Abstract
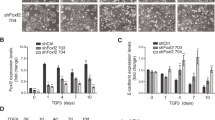
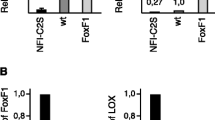
Nuclear Foxc2 is a transcriptional regulator of mesenchymal transformation during developmental epithelial–mesenchymal transition (EMT) and has been associated with EMT in malignant epithelia. Our laboratory has shown that in normal epithelial cells Foxc2 is maintained in the cytoplasm where it promotes an epithelial phenotype. The Foxc2 amino terminus has a consensus casein kinase 2 (CK2) phosphorylation site at serine 124, and we now show that CK2 associates with Foxc2 and phosphorylates this site in vitro. Knockdown or inhibition of the CK2α/α′ kinase subunit in epithelial cells causes de novo accumulation of Foxc2 in the nucleus. Mutation of serine 124 to leucine promotes constitutive nuclear localization of Foxc2 and expression of mesenchymal genes, whereas an S124D phosphomimetic leads to constitutive cytoplasmic localization and epithelial maintenance. In malignant breast cancer cells, the CK2β regulatory subunit is downregulated and FOXC2 is found in the nucleus, correlating with an increase in α-smooth muscle actin (SMA) expression. Restoration of CK2β expression in these cells results in cytoplasmic localization of Foxc2, decreased α-SMA expression and reduced cell migration and invasion. In contrast, knockdown of CK2β in normal breast epithelial cells leads to FOXC2 nuclear localization, decreased E-cadherin expression, increased α-SMA and vimentin expression, and enhanced cell migration and invasion. Based on these findings, we propose that Foxc2 is functionally maintained in the cytoplasm of normal epithelial cells by CK2α/α′-mediated phosphorylation at serine 124, which is dependent on proper targeting of the holoenzyme via the CK2β regulatory subunit.









Similar content being viewed by others
References
Wallin A, Zhang G, Jones TW, Jaken S, Stevens JL . Mechanism of the nephrogenic repair response. Studies on proliferation and vimentin expression after 35S-1,2-dichlorovinyl-L-cysteine nephrotoxicity in vivo and in cultured proximal tubule epithelial cells. Lab Invest 1992; 66: 474–484.
Witzgall R, Brown D, Schwarz C, Bonventre JV . Localization of proliferating cell nuclear antigen, vimentin, c-Fos, and clusterin in the postischemic kidney Evidence for a heterogenous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. J Clin Invest 1994; 93: 2175–2188.
Strutz F, Okada H, Lo CW, Danoff T, Carone RL, Tomaszewski JE et al. Identification and characterization of a fibroblast marker: FSP1. J Cell Biol 1995; 130: 393–405.
Bonventre JV . Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J Am Soc Nephrol 2003; 14: S55–S61.
Kalluri R, Neilson EG . Epithelial–mesenchymal transition and its implications for fibrosis. J Clin Invest 2003; 112: 1776–1784.
Liu Y . Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol 2004; 15: 1–12.
Mani SA, Yang J, Brooks M, Schwaninger G, Zhou A, Miura N et al. Mesenchyme Forkhead 1 (FOXC2) plays a key role in metastasis and is associated with aggressive basal-like breast cancers. Proc Natl Acad Sci USA 2007; 104: 10069–10074.
Birkenkamp KU, Coffer PJ . Regulation of cell survival and proliferation by the FOXO (Forkhead box, class O) subfamily of Forkhead transcription factors. Biochem Soc Trans 2003; 31: 292–297.
Calnan DR, Brunet A . The FoxO code. Oncogene 2008; 27: 2276–2288.
Obsil T, Obsilova V . Structure/function relationships underlying regulation of FOXO transcription factors. Oncogene 2008; 27: 2263–2275.
Arden KC . Multiple roles of FOXO transcription factors in mammalian cells point to multiple roles in cancer. Exp Gerontol 2006; 41: 709–717.
Myatt SS, Lam EW . The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer 2007; 7: 847–859.
Xu L, Massague J . Nucleocytoplasmic shuttling of signal transducers. Nat Rev Mol Cell Biol 2004; 5: 209–219.
Greer EL, Brunet A . FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene 2005; 24: 7410–7425.
Bard JB, Lam MS, Aitken S . A bioinformatics approach for identifying candidate transcriptional regulators of mesenchyme-to-epithelium transitions in mouse embryos. Dev Dyn 2008; 237: 2748–2754.
Hader C, Marlier A, Cantley LG . Mesenchymal–epithelial transition in epithelial response to injury: the role of Foxc2. Oncogene 2010; 29: 1031–1040.
Fang J, Dagenais SL, Erickson RP, Arlt MF, Glynn MW, Gorski JL et al. Mutations in FOXC2 (MFH-1), a forkhead family transcription factor, are responsible for the hereditary lymphedema–distichiasis syndrome. Am J Hum Genet 2000; 67: 1382–1388.
Berry FB, Tamimi Y, Carle MV, Lehmann OJ, Walter MA . The establishment of a predictive mutational model of the forkhead domain through the analyses of FOXC2 missense mutations identified in patients with hereditary lymphedema with distichiasis. Hum Mol Genet 2005; 18: 2619–2627.
Berry FB, Saleem RA, Walter MA . FOXC1 transcriptional regulation is mediated by N- and C-terminal activation domains and contains a phosphorylated transcriptional inhibitory domain. J Biol Chem 2002; 12: 10292–10307.
Pagano MA, Poletto G, Di Maira G, Cozza G, Ruzzene M, Sarno S et al. Tetrabromocinnamic acid (TBCA) and related compounds represent a new class of specific protein kinase CK2 inhibitors. Chembiochem 2007; 8: 129–139.
Tu YF, Kaipparettu BA, Ma Y, Wong LJ . Mitochondria of highly metastatic breast cancer cell line MDA-MB-231 exhibits increased autophagic properties. Biochim Biophys Acta 2011; 1807: 1125–1132.
Deshiere A, Duchemin-Pelletier E, Spreux E, Ciais D, Combes F, Vandenbrouck Y et al. Unbalanced expression of CK2 kinase subunits is sufficient to drive epithelial-to-mesenchymal transition by Snail1 induction. Oncogene 2013; 32: 1373–1383.
Meggio F, Pinna LA . One-thousand-and-one substrates of protein kinase CK2? FASEB J 2003; 17: 349–368.
Mueller T, Breuer P, Schmitt I, Walter J, Evert BO, Wüllner U . CK2-dependent phosphorylation determines cellular localization and stability of ataxin-3. Hum Mol Genet 2009; 17: 3334–3343.
Schwindling SL, Noll A, Montenarh M, Götz C . Mutation of a CK2 phosphorylation site in cdc25C impairs importin alpha/beta binding and results in cytoplasmic retention. Oncogene 2004; 23: 4155–4165.
Barrett RM, Colnaghi R, Wheatley SP . Threonine 48 in the BIR domain of survivin is critical to its mitotic and anti-apoptotic activities and can be phosphorylated by CK2 in vitro. Cell Cycle 2011; 10: 538–548.
Faust M, Montenarh M . Subcellular localization of protein kinase CK2: a key to its function? Cell Tissue Res 2000; 301: 329–340.
von Knethen A, Tzieply N, Jennewein C, Brüne B . Casein-kinase-II-dependent phosphorylation of PPARgamma provokes CRM1-mediated shuttling of PPARgamma from the nucleus to the cytosol. J Cell Sci 2010; 123: 192–201.
MacPherson MR, Molina P, Souchelnytskyi S, Wernstedt C, Martin-Pérez J, Portillo F et al. Phosphorylation of serine 11 and serine 92 as new positive regulators of human Snail1 function: potential involvement of casein kinase-2 and the cAMP-activated kinase protein kinase A. Mol Biol Cell 2010; 21: 244–253.
Acknowledgements
This work was supported by NIH awards to LGC (DK65109) and DG (DK094589).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Golden, D., Cantley, L. Casein kinase 2 prevents mesenchymal transformation by maintaining Foxc2 in the cytoplasm. Oncogene 34, 4702–4712 (2015). https://doi.org/10.1038/onc.2014.395
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2014.395
- Springer Nature Limited
This article is cited by
-
CSNK2 in cancer: pathophysiology and translational applications
British Journal of Cancer (2022)