Abstract
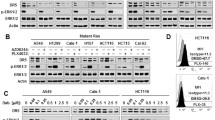
Raf-1 has an important role in cellular antiapoptosis. So far, there is no solid evidence that shows that Raf-1 mutation is associated with cancer development. In the course of further study of Raf-1 signaling, we have reported that Raf-1 hyperphosphorylation inhibits its kinase activity toward its downstream mitogen-activated protein kinase kinase 1/2 (MEK1/2) and proposed a model for negative feedback regulation of Raf-1. Here, we show that there is no hyperphosphorylation in some cancer cells, which results in increased kinase activity and enhances the antiapoptotic ability. Inhibition of either Raf-1 or ALG-2 (apoptosis-linked gene 2) expression results in apoptosis signal-regulating kinase 1/c-Jun N-terminal kinase (ASK1/JNK) signaling activation, and cell sensitivity to chemotherapeutic reagents, indicating that inhibition of ASK1/JNK apoptotic signaling by Raf-1 is mediated by ALG-2. A previous report indicated that extracellular signal-regulated kinase 1/2 (ERK1/2) were responsible for Raf-1 hyperphosphorylation. However, our evidence shows that when ERK1/2 are activated and the Raf-1 gene is not mutated, Raf-1 is not hyperphosphorylated in these cells, indicating that ERK1/2 are not responsible for the Raf-1 hyperphosphorylation in these cancer cell lines. Surprisingly, we also found that Raf-1 is not a necessary kinase for MEK1/2 activation under normal tissue culture conditions, but is required for MEK1/2 activation under apoptosis-inducing conditions. Our research demonstrates that although Raf-1 gene is not mutated, an abnormality of Raf-1 kinase feedback regulation enhances its antiapoptotic function, and Raf-1 can still be a pharmaceutical target to increase chemotherapy or radiotherapy sensitivity in these cancer cells.










Similar content being viewed by others
References
Appert-Collin A, Hubert P, Cremel G, Bennasroune A . Role of ErbB receptors in cancer cell migration and invasion. Front Pharmacol 2015; 6: 283.
Bieker R, Padro T, Kramer J, Steins M, Kessler T, Retzlaff S et al. Overexpression of basic fibroblast growth factor and autocrine stimulation in acute myeloid leukemia. Cancer Res 2003; 63: 7241–7246.
Brady G, Crean SJ, Naik P, Kapas S . Upregulation of IGF-2 and IGF-1 receptor expression in oral cancer cell lines. Int J Oncol. 2007; 31: 875–881.
Culig Z, Bartsch G, Hobisch A . Interleukin-6 regulates androgen receptor activity and prostate cancer cell growth. Mol Cell Endocrinol 2002; 197: 231–238.
Culig Z, Hobisch A, Cronauer MV, Radmayr C, Trapman J, Hittmair A et al. Androgen receptor activation in prostatic tumor cell lines by insulin-like growth factor-I, keratinocyte growth factor, and epidermal growth factor. Cancer Res 1994; 54: 5474–5478.
Cussenot O . Growth factors and prostatic tumors. Ann Endocrinol (Paris) 1997; 58: 370–380.
Kaplan PJ, Leav I, Greenwood J, Kwan PW, Ho SM . Involvement of transforming growth factor alpha (TGFalpha) and epidermal growth factor receptor (EGFR) in sex hormone-induced prostatic dysplasia and the growth of an androgen-independent transplantable carcinoma of the prostate. Carcinogenesis 1996; 17: 2571–2579.
Latil A, Bieche I, Pesche S, Valeri A, Fournier G, Cussenot O et al. VEGF overexpression in clinically localized prostate tumors and neuropilin-1 overexpression in metastatic forms. Int J Cancer 2000; 89: 167–171.
Li R, Younes M, Wheeler TM, Scardino P, Ohori M, Frolov A et al. Expression of vascular endothelial growth factor receptor-3 (VEGFR-3) in human prostate. Prostate 2004; 58: 193–199.
Park SJ, Gu MJ, Lee DS, Yun SS, Kim HJ, Choi JH . EGFR expression in pancreatic intraepithelial neoplasia and ductal adenocarcinoma. Int J Clin Exp Pathol 2015; 8: 8298–8304.
Segaliny AI, Tellez-Gabriel M, Heymann MF, Heymann D . Receptor tyrosine kinases: characterisation, mechanism of action and therapeutic interests for bone cancers. J Bone Oncol 2015; 4: 1–12.
Trojan L, Thomas D, Knoll T, Grobholz R, Alken P, Michel MS . Expression of pro-angiogenic growth factors VEGF, EGF and bFGF and their topographical relation to neovascularisation in prostate cancer. Urol Res 2004; 32: 97–103.
Gille H, Sharrocks AD, Shaw PE . Phosphorylation of transcription factor p62TCF by MAP kinase stimulates ternary complex formation at c-fos promoter. Nature 1992; 358: 414–417.
Roberts TM . Cell biology. A signal chain of events. Nature 1992; 360: 534–535.
Steelman LS, Franklin RA, Abrams SL, Chappell W, Kempf CR, Basecke J et al. Roles of the Ras/Raf/MEK/ERK pathway in leukemia therapy. Leukemia 2011; 25: 1080–1094.
Yu P, Tung JK, Simons M . Lymphatic fate specification: an ERK-controlled transcriptional program. Microvasc Res 2014; 96: 10–15.
Zhang W, Lee JC, Kumar S, Gowen M . ERK pathway mediates the activation of Cdk2 in IGF-1-induced proliferation of human osteosarcoma MG-63 cells. J Bone Miner Res 1999; 14: 528–535.
Huser M, Luckett J, Chiloeches A, Mercer K, Iwobi M, Giblett S et al. MEK kinase activity is not necessary for Raf-1 function. EMBO J 2001; 20: 1940–1951.
Kauffmann-Zeh A, Rodriguez-Viciana P, Ulrich E, Gilbert C, Coffer P, Downward J et al. Suppression of c-Myc-induced apoptosis by Ras signalling through PI(3)K and PKB. Nature 1997; 385: 544–548.
Lehmann K, Janda E, Pierreux CE, Rytomaa M, Schulze A, McMahon M et al. Raf induces TGFbeta production while blocking its apoptotic but not invasive responses: a mechanism leading to increased malignancy in epithelial cells. Genes Dev 2000; 14: 2610–2622.
Mikula M, Schreiber M, Husak Z, Kucerova L, Ruth J, Wieser R et al. Embryonic lethality and fetal liver apoptosis in mice lacking the c-raf- 1 gene. EMBO J 2001; 20: 1952–1962.
Murakami MS, Morrison DK . Raf-1 without MEK? Sci STKE 2001; 2001: E30.
Pritchard C, McMahon M . Raf revealed in life-or-death decisions. Nat Genet 1997; 16: 214–215.
Sewing A, Wiseman B, Lloyd AC, Land H . High-intensity Raf signal causes cell cycle arrest mediated by p21Cip1. Mol Cell Biol 1997; 17: 5588–5597.
Little AS, Smith PD, Cook SJ . Mechanisms of acquired resistance to ERK1/2 pathway inhibitors. Oncogene 2013; 32: 1207–1215.
Osborne JK, Zaganjor E, Cobb MH . Signal control through Raf: in sickness and in health. Cell Res 2012; 22: 14–22.
Poulikakos PI, Persaud Y, Janakiraman M, Kong X, Ng C, Moriceau G et al. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E). Nature 2011; 480: 387–390.
Poulikakos PI, Solit DB . Resistance to MEK inhibitors: should we co-target upstream? Sci Signal 2011; 4 pe16.
Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N . RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature 2010; 464: 427–430.
Alavi A, Hood JD, Frausto R, Stupack DG, Cheresh DA . Role of Raf in vascular protection from distinct apoptotic stimuli. Science 2003; 301: 94–96.
Chen J, Fujii K, Zhang L, Roberts T, Fu H . Raf-1 promotes cell survival by antagonizing apoptosis signal-regulating kinase 1 through a MEK-ERK independent mechanism. Proc Natl Acad Sci USA 2001; 98: 7783–7788.
Yamaguchi O, Watanabe T, Nishida K, Kashiwase K, Higuchi Y, Takeda T et al. Cardiac-specific disruption of the c-raf-1 gene induces cardiac dysfunction and apoptosis. J Clin Invest 2004; 114: 937–943.
Hwang IS, Jung YS, Kim E . Interaction of ALG-2 with ASK1 influences ASK1 localization and subsequent JNK activation. FEBS Lett 2002; 529: 183–187.
Chen C, Sytkowski AJ . Apoptosis-linked gene-2 connects the Raf-1 and ASK1 signalings. Biochem Biophys Res Commun 2005; 333: 51–57.
Chen C, Sytkowski AJ . Erythropoietin regulation of Raf-1 and MEK: evidence for a Ras-independent mechanism. Blood 2004; 104: 73–80.
Dougherty MK, Muller J, Ritt DA, Zhou M, Zhou XZ, Copeland TD et al. Regulation of Raf-1 by direct feedback phosphorylation. Mol Cell 2005; 17: 215–224.
Emuss V, Garnett M, Mason C, Marais R . Mutations of C-RAF are rare in human cancer because C-RAF has a low basal kinase activity compared with B-RAF. Cancer Res 2005; 65: 9719–9726.
Hagemann C, Rapp UR . Isotype-specific functions of Raf kinases. Exp Cell Res 1999; 253: 34–46.
Matallanas D, Birtwistle M, Romano D, Zebisch A, Rauch J, von Kriegsheim A et al. Raf family kinases: old dogs have learned new tricks. Genes Cancer 2011; 2: 232–260.
Acknowledgements
This work was financially supported by Sichuan Scientific and Technology Department Basic Research Fund (2015JY0011) (to CC) and Sichuan Cancer Hospital Research Fund (to CC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Ma, S., Cao, B., Zhang, H. et al. The lack of Raf-1 kinase feedback regulation enhances antiapoptosis in cancer cells. Oncogene 36, 2014–2022 (2017). https://doi.org/10.1038/onc.2016.384
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2016.384
- Springer Nature Limited
This article is cited by
-
Arterial chemotherapy for hepatocellular carcinoma in China: consensus recommendations
Hepatology International (2024)
-
MicroRNA-29b regulates the radiosensitivity of esophageal squamous cell carcinoma by regulating the BTG2-mediated cell cycle
Strahlentherapie und Onkologie (2021)
-
Phase II Study of Sorafenib Combined with Concurrent Hepatic Arterial Infusion of Oxaliplatin, 5-Fluorouracil and Leucovorin for Unresectable Hepatocellular Carcinoma with Major Portal Vein Thrombosis
CardioVascular and Interventional Radiology (2018)
-
A network-based predictive gene-expression signature for adjuvant chemotherapy benefit in stage II colorectal cancer
BMC Cancer (2017)