Key Points
-
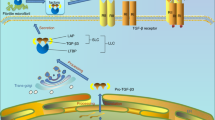
Physiological loading of articular cartilage maintains transforming growth factor-β (TGFβ) signalling and prevents hypertrophic differentiation of articular chondrocytes
-
TGFβ signalling changes with ageing, at least in animal models, resulting in a loss of SMAD2–SMAD3 signalling and making articular cartilage prone to changes associated with osteoarthritis (OA)
-
Loading-induced induction of TGFβ signalling is less efficient in aged cartilage than in young cartilage, impairing the mechanism that blocks chondrocyte hypertrophic differentiation
-
During OA, high levels of active TGFβ in the joint drive fibrosis, osteophyte formation and subchondral bone changes
-
The role of TGFβ changes from protective in a young, healthy joint to pathologic in an osteoarthritic joint
Abstract
Transforming growth factor-β (TGFβ) is a pleiotropic cytokine that is important in the regulation of joint homeostasis and disease. TGFβ signalling is induced by loading and has an important function in maintaining the differentiated phenotype of articular chondrocytes. Concentrations of active TGFβ differ greatly between healthy and osteoarthritic joints, being low in healthy joints and high in osteoarthritic joints, leading to the activation of different signalling pathways in joint cells. The characteristic pathology of osteoarthritic joints, such as cartilage damage, osteophyte formation and synovial fibrosis, seems to be stimulated or even caused by the high levels of active TGFβ, in combination with altered chondrocyte signalling pathways (which are also observed in ageing joints). In this Review, the changing role of TGFβ in normal joint homeostasis, ageing and osteoarthritis is discussed: TGFβ counteracts pathological changes in a young healthy joint, alters its signalling during ageing and is a driving force of pathology in osteoarthritic joints.




Similar content being viewed by others
References
Johnson, V. L. & Hunter, D. J. The epidemiology of osteoarthritis. Best Pract. Res. Clin. Rheumatol. 28, 5–15 (2014).
de Caestecker, M. The transforming growth factor-β superfamily of receptors. Cytokine Growth Factor Rev. 15, 1–11 (2004).
Govinden, R. & Bhoola, K. D. Genealogy, expression, and cellular function of transforming growth factor-β. Pharmacol. Ther. 98, 257–265 (2003).
Unsold, C., Hyytiainen, M., Bruckner-Tuderman, L. & Keski-Oja, J. Latent TGF-β binding protein LTBP-1 contains three potential extracellular matrix interacting domains. J. Cell Sci. 114, 187–197 (2001).
Sheppard, D. Integrin-mediated activation of latent transforming growth factor β. Cancer Metastasis Rev. 24, 395–402 (2005).
Munger, J. S. et al. Latent transforming growth factor-β: structural features and mechanisms of activation. Kidney Int. 51, 1376–1382 (1997).
Wipff, P. J. & Hinz, B. Integrins and the activation of latent transforming growth factor β1 — an intimate relationship. Eur. J. Cell Biol. 87, 601–615 (2008).
Heldin, C. H., Miyazono, K. & ten Dijke, P. TGF-β signalling from cell membrane to nucleus through SMAD proteins. Nature 390, 465–471 (1997).
Goumans, M. J. et al. Balancing the activation state of the endothelium via two distinct TGF-β type I receptors. EMBO J. 21, 1743–1753 (2002).
Finnson, K. W., Parker, W. L., ten Dijke, P., Thorikay, M. & Philip, A. ALK1 opposes ALK5/Smad3 signaling and expression of extracellular matrix components in human chondrocytes. J. Bone Miner. Res. 23, 896–906 (2008).
Blaney Davidson, E. N. et al. Increase in ALK1/ALK5 ratio as a cause for elevated MMP-13 expression in osteoarthritis in humans and mice. J. Immunol. 182, 7937–7945 (2009).
Finnson, K. W. et al. Endoglin differentially regulates TGF-β-induced Smad2/3 and Smad1/5 signalling and its expression correlates with extracellular matrix production and cellular differentiation state in human chondrocytes. Osteoarthritis Cartilage 18, 1518–1527 (2010).
Chacko, B. M. et al. The L3 loop and C-terminal phosphorylation jointly define Smad protein trimerization. Nat. Struct. Biol. 8, 248–253 (2001).
Goumans, M. J. et al. Activin receptor-like kinase (ALK)1 is an antagonistic mediator of lateral TGFβ/ALK5 signaling. Mol. Cell 12, 817–828 (2003).
Daly, A. C., Randall, R. A. & Hill, C. S. Transforming growth factor β-induced Smad1/5 phosphorylation in epithelial cells is mediated by novel receptor complexes and is essential for anchorage-independent growth. Mol. Cell. Biol. 28, 6889–6902 (2008).
Hellingman, C. A. et al. Smad signaling determines chondrogenic differentiation of bone-marrow-derived mesenchymal stem cells: inhibition of Smad1/5/8P prevents terminal differentiation and calcification. Tissue Eng. Part A 17, 1157–1167 (2011).
Keller, B. et al. Interaction of TGFβ and BMP signaling pathways during chondrogenesis. PLoS ONE 6, e16421 (2011).
de Kroon, L. M. et al. Activin receptor-like kinase receptors ALK5 and ALK1 are both required for TGFβ-induced chondrogenic differentiation of human bone marrow-derived mesenchymal stem cells. PLoS ONE 10, e0146124 (2015).
Zhang, Y. E. Non-Smad pathways in TGF-β signaling. Cell Res. 19, 128–139 (2009).
Gao, L. et al. TAK1 regulates SOX9 expression in chondrocytes and is essential for postnatal development of the growth plate and articular cartilages. J. Cell Sci. 126, 5704–5713 (2013).
Jurvelin, J., Kiviranta, I., Tammi, M. & Helminen, J. H. Softening of canine articular cartilage after immobilization of the knee joint. Clin. Orthop. Relat. Res. 207, 246–252 (1986).
Palmoski, M. J., Colyer, R. A. & Brandt, K. D. Joint motion in the absence of normal loading does not maintain normal articular cartilage. Arthritis Rheum. 23, 325–334 (1980).
Hinterwimmer, S. et al. Cartilage atrophy in the knees of patients after seven weeks of partial load bearing. Arthritis Rheum. 50, 2516–2520 (2004).
Ando, A. et al. Increased expression of metalloproteinase-8 and -13 on articular cartilage in a rat immobilized knee model. Tohoku J. Exp. Med. 217, 271–278 (2009).
Vanwanseele, B., Eckstein, F., Knecht, H., Stussi, E. & Spaepen, A. Knee cartilage of spinal cord-injured patients displays progressive thinning in the absence of normal joint loading and movement. Arthritis Rheum. 46, 2073–2078 (2002).
Sanchez-Adams, J., Leddy, H. A., McNulty, A. L., O'Conor, C. J. & Guilak, F. The mechanobiology of articular cartilage: bearing the burden of osteoarthritis. Curr. Rheumatol. Rep. 16, 451 (2014).
Bougault, C. et al. Dynamic compression of chondrocyte-agarose constructs reveals new candidate mechanosensitive genes. PLoS ONE 7, e36964 (2012).
Furumatsu, T. et al. Tensile strain increases expression of CCN2 and COL2A1 by activating TGF-β-Smad2/3 pathway in chondrocytic cells. J. Biomech. 46, 1508–1515 (2013).
Morales, T. I., Joyce, M. E., Sobel, M. E., Danielpour, D. & Roberts, A. B. Transforming growth factor-β in calf articular cartilage organ cultures: synthesis and distribution. Arch. Biochem. Biophys. 288, 397–405 (1991).
Yao, J. Y. et al. Mutation analysis of the Smad3 gene in human osteoarthritis. Eur. J. Hum. Genet. 11, 714–717 (2003).
van de Laar, I. M. et al. Mutations in SMAD3 cause a syndromic form of aortic aneurysms and dissections with early-onset osteoarthritis. Nat. Genet. 43, 121–126 (2011).
Wu, Q. et al. Induction of an osteoarthritis-like phenotype and degradation of phosphorylated Smad3 by Smurf2 in transgenic mice. Arthritis Rheum. 58, 3132–3144 (2008).
Yang, X. et al. TGF-β/Smad3 signals repress chondrocyte hypertrophic differentiation and are required for maintaining articular cartilage. J. Cell Biol. 153, 35–46 (2001).
Serra, R. et al. Expression of a truncated, kinase-defective TGF-β type II receptor in mouse skeletal tissue promotes terminal chondrocyte differentiation and osteoarthritis. J. Cell Biol. 139, 541–552 (1997).
Madej, W., van Caam, A., Blaney Davidson, E. N., van der Kraan, P. M. & Buma, P. Physiological and excessive mechanical compression of articular cartilage activates Smad2/3P signaling. Osteoarthritis Cartilage 22, 1018–1025 (2014).
Madej, W., van Caam, A., Blaney Davidson, E., Buma, P. & van der Kraan, P. M. Unloading results in rapid loss of TGFβ signaling in articular cartilage: role of loading-induced TGFβ signaling in maintenance of articular chondrocyte phenotype? Osteoarthritis Cartilage 24, 1807–1815 (2016).
Ballock, R. T. et al. TGF-β1 prevents hypertrophy of epiphyseal chondrocytes: regulation of gene expression for cartilage matrix proteins and metalloproteases. Dev. Biol. 158, 414–429 (1993).
Albro, M. B. et al. Shearing of synovial fluid activates latent TGF-β. Osteoarthritis Cartilage 20, 1374–1382 (2012).
Hinz, B. The extracellular matrix and transforming growth factor-β1: tale of a strained relationship. Matrix Biol. 47, 54–65 (2015).
Ferguson, C. M. et al. Smad2 and 3 mediate transforming growth factor-β1-induced inhibition of chondrocyte maturation. Endocrinology 141, 4728–4735 (2000).
Li, T. F. et al. Smad3-deficient chondrocytes have enhanced BMP signaling and accelerated differentiation. J. Bone Miner. Res. 21, 4–16 (2006).
Deie, M. et al. The effects of age on rabbit MCL fibroblast matrix synthesis in response to TGF-β1 or EGF. Mech. Ageing Dev. 97, 121–130 (1997).
Iqbal, J., Dudhia, J., Bird, J. L. & Bayliss, M. T. Age-related effects of TGF-β on proteoglycan synthesis in equine articular cartilage. Biochem. Biophys. Res. Commun. 274, 467–471 (2000).
van Beuningen, H. M., van der Kraan, P. M., Arntz, O. J. & van den Berg, W. B. In vivo protection against interleukin-1-induced articular cartilage damage by transforming growth factor-β1: age-related differences. Ann. Rheum. Dis. 53, 593–600 (1994).
Scharstuhl, A., van Beuningen, H. M., Vitters, E. L., van der Kraan, P. M. & van den Berg, W. B. Loss of transforming growth factor counteraction on interleukin 1 mediated effects in cartilage of old mice. Ann. Rheum. Dis. 61, 1095–1098 (2002).
Blaney Davidson, E. N., Scharstuhl, A., Vitters, E. L., van der Kraan, P. M. & van den Berg, W. B. Reduced transforming growth factor-β signaling in cartilage of old mice: role in impaired repair capacity. Arthritis Res. Ther. 7, R1338–R1347 (2005).
Zhao, W. et al. Cartilage degeneration and excessive subchondral bone formation in spontaneous osteoarthritis involves altered TGF-β signaling. J. Orthop. Res. 34, 763–770 (2016).
Hui, W. et al. Oxidative changes and signalling pathways are pivotal in initiating age-related changes in articular cartilage. Ann. Rheum. Dis. 75, 449–458 (2016).
van Caam, A. et al. Expression of TGFβ-family signalling components in ageing cartilage: age-related loss of TGFβ and BMP receptors. Osteoarthritis Cartilage 24, 1235–1245 (2016).
Qureshi, H. Y., Ricci, G. & Zafarullah, M. Smad signaling pathway is a pivotal component of tissue inhibitor of metalloproteinases-3 regulation by transforming growth factor β in human chondrocytes. Biochim. Biophys. Acta 1783, 1605–1612 (2008).
Wang, X. et al. Interaction of ERK1/2 and Smad2/3 signaling pathways in TGF-β1-induced TIMP-3 expression in rat chondrocytes. Arch. Biochem. Biophys. 564, 229–236 (2014).
Madej, W. et al. Ageing is associated with reduction of mechanically-induced activation of Smad2/3P signaling in articular cartilage. Osteoarthritis Cartilage 24, 146–157 (2016).
Gubin, D. G., Weinert, D. & Bolotnova, T. V. Age-dependent changes of the temporal order — causes and treatment. Curr. Aging Sci. 9, 14–25 (2016).
Weinert, D. Age-dependent changes of the circadian system. Chronobiol. Int. 17, 261–283 (2000).
Gossan, N., Boot-Handford, R. & Meng, Q. J. Ageing and osteoarthritis: a circadian rhythm connection. Biogerontology 16, 209–219 (2015).
Dudek, M. et al. The chondrocyte clock gene Bmal1 controls cartilage homeostasis and integrity. J. Clin. Invest. 126, 365–376 (2016).
Jiang, Q., Qiu, Y. T., Chen, M. J., Zhang, Z. Y. & Yang, C. Synovial TGF-β1 and MMP-3 levels and their correlation with the progression of temporomandibular joint osteoarthritis combined with disc displacement: a preliminary study. Biomed. Rep. 1, 218–222 (2013).
Wei, X. & Messner, K. Age- and injury-dependent concentrations of transforming growth factor-β1 and proteoglycan fragments in rabbit knee joint fluid. Osteoarthritis Cartilage 6, 10–18 (1998).
Fava, R., Olsen, N., Keski-Oja, J., Moses, H. & Pincus, T. Active and latent forms of transforming growth factor β activity in synovial effusions. J. Exp. Med. 169, 291–296 (1989).
Metukuri, M. R. et al. Activation of latent transforming growth factor-β1 by nitric oxide in macrophages: role of soluble guanylate cyclase and MAP kinases. Wound Repair Regen. 17, 578–588 (2009).
Lee, J. K., Lee, Y. R., Lee, Y. H., Kim, K. & Lee, C. K. Production of TGF-β1 as a mechanism for defective antigen-presenting cell function of macrophages generated in vitro with M-CSF. Immune Netw. 9, 27–33 (2009).
McGowan, S. E. Influences of endogenous and exogenous TGF-β on elastin in rat lung fibroblasts and aortic smooth muscle cells. Am. J. Physiol. 263, L257–L263 (1992).
Torrego, A., Hew, M., Oates, T., Sukkar, M. & Fan Chung, K. Expression and activation of TGF-β isoforms in acute allergen-induced remodelling in asthma. Thorax 62, 307–313 (2007).
Travis, M. A. & Sheppard, D. TGF-β activation and function in immunity. Annu. Rev. Immunol. 32, 51–82 (2014).
Blaney Davidson, E. N., Vitters, E. L., van der Kraan, P. M. & van den Berg, W. B. Expression of transforming growth factor-β (TGFβ) and the TGFβ signalling molecule SMAD-2P in spontaneous and instability-induced osteoarthritis: role in cartilage degradation, chondrogenesis and osteophyte formation. Ann. Rheum. Dis. 65, 1414–1421 (2006).
Koli, K., Saharinen, J., Hyytiainen, M., Penttinen, C. & Keski-Oja, J. Latency, activation, and binding proteins of TGF-β. Microsc. Res. Tech. 52, 354–362 (2001).
Remst, D. F. et al. TGF-β induces Lysyl hydroxylase 2b in human synovial osteoarthritic fibroblasts through ALK5 signaling. Cell Tissue Res. 355, 163–171 (2014).
Bauge, C. et al. Interleukin-1β impairment of transforming growth factor β1 signaling by down-regulation of transforming growth factor β receptor type II and up-regulation of Smad7 in human articular chondrocytes. Arthritis Rheum. 56, 3020–3032 (2007).
Bauge, C. et al. Interleukin-1β up-regulation of Smad7 via NF-κB activation in human chondrocytes. Arthritis Rheum. 58, 221–226 (2008).
Matsuzaki, K. Smad phospho-isoforms direct context-dependent TGF-β signaling. Cytokine Growth Factor Rev. 24, 385–399 (2013).
Yu, J. S. et al. PI3K/mTORC2 regulates TGF-β/Activin signalling by modulating Smad2/3 activity via linker phosphorylation. Nat. Commun. 6, 7212 (2015).
Yumoto, K. et al. TGF-β-activated kinase 1 (Tak1) mediates agonist-induced Smad activation and linker region phosphorylation in embryonic craniofacial neural crest-derived cells. J. Biol. Chem. 288, 13467–13480 (2013).
Kamato, D. et al. Transforming growth factor-β signalling: role and consequences of Smad linker region phosphorylation. Cell Signal. 25, 2017–2024 (2013).
van den Bosch, M. H. et al. Canonical Wnt signaling skews TGF-β signaling in chondrocytes towards signaling via ALK1 and Smad 1/5/8. Cell Signal. 26, 951–958 (2014).
Studer, R. K. & Chu, C. R. p38 MAPK and COX2 inhibition modulate human chondrocyte response to TGF-β. J. Orthop. Res. 23, 454–461 (2005).
Guo, P., Zhao, K. W., Dong, X. Y., Sun, X. & Dong, J. T. Acetylation of KLF5 alters the assembly of p15 transcription factors in transforming growth factor-β-mediated induction in epithelial cells. J. Biol. Chem. 284, 18184–18193 (2009).
Wilkinson, D. S. et al. A direct intersection between p53 and transforming growth factor β pathways targets chromatin modification and transcription repression of the α-fetoprotein gene. Mol. Cell. Biol. 25, 1200–1212 (2005).
Costamagna, E., Garcia, B. & Santisteban, P. The functional interaction between the paired domain transcription factor Pax8 and Smad3 is involved in transforming growth factor-β repression of the sodium/iodide symporter gene. J. Biol. Chem. 279, 3439–3446 (2004).
Meng, X. M., Nikolic-Paterson, D. J. & Lan, H. Y. TGF-β: the master regulator of fibrosis. Nat. Rev. Nephrol. 12, 325–338 (2016).
Bakker, A. C. et al. Overexpression of active TGF-β-1 in the murine knee joint: evidence for synovial-layer-dependent chondro-osteophyte formation. Osteoarthritis Cartilage 9, 128–136 (2001).
van Beuningen, H. M., Glansbeek, H. L., van der Kraan, P. M. & van den Berg, W. B. Differential effects of local application of BMP-2 or TGF-β 1 on both articular cartilage composition and osteophyte formation. Osteoarthritis Cartilage 6, 306–317 (1998).
Scharstuhl, A., Vitters, E. L., van der Kraan, P. M. & van den Berg, W. B. Reduction of osteophyte formation and synovial thickening by adenoviral overexpression of transforming growth factor β/bone morphogenetic protein inhibitors during experimental osteoarthritis. Arthritis Rheum. 48, 3442–3451 (2003).
Blaney Davidson, E. N., Vitters, E. L., van den Berg, W. B. & van der Kraan, P. M. TGF β-induced cartilage repair is maintained but fibrosis is blocked in the presence of Smad7. Arthritis Res. Ther. 8, R65 (2006).
Remst, D. F. et al. Gene expression analysis of murine and human osteoarthritis synovium reveals elevation of transforming growth factor β-responsive genes in osteoarthritis-related fibrosis. Arthritis Rheumatol. 66, 647–656 (2014).
Remst, D. F. et al. Osteoarthritis-related fibrosis is associated with both elevated pyridinoline cross-link formation and lysyl hydroxylase 2b expression. Osteoarthritis Cartilage 21, 157–164 (2013).
Blaney Davidson, E. N. et al. Connective tissue growth factor/CCN2 overexpression in mouse synovial lining results in transient fibrosis and cartilage damage. Arthritis Rheum. 54, 1653–1661 (2006).
Li, J. et al. Hyaluronan injection in murine osteoarthritis prevents TGFβ1-induced synovial neovascularization and fibrosis and maintains articular cartilage integrity by a CD44-dependent mechanism. Arthritis Res. Ther. 14, R151 (2012).
Plaas, A. et al. The relationship between fibrogenic TGFβ1 signaling in the joint and cartilage degradation in post-injury osteoarthritis. Osteoarthritis Cartilage 19, 1081–1090 (2011).
van der Kraan, P. M. & van den Berg, W. B. Osteophytes: relevance and biology. Osteoarthritis Cartilage 15, 237–244 (2007).
van Beuningen, H. M., van der Kraan, P. M., Arntz, O. J. & van den Berg, W. B. Transforming growth factor-β1 stimulates articular chondrocyte proteoglycan synthesis and induces osteophyte formation in the murine knee joint. Lab. Invest. 71, 279–290 (1994).
Blaney Davidson, E. N. et al. Inducible chondrocyte-specific overexpression of BMP2 in young mice results in severe aggravation of osteophyte formation in experimental OA without altering cartilage damage. Ann. Rheum. Dis. 74, 1257–1264 (2015).
Blaney Davidson, E. N. et al. Resemblance of osteophytes in experimental osteoarthritis to transforming growth factor β-induced osteophytes: limited role of bone morphogenetic protein in early osteoarthritic osteophyte formation. Arthritis Rheum. 56, 4065–4073 (2007).
Uchino, M. et al. Growth factor expression in the osteophytes of the human femoral head in osteoarthritis. Clin. Orthop. Relat. Res. 377, 119–125 (2000).
Sakao, K. et al. Asporin and transforming growth factor-β gene expression in osteoblasts from subchondral bone and osteophytes in osteoarthritis. J. Orthop. Sci. 14, 738–747 (2009).
Scharstuhl, A. et al. Inhibition of endogenous TGF-β during experimental osteoarthritis prevents osteophyte formation and impairs cartilage repair. J. Immunol. 169, 507–514 (2002).
Blitz, E., Sharir, A., Akiyama, H. & Zelzer, E. Tendon-bone attachment unit is formed modularly by a distinct pool of Scx- and Sox9-positive progenitors. Development 140, 2680–2690 (2013).
Li, T. et al. Joint TGF-β type II receptor-expressing cells: ontogeny and characterization as joint progenitors. Stem Cells Dev. 22, 1342–1359 (2013).
Moskowitz, R. W. Bone remodeling in osteoarthritis: subchondral and osteophytic responses. Osteoarthritis Cartilage 7, 323–324 (1999).
Yusup, A. et al. Bone marrow lesions, subchondral bone cysts and subchondral bone attrition are associated with histological synovitis in patients with end-stage knee osteoarthritis: a cross-sectional study. Osteoarthritis Cartilage 23, 1858–1864 (2015).
Quasnichka, H. L., Anderson-MacKenzie, J. M. & Bailey, A. J. Subchondral bone and ligament changes precede cartilage degradation in guinea pig osteoarthritis. Biorheology 43, 389–397 (2006).
Lajeunesse, D., Massicotte, F., Pelletier, J. P. & Martel-Pelletier, J. Subchondral bone sclerosis in osteoarthritis: not just an innocent bystander. Mod. Rheumatol. 13, 7–14 (2003).
Zhen, G. et al. Inhibition of TGF-β signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat. Med. 19, 704–712 (2013).
Jiao, K. et al. Overexpressed TGF-β in subchondral bone leads to mandibular condyle degradation. J. Dent. Res. 93, 140–147 (2014).
Xie, L. et al. Systemic neutralization of TGF-β attenuates osteoarthritis. Ann. NY Acad. Sci. 1376, 53–64 (2016).
Letterio, J. J. et al. Autoimmunity associated with TGF-β1-deficiency in mice is dependent on MHC class II antigen expression. J. Clin. Invest. 98, 2109–2119 (1996).
Lee, K. H. et al. Regeneration of hyaline cartilage by cell-mediated gene therapy using transforming growth factor β1-producing fibroblasts. Hum. Gene Ther. 12, 1805–1813 (2001).
Song, S. U. et al. Hyaline cartilage regeneration using mixed human chondrocytes and transforming growth factor-β1- producing chondrocytes. Tissue Eng. 11, 1516–1526 (2005).
Guo, X. et al. Repair of full-thickness articular cartilage defects by cultured mesenchymal stem cells transfected with the transforming growth factor β1 gene. Biomed. Mater. 1, 206–215 (2006).
Ivkovic, A. et al. Articular cartilage repair by genetically modified bone marrow aspirate in sheep. Gene Ther. 17, 779–789 (2010).
Zhang, P., Zhong, Z. H., Yu, H. T. & Liu, B. Exogenous expression of IL-1Ra and TGF-β1 promotes in vivo repair in experimental rabbit osteoarthritis. Scand. J. Rheumatol. 44, 404–411 (2015).
Chen, R. et al. Attenuation of the progression of articular cartilage degeneration by inhibition of TGF-β1 signaling in a mouse model of osteoarthritis. Am. J. Pathol. 185, 2875–2885 (2015).
Principe, D. R. et al. TGF-β: duality of function between tumor prevention and carcinogenesis. J. Natl Cancer Inst. 106, djt369 (2014).
Blaney Davidson, E. N. et al. TGF-β is a potent inducer of Nerve Growth Factor in articular cartilage via the ALK5-Smad2/3 pathway. Potential role in OA related pain? Osteoarthritis Cartilage 23, 478–486 (2015).
Chen, G., Park, C. K., Xie, R. G. & Ji, R. R. Intrathecal bone marrow stromal cells inhibit neuropathic pain via TGF-β secretion. J. Clin. Invest. 125, 3226–3240 (2015).
Lantero, A. et al. TGF-β and opioid receptor signaling crosstalk results in improvement of endogenous and exogenous opioid analgesia under pathological pain conditions. J. Neurosci. 34, 5385–5395 (2014).
Chen, N. F. et al. TGF-β1 attenuates spinal neuroinflammation and the excitatory amino acid system in rats with neuropathic pain. J. Pain 14, 1671–1685 (2013).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
van der Kraan, P. The changing role of TGFβ in healthy, ageing and osteoarthritic joints. Nat Rev Rheumatol 13, 155–163 (2017). https://doi.org/10.1038/nrrheum.2016.219
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2016.219
- Springer Nature Limited
This article is cited by
-
Specific isoforms of the ubiquitin ligase gene WWP2 are targets of osteoarthritis genetic risk via a differentially methylated DNA sequence
Arthritis Research & Therapy (2024)
-
Inhibition of SMAD3 effectively reduces ADAMTS-5 expression in the early stages of osteoarthritis
BMC Musculoskeletal Disorders (2023)
-
SET8 is a novel negative regulator of TGF-β signaling in a methylation-independent manner
Scientific Reports (2023)
-
Skin aging from the perspective of dermal fibroblasts: the interplay between the adaptation to the extracellular matrix microenvironment and cell autonomous processes
Journal of Cell Communication and Signaling (2023)
-
Focusing on the hypoxia-inducible factor pathway: role, regulation, and therapy for osteoarthritis
European Journal of Medical Research (2022)