Abstract
Atg101 is an essential component of the autophagy-initiating ULK complex in higher eukaryotes, but it is absent from the functionally equivalent Atg1 complex in budding yeast. Here, we report the crystal structure of the fission yeast Atg101–Atg13 complex. Atg101 has a Hop1, Rev7 and Mad2 (HORMA) architecture similar to that of Atg13. Mad2 HORMA has two distinct conformations (O-Mad2 and C-Mad2), and, intriguingly, Atg101 resembles O-Mad2 rather than the C-Mad2–like Atg13. Atg13 HORMA from higher eukaryotes possesses an inherently unstable fold, which is stabilized by Atg101 via interactions analogous to those between O-Mad2 and C-Mad2. Mutational studies revealed that Atg101 is responsible for recruiting downstream factors to the autophagosome-formation site in mammals via a newly identified WF finger. These data define the molecular functions of Atg101, providing a basis for elucidating the molecular mechanisms of mammalian autophagy initiation by the ULK complex.







Similar content being viewed by others
References
Mizushima, N., Yoshimori, T. & Ohsumi, Y. The role of Atg proteins in autophagosome formation. Annu. Rev. Cell Dev. Biol. 27, 107–132 (2011).
Mizushima, N. & Komatsu, M. Autophagy: renovation of cells and tissues. Cell 147, 728–741 (2011).
Mizushima, N., Levine, B., Cuervo, A.M. & Klionsky, D.J. Autophagy fights disease through cellular self-digestion. Nature 451, 1069–1075 (2008).
Kundu, M. & Thompson, C.B. Autophagy: basic principles and relevance to disease. Annu. Rev. Pathol. 3, 427–455 (2008).
Nakatogawa, H., Suzuki, K., Kamada, Y. & Ohsumi, Y. Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat. Rev. Mol. Cell Biol. 10, 458–467 (2009).
Feng, Y., He, D., Yao, Z. & Klionsky, D.J. The machinery of macroautophagy. Cell Res. 24, 24–41 (2014).
Mizushima, N. The role of the Atg1/ULK1 complex in autophagy regulation. Curr. Opin. Cell Biol. 22, 132–139 (2010).
Ragusa, M.J., Stanley, R.E. & Hurley, J.H. Architecture of the Atg17 complex as a scaffold for autophagosome biogenesis. Cell 151, 1501–1512 (2012).
Hosokawa, N. et al. Atg101, a novel mammalian autophagy protein interacting with Atg13. Autophagy 5, 973–979 (2009).
Mercer, C.A., Kaliappan, A. & Dennis, P.B. A novel, human Atg13 binding protein, Atg101, interacts with ULK1 and is essential for macroautophagy. Autophagy 5, 649–662 (2009).
Itakura, E. & Mizushima, N. Characterization of autophagosome formation site by a hierarchical analysis of mammalian Atg proteins. Autophagy 6, 764–776 (2010).
Itakura, E., Kishi-Itakura, C., Koyama-Honda, I. & Mizushima, N. Structures containing Atg9A and the ULK1 complex independently target depolarized mitochondria at initial stages of Parkin-mediated mitophagy. J. Cell Sci. 125, 1488–1499 (2012).
Suzuki, K., Kubota, Y., Sekito, T. & Ohsumi, Y. Hierarchy of Atg proteins in pre-autophagosomal structure organization. Genes Cells 12, 209–218 (2007).
Kawamata, T., Kamada, Y., Kabeya, Y., Sekito, T. & Ohsumi, Y. Organization of the pre-autophagosomal structure responsible for autophagosome formation. Mol. Biol. Cell 19, 2039–2050 (2008).
Cheong, H., Nair, U., Geng, J. & Klionsky, D.J. The Atg1 kinase complex is involved in the regulation of protein recruitment to initiate sequestering vesicle formation for nonspecific autophagy in Saccharomyces cerevisiae. Mol. Biol. Cell 19, 668–681 (2008).
Suzuki, S.W. et al. Atg13 HORMA domain recruits Atg9 vesicles during autophagosome formation. Proc. Natl. Acad. Sci. USA 112, 3350–3355 (2015).
Jao, C.C., Ragusa, M.J., Stanley, R.E. & Hurley, J.H.A. HORMA domain in Atg13 mediates PI 3-kinase recruitment in autophagy. Proc. Natl. Acad. Sci. USA 110, 5486–5491 (2013).
Fujioka, Y. et al. Structural basis of starvation-induced assembly of the autophagy initiation complex. Nat. Struct. Mol. Biol. 21, 513–521 (2014).
Sun, L.L. et al. Global analysis of fission yeast mating genes reveals new autophagy factors. PLoS Genet. 9, e1003715 (2013).
Aravind, L. & Koonin, E.V. The HORMA domain: a common structural denominator in mitotic checkpoints, chromosome synapsis and DNA repair. Trends Biochem. Sci. 23, 284–286 (1998).
Hegedu˝s, K., Nagy, P., Gaspari, Z. & Juhasz, G. The putative HORMA domain protein Atg101 dimerizes and is required for starvation-induced and selective autophagy in Drosophila. Biomed Res. Int. 2014, 470482 (2014).
Holm, L., Kaariainen, S., Rosenstrom, P. & Schenkel, A. Searching protein structure databases with DaliLite v.3. Bioinformatics 24, 2780–2781 (2008).
Mapelli, M., Massimiliano, L., Santaguida, S. & Musacchio, A. The Mad2 conformational dimer: structure and implications for the spindle assembly checkpoint. Cell 131, 730–743 (2007).
Luo, X. & Yu, H. Protein metamorphosis: the two-state behavior of Mad2. Structure 16, 1616–1625 (2008).
Luo, X. et al. Structure of the Mad2 spindle assembly checkpoint protein and its interaction with Cdc20. Nat. Struct. Biol. 7, 224–229 (2000).
Luo, X., Tang, Z., Rizo, J. & Yu, H. The Mad2 spindle checkpoint protein undergoes similar major conformational changes upon binding to either Mad1 or Cdc20. Mol. Cell 9, 59–71 (2002).
Sironi, L. et al. Crystal structure of the tetrameric Mad1-Mad2 core complex: implications of a ′safety belt′ binding mechanism for the spindle checkpoint. EMBO J. 21, 2496–2506 (2002).
Mizushima, N., Yoshimori, T. & Levine, B. Methods in mammalian autophagy research. Cell 140, 313–326 (2010).
Demishtein, A., Porat, Z., Elazar, Z. & Shvets, E. Applications of flow cytometry for measurement of autophagy. Methods 75, 87–95 (2015).
Hara, T. et al. FIP200, a ULK-interacting protein, is required for autophagosome formation in mammalian cells. J. Cell Biol. 181, 497–510 (2008).
Itakura, E. & Mizushima, N. p62 targeting to the autophagosome formation site requires self-oligomerization but not LC3 binding. J. Cell Biol. 192, 17–27 (2011).
Kabeya, Y. et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 19, 5720–5728 (2000).
Proikas-Cezanne, T., Takacs, Z., Donnes, P. & Kohlbacher, O. WIPI proteins: essential PtdIns3P effectors at the nascent autophagosome. J. Cell Sci. 128, 207–217 (2015).
Axe, E.L. et al. Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J. Cell Biol. 182, 685–701 (2008).
Obara, K., Sekito, T., Niimi, K. & Ohsumi, Y. The Atg18-Atg2 complex is recruited to autophagic membranes via phosphatidylinositol 3-phosphate and exerts an essential function. J. Biol. Chem. 283, 23972–23980 (2008).
McNicholas, S., Potterton, E., Wilson, K.S. & Noble, M.E. Presenting your structures: the CCP4mg molecular-graphics software. Acta Crystallogr. D Biol. Crystallogr. 67, 386–394 (2011).
Matthews, B.W. Solvent content of protein crystals. J. Mol. Biol. 33, 491–497 (1968).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
Adams, P.D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Murshudov, G.N., Vagin, A.A. & Dodson, E.J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D Biol. Crystallogr. 53, 240–255 (1997).
Winn, M.D. et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. D Biol. Crystallogr. 67, 235–242 (2011).
Brünger, A.T. et al. Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D Biol. Crystallogr. 54, 905–921 (1998).
Lovell, S.C. et al. Structure validation by Calpha geometry: phi,psi and Cbeta deviation. Proteins 50, 437–450 (2003).
Kitamura, T. et al. Retrovirus-mediated gene transfer and expression cloning: powerful tools in functional genomics. Exp. Hematol. 31, 1007–1014 (2003).
Quy, P.N., Kuma, A., Pierre, P. & Mizushima, N. Proteasome-dependent activation of mammalian target of rapamycin complex 1 (mTORC1) is essential for autophagy suppression and muscle remodeling following denervation. J. Biol. Chem. 288, 1125–1134 (2013).
Mali, P. et al. RNA-guided human genome engineering via Cas9. Science 339, 823–826 (2013).
Hosokawa, N. et al. Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol. Biol. Cell 20, 1981–1991 (2009).
Saitoh, T., Nakano, H., Yamamoto, N. & Yamaoka, S. Lymphotoxin-beta receptor mediates NEMO-independent NF-kappaB activation. FEBS Lett. 532, 45–51 (2002).
Acknowledgements
We thank T. Kitamura (University of Tokyo) and S. Yamaoka (Tokyo Medical and Dental University) for retroviral vectors and Plat-E cells and S. Sugano (University of Tokyo) for the pEF321-T plasmid. Synchrotron radiation experiments were performed at beamline BL41XU at SPring-8, Japan. This work was supported by Grants-in-Aid for Scientific Research on Priority Areas 25111005 (to N.M.) and 25111004 (to N.N.N.) and by funding from the Platform for Drug Discovery, Informatics and Structural Life Science (to N.N.N.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Author information
Authors and Affiliations
Contributions
H.S. and N.N.N. performed structural studies; H.S. performed in vitro experiments; T.K. performed experiments in mammalian cells; H.S., T.K., N.M. and N.N.N. analyzed data; and H.S. and N.N.N. wrote the manuscript. All authors discussed the results and commented on the manuscript. N.N.N. and N.M. supervised the work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
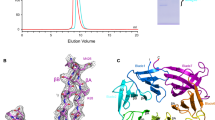
Supplementary Figure 1 Crystallographic study of the Atg101–Atg13 complex.
(a) Oligomerization states of the SpAtg101, SpAtg13HORMA, and SpAtg101-SpAtg13HORMA complex studied by gel filtration chromatography. Absorbance at 280 nm of SpAtg101 alone, SpAtg13HORMA alone, and SpAtg101-SpAtg13HORMA mixture are shown with blue, green, and red lines, respectively. The apparent molecular mass of the proteins estimated from the elution volumes was indicated in parentheses. (b) Oligomerization states of the SpAtg101, SpAtg13HORMA, and SpAtg101-SpAtg13HORMA complex studied by SAXS. Top panel shows experimental scattering patterns and middle four panels show the concentration dependence of I(0)/Concentration, where I(0) is the forward scattering intensity derived using the Guinier law. (c) Sequence alignment of Atg101 homologs and human Mad2. Secondary structure elements that are colored as in Fig. 1a are shown below the sequence. The residues des are shown below the sequence. The residues interacting with SpAtg13HORMA are surrounded with red (Atg13 binding surface) or blue (WF finger) squares. Species are abbreviated as follows: S. pombe (Sp), Dorosophila melanogaster (Dm), Mus musculus (Mm), and Homo sapiens (Hs). As for Mad2, sequence of human Mad2 structurally aligned with SpAtg101 was shown, in which unaligned residues were shaded. (d) Structure and topology of LtAtg13HORMA (PDB ID 4J2G). The C-terminal segments including the safety belt and the cap are purple and yellow, respectively. (e) Close-up stereo view of the interaction of β1 with α-helices in SpAtg101. β1 is colored orange while the other regions are colored light blue. The side-chains of the residues involved in the interactions are shown by a stick model. (f) Close-up stereo view of the interaction of β1 with α-helices in O-Mad2 (PDB ID 2V64). β1 is colored orange while the other regions are colored light blue. The side-chains of the residues involved in the interactions are shown by a stick model. (g) Close-up stereo view of the interaction between the cap and the safety belt in LtAtg13HORMA (PDB ID 4J2G). Coloring is as in d. The side-chains of the residues involved in the interactions are shown by a stick model. (h) Sequence alignment of the HORMA domain of Atg13 homologs and human Mad2. Secondary structure elements that are colored as in Fig. 1a are shown below the sequence. The residues described in g are marked by dots on the secondary structure elements. Species are abbreviated as in c and as follows: S. cerevisiae (Sc), L. thermotolerans (Lt), Kluyveromyces marxianus (Km), Kluyveromyces lactis (Kl), Candida glabrata (Cg).
Supplementary Figure 2 Interactions between SpAtg101 and SpAtg13HORMA observed in the crystal.
(a) Close-up stereo view of the intermolecular β-sheet between SpAtg101 and SpAtg13HORMA. The main-chain of the residues forming the intermolecular β-sheet is shown with a stick model. Coloring is as in Fig. 1a. (b) Artificial lattice contact between SpAtg101 and SpAtg13HORMA. Coloring is as in Fig. 1a except for the neighboring SpAtg13HORMA, which is colored sea green. (c) Close-up stereo view of the lattice contact interaction between the WF finger of SpAtg101 and a neighboring SpAtg13HORMA molecule in the crystal. Coloring is as in (b). (d) In vitro pulldown assay between GST-fused SpAtg101 and SpAtg13HORMA. Uncropped images of SDS-PAGE gels are shown in Supplementary Data Set 1.
Supplementary Figure 3 Mutational analyses of the Atg101-Atg13 interaction.
(a) Gel filtration profiles of SpAtg101 mutants mixed with wild-type SpAtg13HORMA or SpAtg13HORMA mutants mixed with wild-type SpAtg101. Black line denotes the absorption at 220nm. SDS-PAGE analysis of the fraction No. 1 and 2 stained with Oriole (Bio-rad) was also shown. (b) CD spectra of wild-type and representative mutants of SpAtg101 (top panel) and SpAtg13HORMA (bottom panel). (c) Mutation detected in Atg101 KO MEFs. The sequences of the wild-type Atg101 allele and mutated alleles identified in Atg101 KO MEFs. The position of the PAM sequence used for CRISPR-mediated gene targeting is shown with a red line. (d) Close-up stereo view of the interaction between SpAtg101 and β8’ of SpAtg13HORMA. The side-chain of the residues involved in the interaction is shown with a stick model. Coloring is as in Fig. 1a. (e) Close-up stereo view of the interaction between O-Mad2 and β8’ of C-Mad2. The side-chain of the residues involved in the interaction is shown with a stick model. Coloring is as in Fig. 1b. (f) The cytosolic fractions of wild-type or Atg101 KO MEFs were separated by size exclusion chromatography. Each fraction was analyzed by immunoblotting with indicated antibodies. Positions of the molecular mass standards (in kDa) are shown. V, void fraction. (g) Cytosolic fractions of Atg101 KO MEFs stably expressing Atg101 W110A P111A F112A separated by gel filtration chromatography as in Fig. 3e. Uncropped images of SDS-PAGE gels and immunoblotting are shown in Supplementary Data Set 1.
Supplementary Figure 4 Low expression of wild-type Atg101 can restore Atg13 stability and autophagy.
Western blotting analyses for detecting LC3 turnover, p62 accumulation, and expression level of Atg13. Samples are lysates of Atg101 KO MEFs stably expressing wild type HA-HsAtg101 with a high expression level (used in the main Figure) or low expression levels (clones 3, 6, and 10), and Atg101 KO MEFs stably expressing the L30R/H31R mutant of HA-HsAtg101 cultured in regular or starvation medium in the presence or absence of 100 nM bafilomycin A1 for 2 h. Uncropped images of immunoblotting are shown in Supplementary Data Set 1.
Supplementary Figure 5 Colocalization between Atg13 and FIP200 in Atg101 mutant–expressing cells.
Colocalization analysis of GFP-Atg13 and FIP200 by immunostaining using anti-GFP and anti-FIP200 antibodies. Samples are Atg101 KO MEFs stably expressing GFP-Atg13 and indicated HA-Atg101 mutants cultured in regular medium or starvation medium for 1 h. Scale bars, white: 20 µm, yellow (insets): 1 µm.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 (PDF 6298 kb)
Supplementary Data Set 1
Uncropped images for western blots and SDS-PAGE gels (PDF 3375 kb)
Rights and permissions
About this article
Cite this article
Suzuki, H., Kaizuka, T., Mizushima, N. et al. Structure of the Atg101–Atg13 complex reveals essential roles of Atg101 in autophagy initiation. Nat Struct Mol Biol 22, 572–580 (2015). https://doi.org/10.1038/nsmb.3036
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.3036
- Springer Nature America, Inc.
This article is cited by
-
Autophagy deficiency in neurodevelopmental disorders
Cell & Bioscience (2021)
-
CircRAB11FIP1 promoted autophagy flux of ovarian cancer through DSC1 and miR-129
Cell Death & Disease (2021)
-
Autophagy and its role in regeneration and remodeling within invertebrate
Cell & Bioscience (2020)
-
Autophagy and autophagy-related proteins in cancer
Molecular Cancer (2020)
-
An antibody for analysis of autophagy induction
Nature Methods (2020)