Abstract
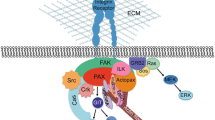
Vaccinia H1-related phosphatase (VHR/DUSP3) is a member of the dual-specificity phosphatase family. Deregulation of VHR is observed in various malignant diseases. We identified focal adhesion kinase (FAK) as a VHR-interacting molecule. Over-expression of VHR decreased tyrosine phosphorylation of FAK and decreasing VHR promoted FAK tyrosine phosphorylation. In vitro assays proved that recombinant VHR directly dephosphorylated FAK and paxillin. VHR-knockout mice did not have obvious abnormality; however, VHR-knockout cells showed decreased expression of integrins and FAK but stronger FAK and paxillin phosphorylation upon attachment to fibronectin. Additionally, VHR-knockout fibroblast and lung epithelial cells had elevated ligand-induced epidermal growth factor receptor (EGFR) phosphorylation. Inducible expression of VHR suppressed directional cell migration, and VHR deficiency resulted in a higher cell migratory ability. VHR-knockout cells have stronger FAK phosphorylation in cell adhesions, long-lasting trailing ends and slower turnover of focal adhesions. These collective data indicate that VHR is a FAK phosphatase and participates in regulating the formation and disassembly of focal adhesions.







Similar content being viewed by others
References
Mitra SK, Hanson DA, Schlaepfer DD . Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol 2005; 6: 56–68.
Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N et al. Reduced cell motility and enhanced focal adhesion contect formation in cells form FAK-deficient mice. Nature 1995; 377: 539–544.
Sieg DJ, Hauck CR, Schlaepfer DD . Required role of focal adhesion kinase (FAK) for integrin-stimulated cell migration. J Cell Sci 1999; 112: 2677–2691.
Calalb MB, Polte TR, Hanks SK . Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src family kinases. Mol Cell Biol 1995; 15: 954–963.
Schaller MD, Hildebrand JD, Shannon JD, Fox JW, Vines RR, Parsons JT . Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol Cell Biol 1994; 14: 1680–1688.
Yu D-H, Qu C-K, Henegariu O, Lu X, Feng G-S . Protein-tyrosine phosphatase Shp-2 regulates cell spreading, migration, and focal adhesion. J Biol Chem 1998; 273: 21125–21131.
Davidson D, Veillette A . PTP-PEST, a scaffold protein phosphatase, negatively regulates lymphocyte activation by targeting a unique set of substrates. EMBO J 2001; 20: 3414–3426.
Klinghoffer RA, Sachsenmaier C, Cooper JA, Soriano P . Src family kinases are required for integrin but not PDGFR signal transduction. EMBO J 1999; 18: 2459–2471.
Angers-Loustau A, Cote J-F, Charest A, Dowbenko D, Spencer S, Lasky LA et al. Protein tyrosine phosphatase-PEST regulates focal adhesion disassembly, migration, and cytokinesis in fibroblasts. J Cell Biol 1999; 144: 1019–1031.
Camps M, Nichols A, Arkinstall S . Dual specificity phosphatases: a gene family for control of MAP kinase function. FASEB J 1999; 14: 6–16.
Alonso A, Sasin J, Bottini N, Friedberg I, Friedberg I, Osterman A et al. Protein tyrosine phosphatases in the human genome. Cell 2004; 117: 699–711.
Huang C-Y, Tan T-H . DUSPs, to MAP kinases and beyond. Cell Biosci 2012; 2: 24–34.
Chen AJ, Zhou G, Juan T, Colicos SM, Cannon JP, Cabriera-Hansen M et al. The dual specificity JKAP specifically activates the c-Jun N-terminal kinase pathway. J Biol Chem 2002; 277: 36592–36601.
Hood KL, Tobin JF, Yoon C . Identification and characterization of two novel low-molecular-weight dual specificity phosphatases. Biochem Biophys Res Commun 2002; 298: 545–551.
Niwa R, Nagata-Ohashi K, Takeichi M, Mizuno K, Uemura T . Control of actin reorganization by Slingshot, a family of phosphatases that dephosphorylates ADF/Coffilin. Cell 2002; 108: 233–246.
Wang JY, Lin C-H, Yang C-H, Tan TH, Chen YR . Biochemical and biological characterization of a neuroendocrine-associated phosphatase. J Neurochem 2006; 98: 89–101.
Lee J, Hun Yun J, Lee J, Choi C, Hoon Kim J . Blockade of dual-specificity phosphatase 28 decreases chemoresistance and migration in human pancreatic cancer cells. Sci Rep 2015; 5: 12296–12307.
Hoyt R, Zhu W, cerignoli F, Alonso A, Mustelin T, David M . Selective tyrosine dephosphorylation of interferon-activated nuclear STAT5 by the VHR phosphatase. J Immunol 2007; 179: 3402–3406.
Li J-P, Fu Y-N, Chen Y-R, Tan T-H . JNK pathway-associated phosphatase dephosphorylates focal adhesion kinase and suppresses cell migration. J Biol Chem 2010; 285: 5472–5478.
Wang J-Y, Yeh C-L, Chou H-C, Yang C-H, Fu Y-N, Chen Y-T et al. Vaccinia H1-related phosphatase is a phosphatase of ErbB receptors and Is down-regulated in non-small cell lung cancer. J Biol Chem 2011; 286: 10177–10184.
Li J-P, Yang C-Y, Chuang H-C, Lan J-L, Chen D-Y, Chen Y-M et al. The phosphatase JKAP/DUSP22 inhibits T-cell receptor signalling and autoimmunity by inactivating Lck. Nat Commun 2014; 5: 3618–3631.
Gallegos LL, Ng MR, Sowa ME, Selfors LM, White A, Zervantonakis IK et al. A protein interaction map for cellcell adhesion regulators identifies DUSP23 as a novel phosphatase for β-catenin. Sci Rep 2016; 6: 27114–27128.
Ishibashi T, Bottaro DP, Chan A, Miki T, Aaronson SA . Expression cloing of a human dual-specificity phosphatase. Proc Natl Acad Sci USA 1992; 89: 12170–12174.
Todd JL, Rigas JD, Rafty LA, Denu JM . Dual-specificity protein tyrosine phosphatase VHR down-regulates c-Jun N-terminal kinase (JNK). Oncogene 2002; 21: 2573–2583.
Todd JL, Tanner KG, Denu JM . Extracellular regulated kinases (ERK) 1 and ERK 2 are authetic substrates for the dual specificity protein-tyrosine phosphatase VHR. J Biol Chem 1999; 274: 13271–13280.
Alonso A, Saxena M, Williams S, Mustellin T . Inhibitory role for dual-specificity phosphatase VHR in T cell antigen receptor and CD28-induced ERK and JNK activation. J Biol Chem 2001; 276: 4766–4771.
Rahmouni S, Cerignoli F, Alonso A, Tsutji T, Henkens R, Zhu C et al. Loss of the VHR dual-specific phosphatase causes cell-cycle arrest and senescence. Nat Cell Biol 2006; 8: 524–531.
Yang CH, Chou HC, Fu YN, Yeh CL, Cheng HW, Chang IC et al. EGFR over-expression in non-small cell lung cancers harboring EGFR mutations is associated with marked down-regulation of CD82. BBA-Mol Biol Dis 2015; 1852: 1540–1549.
Schumacher MA, Todd JL, Rice AE, Tanner KG, Denu JM . Structural basis for the recognition of a bisphosphorylated MAP kinase peptide by human VHR protein phosphatase. Biochemistry 2002; 41: 3009–3017.
Xiao Q, Luechapanichkul R, Zhai Y, Pei D . Specificity profiling of protein phosphatases toward phosphoseryl and phosphothreonyl peptides. J Am Chem Soc 2013; 135: 9760–9767.
Pavic K, Duan G, Kohn M . VHR/DUSP3 phosphatase: structure, function and regulation. FEBS J 2015; 282: 1871–1890.
Hoyt R, Zhu W, Cerignoli F, Alonso A, Mustelin T, David M . Selective tyrosine dephosphorylation of interferon-activated nuclear STAT5 by the VHR phosphatase. J Immunol 2007; 179: 3402–3406.
Amand M, Erpicum C, Bajou K, Cerignoli F, Blacher S, Martin M et al. DUSP3/VHR is a pro-angiogenic atypical dual-specificity phosphatase. Mol Cancer 2014; 13: 108–125.
Musumeci L, Kuijpers MJ, Gilio K, Hego A, Théâtre E, Maurissen L et al. Dual-specificity phosphatase 3 deficiency or inhibition limits platelet activation and arterial thrombosis. Circulation 2015; 131: 656–668.
Singh P, Dejager L, Amand M, Theatre E, Vandereyken M, Zurashvili T et al. DUSP3 genetic de;etion confers M2-like macrophage-dependent tolerance to septic shock. J Immunol 2015; 194: 4951–4962.
Hao L, ElShamy WM . BRCA1-IRIS activates cyclin D1 expression in breast cancer cells by downregulating the JNK phosphatase DUSP3/VHR. Int J Cancer 2007; 121: 39–46.
Henkens R, Delvenne P, Arafa M, Moutschen M, Zeddou M, Tautz L et al. Cervix carcinoma is associated with an up-regulation and nuclear localization of the dual-specificity protein phosphatase VHR. BMC Cancer 2008; 8: 47–55.
Arnoldussen YJ, Lorenzo PI, Pretious ME, Waehre H, Risberg B, Maelandsmo GM et al. The mitogen-activated protein kinase phosphatase Vaccinia H1-related protein inhibits apoptosis in prostate cancer cells and is overexpressed in prostate cancer. Cancer Res 2008; 68: 9255–9264.
Chu Y-W, Yang P-C, Yang S-C, Shyu Y-C, Hendrix MJC, Wu R et al. Selection of invasive and metastatic subpopulations from a human lung adenocarcinoma cell line. Am J Respir Cell Mol Biol 1997; 17: 353–360.
Fredriksson S, Gullberg M, Jarvius J, Olsson C, Pietras K, Gustafsdottir SM et al. Protein detection using proximity-dependent DNA ligation assays. Nat Biotechnol 2002; 20: 473–477.
Wang X, Li JP, Kuo HK, Chiu LL, Dement GA, Lan JL et al. Down-regulation of B cell receptor signaling by hematopoietic progenitor kinase 1 (HPK1)-mediated phosphorylation and ubiquitination of activated B cell linker protein (BLNK). J Biol Chem 2012; 287: 11037–11048.
Acknowledgements
We thank National Health Research Institutes Transgenic Animal Core for assistance in the mouse blastocyst injection and Optical Biology Core for the assistance in confocal microscopy and time-lapsed photography analyses. This work was supported by grants from National Health Research Institutes, Taiwan (MG-102-PP-03 and MG-103-PP-03 to Y-R Chen; 105-IMPP01 and 105-IMSP01 to T-H Tan), and grants from Ministry of Science and Technology, Taiwan (102-2628-B-400-003 to Y-R Chen and 105-2314-B-400-027 to T-H Tan).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Chen, YR., Chou, HC., Yang, CH. et al. Deficiency in VHR/DUSP3, a suppressor of focal adhesion kinase, reveals its role in regulating cell adhesion and migration. Oncogene 36, 6509–6517 (2017). https://doi.org/10.1038/onc.2017.255
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2017.255
- Springer Nature Limited
This article is cited by
-
Dual-specificity phosphatases 22-deficient T cells contribute to the pathogenesis of ankylosing spondylitis
BMC Medicine (2023)
-
DUSP3 regulates phosphorylation-mediated degradation of occludin and is required for maintaining epithelial tight junction
Journal of Biomedical Science (2022)
-
UV Radiation-induced Impairment of Cellular Morphology and Motility is Enhanced by DUSP3/VHR Loss and FAK Activation
Cell Biochemistry and Biophysics (2021)