP plates. In particular, the VUV-modified COP plates were bonded with sufficient adhesion strength at low temperatures below the glass transition temperature (Tg) of COP through attractive interactions among the functional groups. To bond a pair of plastic plates, the plastic surfaces must come into contact at a molecular scale to form intramolecular entanglements or chemical interactions, including covalent and non-covalent bond formations at the interface. For this purpose, certain degrees of pressure and heat are commonly applied to achieve sufficient adhesion, which results in the deformation and loss of small features of the products to be bonded. Low-temperature bonding is an effective method for the fabrication of devices such as microchips with shallow microchannels that can preserve the original submicrometer-scale features in COP plates.15
In this study, we investigated the mechanism of the low-temperature direct bonding of VUV-treated COP by performing electron microscopy characterizations. The techniques used in this work were reported in our previous papers.16, 17 The fracture mechanism was studied by investigating fracture surfaces with nanometer scale (nanofractography) using low-voltage scanning electron microscopy. The cross-sections of the bonded interface are investigated using energy-filtered transmission electron microscopy (EFTEM) with electron energy-loss spectroscopy and scanning transmission electron microscopy (STEM) with energy-dispersive X-ray spectrometry (EDX). The interfacial adhesion strength was also evaluated, using the double cantilever beam (DCB) test, which estimates the interfacial fracture energy to elucidate the effect of the irradiation condition and moisture on the adhesion properties. Combining those experimental works, we propose the mechanism of low-temperature bonding of COP.
Experimental procedure
VUV-light treatment on COP surface
COP (ZEONEX 480R; ZEON Corp., Tokyo, Japan) was injection molded into transparent plates 1 mm thick. The UER20-172 VUV irradiation apparatus (Ushio, Tokyo, Japan) generates VUV light at a wavelength of 172 nm with the intensity of 10 mW cm−2. The details of the apparatus were previously described.14 The COP plates were placed on the sample stage in the irradiation chamber in ambient air. The distance between the lamp window and the sample surface was fixed at 5 mm, and the irradiation time was varied from 2 to 40 min.
Low-temperature direct bonding of COP plates
Immediately after the VUV treatment, the COP plates were bonded under a pressure of 2.4 MPa at 110 °C for 10 min. Tg of COP is ~140 °C;1 thus, the bonding temperature is sufficiently lower than Tg, which caused no significant deformation of the plates during the bonding process. We also investigated the bonding behavior under a conventional melt condition. Two plates (5 cm × 5 cm) were laminated at 150 °C for different periods in nitrogen atmosphere. To ensure good contact between the two surfaces, two plates were joined together under slight pressure for the first 5 min, and then the plates were simply welded by spontaneous interdiffusion of the polymers under no additional pressure.
Measurements of interfacial toughness
The interfacial adhesion strength was evaluated using the DCB test. The detailed procedure and effectiveness of this method for the evaluation of interfacial toughness are described elsewhere.18, 19 After quenching the specimen to room temperature to freeze the interdiffusion, a razor blade with thickness Δ was driven into the interface between the two plastic sheets to propagate an interfacial crack, as shown in Figure 2. When the crack propagation stopped, the length a in front of the blade was measured under a microscope. The blade was then inserted further, and another crack was made. The critical interfacial toughness Gc was calculated using the equation described by Brown and co-workers,18 where C=1+0.64(h/a), E is the modulus (1.83 GPa) and h is the thickness of the beam. The average and s.d.s were calculated with at least 10 data points for each laminate.

Electron microscopy
A Carl Zeiss ULTRA55 field emission scanning electron microscope (Carl Zeiss SMT, Oberkochen, Germany), integrating the in-lens detector system, was operated at an accelerating voltage of 2 kV to observe the fracture surfaces produced by the DCB tests. To avoid the charging problem, the samples were coated with osmium using an OP80NT osmium plasma coater (Filgen, Nagoya, Japan) with OsO4 as the source material.
EFTEM and STEM were performed for the imaging and chemical analysis of the interfaces between the COP plates. Thin sections (<70 nm) were cut using ultramicrotomy; the cutting direction was adjusted to an angle of 90° between the interface and the edge of the diamond knife. For the focus adjustment during the TEM operation and drift correction for element mapping, gold nanoparticles with 10 nm diameter were dispersed onto the thin sections using a dilute gold colloid aqueous solution. EFTEM was performed with Libra920MC (Carl Zeiss SMT), which was equipped with a Schottky field emission system and a Corrected-Omega-type energy filter. The detailed procedure for electron energy-loss spectroscopy and elemental mapping was described in our papers.20, 21, 22 STEM was performed with TECNAI Osiris (FEI Company, Hillsboro, OR, USA), which was equipped with a bright-field (BF) detector, annular dark-field (ADF) detectors and four windowless silicon drift EDX detectors, which were placed symmetrically around the optical axis near the specimen area. This EDX system (Super-X) significantly enhances the EDX detection sensitivity, particularly for light elements, which enables us to rapidly detect a small amount of light elements in polymer materials.23 All work was performed at an accelerating voltage of 200 kV.
Results and discussion
Interfacial fracture behavior of high-temperature bonded COP
To study the low-temperature bonding of COP, we first investigated the adhesion and fracture behavior of COP welded at a temperature above Tg. Figure 3 shows the interfacial fracture toughness (Gc) between COP plates welded at 150 °C as a function of the welding time. Gc steadily increases with welding time. The welding of identical polymers has been well studied with various polymers.24, 25, 26 There are three stages associated with the Gc dependence on the welding time. In the first stage, Gc is quite low, and its increase is less steep with time. In the second stage, Gc markedly increases in a short period and abruptly jumps to a value near the toughness of the bulk material. In the third stage, Gc remains nearly constant and becomes independent of the welding time. In the case of welding identical polymers, the toughness of the interface increases via mutual interdiffusion of the polymer chains at the interface. However, long periods are required to achieve sufficient interdiffusion to obtain high interfacial toughness because the interdiffusion of the polymer chains between identical polymers is driven only by entropic force, and a high level of entropy cannot be expected in the mixing of polymers.
We previously investigated the correlation between the welding behavior of polystyrene (PS) and the interfacial thickness.16 When PS was welded at 120 °C, the Gc values were <20 J m−2 in the initial 200 min, and then it abruptly increased to ~800 J m−2 after 1000 min, which is close to the toughness of bulk PS. In the final stage, the Gc values were nearly constant and independent of the welding time. The reported PS/PS interfacial thickness data, measured using neutron reflectivity,27 indicated that the increase in toughness occurs over a relatively narrow range of interfacial thickness of 9–12 nm and further interdiffusion of the polymers does not contribute to the toughness enhancement.
It is known that the marked increase in interfacial toughness occurs as a result of a shift in the molecular failure mechanism. When the interfacial thickness increases, the failure mechanism changes from 'chain pullout' to crazing. The critical interfacial width (9–12 nm) at which the failure mode transition occurs is the comparable distance between the entanglement points, which was estimated to be 9.3 nm for PS. Thus, sufficient interfacial toughness can be achieved with narrow interfaces, which is considerably smaller than the radius of gyration of the polymers. Therefore, we understand that the required interdiffusion to achieve maximum toughness is approximately equal to the entanglement distance.
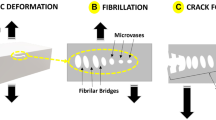
Considering those facts, the measured Gc values in the COP welding experiments in Figure 3 are in the initial stage and have not reached the high values near the toughness of COP bulk materials. Figure 3 shows the SEM micrographs of the fracture surfaces that appeared in front of the cracks after the DCB tests at different welding times, and Figure 4a is a micrograph of the original surface before bonding. Figures 4b–d are micrographs of the production of many nano-sized fibrils after the fracture of the interfaces welded for 5, 15 and 45 min, respectively. With the increase in welding time, the number of fibrils increased and the fibrils seemed to elongate along the crack opening direction. We previously found similar fracture features in the initial stage of PS/PS welding and in the fracture of immiscible polymer pairs such as polymethylmethacrylate/polycarbonate and polymethylmethacrylate/styrene-acrylonitrile random copolymer.20, 21 We have confirmed in our previous works that such a 'nanofibril' pattern is created when the 'chain pullout' is the dominant fracture mechanism. After the fracture mode transitions from 'chain pullout' to crazing, the 'nanofibrils' are eliminated and micrometer-scale 'dimples', which are a typical pattern produced via crazing, are dominant. We can interpret that the 'nanofibril' fracture pattern is created when the narrow interfaces formed during the initial stage of the interdiffusion fracture before the marked increase in toughness. Therefore, the 'nanofibril' fracture pattern can be formed when the polymer chains are disentangled at the interface, before the crazing starts where mechanically effective entanglements cannot be formed.
Interfacial fracture behavior of low-temperature-bonded COP
This nanoscale fractography study enables us to qualitatively investigate the entanglement structures at polymer/polymer interfaces formed in melt conditions. Next, we attempted to extend this technique to the interfaces formed via the bonding of surface-activated polymers. Figure 5 summarizes the effect of the VUV irradiation time on the interfacial toughness caused by low-temperature bonding at 110 °C. The maximum Gc was obtained via 5 min of VUV irradiation; the longer irradiation time did not contribute to the enhancement but resulted in the decrease in Gc. We also evaluated the effect of humidity on the interfacial toughness by measuring the crack growth in the remaining specimen at 25 °C and 60% relative humidity for 12 h. As shown in Figure 5, the Gc values decreased from the initial values in all VUV irradiation conditions. When the razor blade was pushed into the interface of the as-prepared laminates, crack propagation initiated ahead of the razor blade and no significant increase in crack length was detected after an hour. The initial Gc values were calculated using the obtained crack length. However, the crack was gradually propagated when the specimen with an inserted razor blade was left under the aforementioned condition. This means that water vapor degrades the interfacial toughness. Sugimura and co-workers14 reported that the content of oxygen-containing functional groups on the COP surfaces increased and the water contact angle decreased with the increase in VUV irradiation time up to 40 min. The results suggest that the optimal condition for bonding is not equal to that for the surface activation.
Figure 6 shows the SEM micrographs of the fracture surfaces of the DCB specimens. Figures 6a–d are the as-prepared specimens that were VUV-irradiated for 2, 5, 20 and 40 min, respectively, and Figure 6e is the specimen VUV-irradiated for 5 min after the exposure to the high-humidity condition. We have confirmed that the surface of the VUV-irradiated specimens before bonding show no surface features as shown in Figure 4a. However, a large number of nanofibrils were produced on the fracture surfaces of the specimens irradiated for 2 and 5 min, which have relatively high Gc values. The results indicate that similar surface features are obtained in the surface-activated system as observed on the fracture surface of the interfaces formed in melt conditions. The features of the fibrils are characterized by the degree of elongation, thickness and density. These features have not yet been quantitatively correlated with the interfacial structures. Similar surface features have been obtained in the initial welding stage of PS–PS laminates with extremely low Gc values.16 The Gc values obtained by low-temperature bonding are lower compared with those obtained by high-temperature bonding. The interface cannot be sufficiently strengthened as compared with the high-temperature bonding. Therefore, large amounts of energy cannot be dissipated during the fracture, and the fibrils are not elongated to a great degree.
Scanning electron microscopy (SEM) micrograph of the fracture surfaces of cyclo-olefin polymer (COP) laminates, which were bonded at 110 °C. The vacuum ultraviolet (VUV) irradiation times are (a) 2, (b) 5, (c) 10 and (d) 20 min. (e) After high-humidity exposure at 25 °C, 60% relative humidity (RH) for 12 h of (a).
However, the fibrils can barely be observed on the fracture surfaces of the specimens irradiated for 20 and 40 min, which have extremely low Gc values. In addition, the fracture surface produced during the exposure to high humidity shows features that are markedly different from that produced in the as-prepared specimen, where the surface features with similar length scales were produced but the height of the fibrils are quite small, as shown in Figure 6e.
The generation of nanosize fibrils on the fracture surfaces of the specimen VUV-irradiated for 2 and 5 min suggests that the interfaces were fractured via 'chain pullout'. The bonding temperature (110 °C) is significantly lower than Tg of COP (140 °C), and the bonding time is not long (10 min). Thus, it is reasonable to think that mutual interdiffusion could not occur to form sufficient entanglement at the interface. It is anticipated that hydrogen bonds formed at the interface between the oxygen-containing polar functional groups and created the polymer chains that bridge the interface. Those polymer chains can reinforce the interface and are pulled out during the fracture.
The longer VUV irradiation time produces a large number of polar functional groups on the surface, as investigated by Sugimura and co-workers,14 which can produce a larger number of polymer chains across the interface. However, longer VUV irradiation is assumed to degrade the polymers, which may yield fragments with lower molecular weight compared with the average molar weight between the entanglement points. Under such conditions, even if the number of the polymer chains across the interface increases, mechanically effective interfacial entanglement cannot be formed, which results in the poor interfacial toughness. Under the high-humidity condition, water molecules invade the interface and disconnect the hydrogen bonds at the interface, which may cancel the coupling at the interface; thus, the interfacial toughness decreases.
The reinforcement of polymer interfaces by localizing block copolymers at the interfaces has been well studied. The areal density of block copolymers at the interface has an important role in the toughness of the interfaces.28 The low Gc values obtained by low-temperature bonding in this study may be a result of the relatively low concentration of polymer chains that can reinforce the interface as compared with the case of high-temperature bonding.
Imaging the interface with electron microscopy
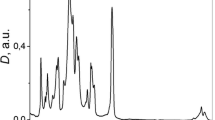
Before we discuss the low-temperature bonding mechanism, the bonded interface of COP bilayer specimens was inspected with two electron microscopy techniques. The interfaces between identical polymers are difficult to directly image using conventional transmission electron microscopy because no contrast can be obtained between the components. In our case, oxygen is introduced only in the region underneath the surface with a certain depth; thus, the VUV-modified region can be imaged by tracing the location of the oxygen element. Figures 7a and b show energy-filtered images of the sample that was VUV-irradiated for 5 min using EFTEM at energy losses (ΔE) of 0±5 (zero-loss image) and 150±5 eV, respectively. The zero-loss image did not clearly show the interfacial region; the high-contrast image was obtained at ΔE of 150 eV, where the interfacial region appears as a dark, ~200-nm-thick layer. Figure 7c shows the electron energy-loss spectroscopy spectra, which include the O-K edge at 535 eV, of the regions inside and outside the interfacial region. The inset of Figure 7 shows the O-K edges after the background subtraction, which was calculated using the power-law function (dotted curves in the as-measured spectra). Oxygen was localized in the interfacial region because polar functional groups were produced by VUV irradiation. A small amount of oxygen was also detected over the entire region in the specimen, which might come from the organic compounds in the gold colloid, which was added as a stabilizing agent. Figure 7d is an oxygen elemental map, which was calculated using the three-window method with three energy-loss images at 450±5, 500±5 and 550±5 eV. Each image was recorded with an acquisition time of 5 s, and the image size is 256x256 pixel. The first two energy windows below the O-K edge (pre-edge images) were used to calculate the background image that was subtracted from the third one beyond the O-K edge (core-loss image). The elemental map indicates that the interphase was formed with a uniform thickness of 200 nm; hence, the estimated depth of the surface modification was ~100 nm.
Energy-filtered transmission electron microscopy (EFTEM) analysis of the interface between the cyclo-olefin polymer (COP) laminate with low-temperature bonding. The vacuum ultraviolet (VUV) irradiation time is 5 min. (a, b) Energy-filtered images at 0±5 and at 150±5 eV energy losses, respectively. (c) O-K edges in the electron energy-loss spectroscopy (EELS) spectra acquired inside and outside the interfacial region in Figure 7b. The inset shows the background-subtracted O-K edges, where the backgrounds are calculated as dotted curves in Figure 7c. (d) Oxygen map, which was created using the three-window method.
STEM was also used to image the interfacial region. Figures 8a and b are bright-field (BF) and annular dark-field (ADF) images, respectively, which clearly show a similar interfacial layer as observed by EFTEM. It is worth mentioning that even the BF image provides sufficient contrast to the interfacial region (Figure 8a). In the TEM mode, there is an objective lens below the specimen, and electrons of different energies are focused at different focal positions, which is known as chromatic aberration. This causes blurring of the image and a loss of contrast. In STEM, on the other hand, chromatic aberration has a relatively minor effect on the image qualities because STEM images are created by simple detection of the signals at each probe position without an objective lens. The ADF image (Figure 8a) is much clearer than the BF image (Figure 8b) and shows the high-contrast interfacial layer. The contrast of an ADF image is related to the mass thickness and atomic numbers in the specimen. In this experiment, the difference in chemical composition between the interfacial region and the bulk part gives a high-contrast image.
Scanning transmission electron microscopy (STEM) (a) bright-field (BF) and (b) annular dark-field (ADF) images of the interface between the cyclo-olefin polymer (COP) laminate with low-temperature bonding. The vacuum ultraviolet (VUV) irradiation time is 5 min. (c) energy-dispersive X-ray spectrometry (EDX) spectra from the point inside (straight line) and outside (dotted line) the interfacial region. (d) STEM-ADF image of the interfacial region of the COP laminate. The VUV irradiation time is 40 min.
The beam was localized on the points inside and outside the interfacial layer, and EDX microanalysis was performed as shown in Figure 8c, where oxygen was detected with sufficient intensity using a beam current of 1 nA for 100 s. Thus, the detected bright layer in the ADF image can be confirmed as an oxygen-rich region. Figure 8d is the ADF image of the sample that was VUV-irradiated for 40 min, which indicates that longer irradiation time causes no significant increase in the thickness of the layer. Sugimura and co-workers14 investigated the COP surface oxidation of VUV using X-ray photoelectron spectroscopy and Fourier transform infrared and found that the highest concentration of oxygen functional groups was obtained with 40 min of VUV irradiation. Even with such a long irradiation time, the VUV treatment was limited to within the region 100 nm below the surface.
Mechanism of low-temperature COP bonding
Figure 9 is a schematic illustration of our proposed low-temperature bonding mechanism of the VUV-irradiated COP. The VUV irradiation on COP plates yields a large number of polar functional groups such as –OH, CHO and COOH on the surface. The estimated modification depth is ~100 nm, which is almost independent of the irradiation time. When the VUV irradiation time is shorter than 5 min, the polymer chains across the interface are formed through the hydrogen bonding among functionalized polymer chains on both sides. Those polymer chains can reinforce the interface, and some energy is dissipated during the fracture of the interface via chain pullout. During the exposure of the COP laminates to the humidity condition, the hydrogen bonds are disconnected by water insertion; then, the interfacial entanglement is canceled, which decreases toughness. The longer VUV irradiation produces a large number of functional polar groups, but it may also degrade the polymers and produce low-molar-weight species. Although polymer chains are formed across the interface, mechanically effective entanglements cannot be achieved at the interface; thus, the interface toughness is quite low. The interfacial fracture toughness was markedly reduced by the 10-min VUV irradiation. It is speculated that the molecular weight decreases to lower values than the average molecular weight between the entanglement points of COP when the irradiation is 5–10 min.
Conclusion
The surfaces of COP are photoactivated by VUV light and can be bonded with a practical bonding strength at low temperatures below Tg. The VUV irradiation condition was optimized, the maximum interfacial toughness was obtained for 5 min of irradiation and a longer irradiation time resulted in the decrease in toughness. We also found that a high-humidity environment causes the decrease in toughness. We investigated the mechanism of the low-temperature bonding of COP by characterizing the fracture surfaces using high-resolution SEM. The fracture surfaces exhibited a large number of nanofibrils, whose features are similar to the ones we previously observed in the polymer–polymer interfaces that were welded in melt conditions. It is interpreted that the interfacial failure occurs where the polymer chains connected by hydrogen bonding are pulled out. Thus, the interfacial entanglement of the polymers that are formed via hydrogen bonding has an important role in the bonding performance of polymers via surface-activation processes.
We also successfully imaged the interfacial regions by probing the oxygen distribution using EFTEM and STEM-ADF imaging with EDX microanalysis, which enabled us to estimate the depth of the VUV surface modification. This study is the first to apply the STEM technique to the analysis of a polymer interface.
References
Yamazaki, M. Industrialization and application development of cyclo-olefin polymer. J. Mol. Catal. A 213, 81–87 (2004).
Owens, D. K. The mechanism of corona and ultraviolet light-induced self-adhesion of poly(ethylene terephthalate) film. J. Appl. Polym. Sci. 19, 3315–3326 (1975).
Carly, J. F. & Kitze, P. T. Corona-discharge treatment of polymeric films, II: Chemical studies. Polym. Eng. Sci. 20, 330–338 (1980).
Iwata, H., Kishida, A., Suzuki, M., Hata, Y. & Ikada, Y. Oxidation of polyethylene surface by corona discharge and the subsequent graft polymerization. J. Polym. Sci. Polym. Chem. Ed. 26, 3309–3322 (1988).
Yasuda, H., Marsh, H. C., Brandt, S. & Reilley, C. N. ESCA study of polymer surfaces treated by plasma. J. Polym. Sci. Polym. Chem. Ed. 15, 991–1019 (1977).
Schonhorn, H. & Hansen, R. H. Surface treatment of polymers for adhesive bonding. J. Appl. Polym. Sci. 11, 1461–1474 (1967).
Hudis, M. Surface crosslinking of polyethylene using a hydrogen glow discharge. J. Appl. Polym. Sci. 16, 2397–2415 (1972).
Nelson, E. R., Kilduff, T. J. & Benderly, A. A. Bonding of Teflon. Ind. Eng. Chem. 50, 329–330 (1958).
Tazuke, S. & Kimura, H. Surface photografting, 2. Modification of polypropylene film surface by graft polymerization of acrylamide. Macromol. Chem. Phys. 179, 2603–2612 (1978).
Allméar, K., Hult, A. & Rårnby, B. Surface modification of polymers. I. Vapour phase photografting with acrylic acid. J. Polym. Sci. Part A: Polym. Chem. 26, 2099–2111 (1988).
Yamada, K., Tsutaya, H., Tatekawa, S. & Hirata, M. Hydrophilic and adhesive properties of polyethylene plates grafted with hydrophilic monomers. J. Appl. Polym. Sci. 46, 1065–1085 (1992).
Hamilton, L. M., Green, A., Edge, S. J., Badyal, P. S., Feast, W. J. & Pacynko, W. F. The surface modification of polyethylene by solution-phase photochemical grafting using short irradiation times. J. Appl. Polym. Sci. 52, 413–419 (1994).
Mingbo, H. & Xingzhou, H. Photo-stabilization of polypropylene film by surface photo-grafting. Polym. Degrad. Stab. 18, 321–328 (1987).
Kim, Y.-J, Taniguchi, Y., Murase, K., Taguchi, Y. & Sugimura, H. Vacuum ultraviolet-induced surface modification of cyclo-olefin polymer substrates for photochemical activation bonding. App. Surface Sci. 255, 3648–3654 (2009).
Taguchi, Y., Kim, Y.-J., Hagioi, M., Taniguchi, Y. & Sugimura, H. Photo-activation bonding of cyclo-olefin polymer plates: evaluation of the bonding strength and application to micro fluidic chips. Hyoumen Gijutsu 65, 234–239 (2014).
Horiuchi, S., Nakagawa, A., Liao, Y. & Ougizawa, T. Interfacial entanglements between glassy polymers investigated by nanofractography with high-resolution scanning electron microscopy. Macromolecules 41, 8063–8071 (2008).
Horiuchi, S. Study of polymer interfacial entanglements and adhesion by high-resolution scanning electron microscopy. Kobunshi Ronbunshu 69, 326–333 (2012).
Creton, C., Kramer, E. J., Hui, C. Y. & Brown, H. R. Failure mechanisms of polymer interfaces reinforced with block copolymers. Macromolecules 25, 3075–3088 (1992).
Janarthanan, V., Stein, R. S. & Garret, P. D. Role of entanglements on the fracture toughness of incompatible polymer interfaces. Macromolecules 27, 4855–4858 (1994).
Horiuchi, S., Yin, D. & Ougizawa, T. Nanoscale analysis of polymer interfaces by energy-filtering transmission electron microscopy. Macromol. Chem. Phys. 206, 725–731 (2005).
Horiuchi, S., Liao, Y., Yin, D. & Ougizawa, T. Study of adhesion and fracture of polymer laminates by imaging of interfaces. Macromol. Rapid Commun. 28, 915–921 (2007).
Liao, Y., Nakagawa, A., Horiuchi, S. & Ougizawa, T. Interdiffusion at homopolymer/random copolymer interfaces investigated by energy-filtering transmission electron microscopy. Macromolecules 40, 7966–7972 (2007).
Genc, A., Cheng, H., Winterstein, J., Pullan, L. & Freitag, B. Macrosc. Anal. 26, 23–25 (2012).
Schnell, R., Stamm, M. & Creton, C. Mechanical properties of homopolymer interfaces: transition from simple pullout to crazing with increasing interfacial width. Macromolecules 32, 3420–3425 (1999).
Schnell, R., Stamm, M. & Creton, C. Direct correlation between interfacial width and adhesion in glassy polymers. Macromolecules 31, 2284–2292 (1998).
Cole, P. J., Cook, R. F. & Macosko, C. W. Adhesion between immiscible polymers correlated with interfacial entanglements. Macromolecules 36, 2808–2815 (2003).
Stamm, M., Hüttenbach, S., Reiter, G. & Springer, T. Initial stages of polymer interdiffusion studied by neutron reflectometry. Eur. Phys. Lett. 14, 451–456 (1991).
Creton, C., Brown, H. R. & Deline, V. R. Influence of chain entanglement on the failure modes in block copolymer toughened interfaces. Macromolecules 27, 1774–1780 (1994).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Horiuchi, S., Hakukawa, H., Jong Kim, Y. et al. Study of the adhesion and interface of the low-temperature bonding of vacuum ultraviolet-irradiated cyclo-olefin polymer using electron microscopy. Polym J 48, 473–479 (2016). https://doi.org/10.1038/pj.2016.3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pj.2016.3
- Springer Nature Limited