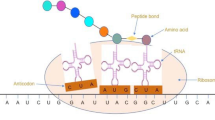
Key Points
-
The genetic code is not frozen.
-
Genetic code variations found in microorganisms include codon bias, codon reassignment, ambiguous decoding and natural genetic code expansion.
-
Codon bias, which is present in all sequenced genomes, modulates the rate of protein synthesis, which in turn regulates many biological processes.
-
Codon reassignment involves distinct mechanisms to completely redefine codon meaning.
-
Ambiguous decoding, which is detrimental at high levels, can be advantageous under stress conditions by increasing proteome diversity and activating stress responses.
-
Natural genetic code expansion with selenocysteine and pyrrolysine enables certain organisms to synthesize proteins with 21 or 22 amino acids, with consequences for enzymatic and metabolic efficiency that are just beginning to be understood.
-
The impact of genetic code evolution on microbial physiology is an emerging field, which is ripe for new discoveries.
Abstract
The genetic code, initially thought to be universal and immutable, is now known to contain many variations, including biased codon usage, codon reassignment, ambiguous decoding and recoding. As a result of recent advances in the areas of genome sequencing, biochemistry, bioinformatics and structural biology, our understanding of genetic code flexibility has advanced substantially in the past decade. In this Review, we highlight the prevalence, evolution and mechanistic basis of genetic code variations in microorganisms, and we discuss how this flexibility of the genetic code affects microbial physiology.





Similar content being viewed by others
References
Nirenberg, M. et al. RNA codewords and protein synthesis, VII. On the general nature of the RNA code. Proc. Natl Acad. Sci. USA 53, 1161–1168 (1965).
Söll, D. et al. Studies on polynucleotides, XLIX. Stimulation of the binding of aminoacyl-sRNA's to ribosomes by ribotrinucleotides and a survey of codon assignments for 20 amino acids. Proc. Natl Acad. Sci. USA 54, 1378–1385 (1965).
Li, M. & Tzagoloff, A. Assembly of the mitochondrial membrane system: sequences of yeast mitochondrial valine and an unusual threonine tRNA gene. Cell 18, 47–53 (1979).
Macino, G., Coruzzi, G., Nobrega, F. G., Li, M. & Tzagoloff, A. Use of the UGA terminator as a tryptophan codon in yeast mitochondria. Proc. Natl Acad. Sci. USA 76, 3784–3785 (1979). First discovery of codon reassignment in microorganisms.
Ambrogelly, A., Palioura, S. & Söll, D. Natural expansion of the genetic code. Nat. Chem. Biol. 3, 29–35 (2007).
Plotkin, J. B. & Kudla, G. Synonymous but not the same: the causes and consequences of codon bias. Nat. Rev. Genet. 12, 32–42 (2011).
Quax, T. E., Claassens, N. J., Söll, D. & van der Oost, J. Codon bias as a means to fine-tune gene expression. Mol. Cell 59, 149–161 (2015).
Pan, T. Adaptive translation as a mechanism of stress response and adaptation. Annu. Rev. Genet. 47, 121–137 (2013). Excellent review on benefits of ambiguous decoding under stress conditions.
Atkins, J. F. & Baranov, P. V. The distinction between recoding and codon reassignment. Genetics 185, 1535–1536 (2010).
Atkins, J. F., Gesteland, R. F., Reid, B. R. & Anderson, C. W. Normal tRNAs promote ribosomal frameshifting. Cell 18, 1119–1131 (1979). One of the first studies to demonstrate frameshifting and genetic code variability.
Grantham, R., Gautier, C., Gouy, M., Mercier, R. & Pave, A. Codon catalog usage and the genome hypothesis. Nucleic Acids Res. 84, r49–r62 (1980).
Shabalina, S. A., Spiridonov, N. A. & Kashina, A. Sounds of silence: synonymous nucleotides as a key to biological regulation and complexity. Nucleic Acids Res. 41, 2073–2094 (2013).
Bulmer, M. The selection-mutation-drift theory of synonymous codon usage. Genetics 129, 897–907 (1991).
Li, G. W., Oh, E. & Weissman, J. S. The anti-Shine-Dalgarno sequence drives translational pausing and codon choice in bacteria. Nature 484, 538–541 (2012).
Subramaniam, A. R., Pan, T. & Cluzel, P. Environmental perturbations lift the degeneracy of the genetic code to regulate protein levels in bacteria. Proc. Natl Acad. Sci. USA 110, 2419–2424 (2013).
Goodman, D. B., Church, G. M. & Kosuri, S. Causes and effects of N-terminal codon bias in bacterial genes. Science 342, 475–479 (2013).
Subramaniam, A. R. et al. A serine sensor for multicellularity in a bacterium. eLife 2, e01501 (2013).
Xu, Y. et al. Non-optimal codon usage is a mechanism to achieve circadian clock conditionality. Nature 495, 116–120 (2013). Suggested that non-optimal codons are used as a regulatory mechanism.
Zhou, M. et al. Non-optimal codon usage affects expression, structure and function of clock protein FRQ. Nature 495, 111–115 (2013). Suggested that non-optimal codons are used as a regulatory mechanism.
Lang, B. F., Lavrov, D., Beck, N. & Steinberg, S. V. in Organelle Genetics (ed. Bullerwell, C. E.) 431–474 (Springer, 2012).
Sengupta, S., Yang, X. & Higgs, P. G. The mechanisms of codon reassignments in mitochondrial genetic codes. J. Mol. Evol. 64, 662–688 (2007).
Alfonzo, J. D., Blanc, V., Estevez, A. M., Rubio, M. A. & Simpson, L. C to U editing of the anticodon of imported mitochondrial tRNATrp allows decoding of the UGA stop codon in Leishmania tarentolae. EMBO J. 18, 7056–7062 (1999). Demonstrated that RNA editing is responsible for UGA reassignment in Leishmania tarentolae mitochondria.
Muramatsu, T. et al. A novel lysine-substituted nucleoside in the first position of the anticodon of minor isoleucine tRNA from Escherichia coli. J. Biol. Chem. 263, 9261–9267 (1988).
Mandal, D. et al. Agmatidine, a modified cytidine in the anticodon of archaeal tRNAIle, base pairs with adenosine but not with guanosine. Proc. Natl Acad. Sci. USA 107, 2872–2877 (2010).
Ikeuchi, Y. et al. Agmatine-conjugated cytidine in a tRNA anticodon is essential for AUA decoding in archaea. Nat. Chem. Biol. 6, 277–282 (2010).
Tomita, K. et al. Codon reading patterns in Drosophila melanogaster mitochondria based on their tRNA sequences: a unique wobble rule in animal mitochondria. Nucleic Acids Res. 27, 4291–4297 (1999).
Cantara, W. A., Murphy, F. V., Demirci, H. & Agris, P. F. Expanded use of sense codons is regulated by modified cytidines in tRNA. Proc. Natl Acad. Sci. USA 110, 10964–10969 (2013).
Marniemi, J. & Parkki, M. G. Radiochemical assay of glutathione S-epoxide transferase and its enhancement by phenobarbital in rat liver in vivo. Biochem. Pharmacol. 24, 1569–1572 (1975).
Su, D. et al. An unusual tRNAThr derived from tRNAHis reassigns in yeast mitochondria the CUN codons to threonine. Nucleic Acids Res. 39, 4866–4874 (2011).
Ling, J., Daoud, R., Lajoie, M. J., Söll, D. & Lang, B. F. Natural reassignment of CUU and CUA sense codons to alanine in Ashbya mitochondria. Nucleic Acids Res. 42, 499–508 (2014).
Ling, J. et al. Yeast mitochondrial threonyl-tRNA synthetase recognizes tRNA isoacceptors by distinct mechanisms and promotes CUN codon reassignment. Proc. Natl Acad. Sci. USA 109, 3281–3286 (2012).
Ivanova, N. N. et al. Stop codon reassignments in the wild. Science 344, 909–913 (2014). Uncovered widespread stop codon reassignment events in microorganisms.
Mukai, T. et al. Codon reassignment in the Escherichia coli genetic code. Nucleic Acids Res. 38, 8188–8195 (2010).
Johnson, D. B. et al. Release factor one is nonessential in Escherichia coli. ACS Chem. Biol. 7, 1337–1344 (2012).
Heinemann, I. U. et al. Enhanced phosphoserine insertion during Escherichia coli protein synthesis via partial UAG codon reassignment and release factor 1 deletion. FEBS Lett. 586, 3716–3722 (2012).
Lajoie, M. J. et al. Genomically recoded organisms expand biological functions. Science 342, 357–360 (2013). Created the first synthetic microorganism with complete removal of UAG stop codons.
Mukai, T. et al. Highly reproductive Escherichia coli cells with no specific assignment to the UAG codon. Sci. Rep. 5, 9699 (2015).
Mukai, T. et al. Reassignment of a rare sense codon to a non-canonical amino acid in Escherichia coli. Nucleic Acids Res. 43, http://dx.doi.org/10.1093/nar/gkv787 (2015).
Aerni, H. R., Shifman, M. A., Rogulina, S., O'Donoghue, P. & Rinehart, J. Revealing the amino acid composition of proteins within an expanded genetic code. Nucleic Acids Res. 43, e8 (2015).
Hammerling, M. J. et al. Bacteriophages use an expanded genetic code on evolutionary paths to higher fitness. Nat. Chem. Biol. 10, 178–180 (2014).
Schultz, D. W. & Yarus, M. Transfer RNA mutation and the malleability of the genetic code. J. Mol. Biol. 235, 1377–1380 (1994).
Ling, J., Reynolds, N. & Ibba, M. Aminoacyl-tRNA synthesis and translational quality control. Annu. Rev. Microbiol. 63, 61–78 (2009).
Rodnina, M. V. & Wintermeyer, W. Ribosome fidelity: tRNA discrimination, proofreading and induced fit. Trends Biochem. Sci. 26, 124–130 (2001).
Zaher, H. S. & Green, R. Fidelity at the molecular level: lessons from protein synthesis. Cell 136, 746–762 (2009).
Netzer, N. et al. Innate immune and chemically triggered oxidative stress modifies translational fidelity. Nature 462, 522–526 (2009).
Mascarenhas, A. P., An, S., Rosen, A. E., Martinis, S. A. & Musier-Forsyth, K. in Protein Engineering (eds RajBhandary, U. L. & Köhrer, C.) 153–200 (Springer, 2008).
Roy, H., Ling, J., Alfonzo, J. & Ibba, M. Loss of editing activity during the evolution of mitochondrial phenylalanyl-tRNA synthetase. J. Biol. Chem. 280, 38186–38192 (2005).
Li, L. et al. Naturally occurring aminoacyl-tRNA synthetases editing-domain mutations that cause mistranslation in Mycoplasma parasites. Proc. Natl Acad. Sci. USA 108, 9378–9383 (2011). Suggested that Mycoplasma spp. may use ambiguous decoding to defend against the host immune response.
Yadavalli, S. S. & Ibba, M. Selection of tRNA charging quality control mechanisms that increase mistranslation of the genetic code. Nucleic Acids Res. 41, 1104–1112 (2013).
Bezerra, A. R. et al. Reversion of a fungal genetic code alteration links proteome instability with genomic and phenotypic diversification. Proc. Natl Acad. Sci. USA 110, 11079–11084 (2013).
Miranda, I. et al. Candida albicans CUG mistranslation is a mechanism to create cell surface variation. mBio 4, e00285-13 (2013).
Javid, B. et al. Mycobacterial mistranslation is necessary and sufficient for rifampicin phenotypic resistance. Proc. Natl Acad. Sci. USA 111, 1132–1137 (2014). Demonstrated that ambiguous decoding increases resistance to an antibiotic in mycobacteria.
Fan, Y. et al. Protein mistranslation protects bacteria against oxidative stress. Nucleic Acids Res. 43, 1740–1748 (2015).
Wu, J., Fan, Y. & Ling, J. Mechanism of oxidant-induced mistranslation by threonyl-tRNA synthetase. Nucleic Acids Res. 42, 6523–6531 (2014).
Crick, F. H. The Croonian lecture, 1966. The genetic code. Proc. R. Soc. Lond. B 167, 331–347 (1967).
Turanov, A. A. et al. Genetic code supports targeted insertion of two amino acids by one codon. Science 323, 259–261 (2009).
Arner, E. S. Selenoproteins — what unique properties can arise with selenocysteine in place of cysteine? Exp. Cell Res. 316, 1296–1303 (2010).
Kim, H. Y. & Gladyshev, V. N. Different catalytic mechanisms in mammalian selenocysteine- and cysteine-containing methionine-R-sulfoxide reductases. PLoS. Biol. 3, e375 (2005).
Snider, G. W., Ruggles, E., Khan, N. & Hondal, R. J. Selenocysteine confers resistance to inactivation by oxidation in thioredoxin reductase: comparison of selenium and sulfur enzymes. Biochemistry 52, 5472–5481 (2013).
Metanis, N. & Hilvert, D. Natural and synthetic selenoproteins. Curr. Opin. Chem. Biol. 22, 27–34 (2014).
Yoshizawa, S. & Böck, A. The many levels of control on bacterial selenoprotein synthesis. Biochim. Biophys. Acta 1790, 1404–1414 (2009). An excellent review of bacterial selenoproteins.
Bröcker, M. J., Ho, J. M., Church, G. M., Söll, D. & O'Donoghue, P. Recoding the genetic code with selenocysteine. Angew. Chem. Int. Ed. Engl. 53, 319–323 (2014).
Aldag, C. et al. Rewiring translation for elongation factor Tu-dependent selenocysteine incorporation. Angew. Chem. Int. Ed. Engl. 52, 1441–1445 (2013).
Su, D. et al. How an obscure archaeal gene inspired the discovery of selenocysteine biosynthesis in humans. IUBMB Life 61, 35–39 (2009).
Thyer, R., Robotham, S. A., Brodbelt, J. S. & Ellington, A. D. Evolving tRNASec for efficient canonical incorporation of selenocysteine. J. Am. Chem. Soc. 137, 46–49 (2015).
Miller, C. et al. A synthetic tRNA for EF-Tu mediated selenocysteine incorporation in vivo and in vitro. FEBS Lett. 589, 2194–2199 (2015).
Haruna, K., Alkazemi, M. H., Liu, Y., Söll, D. & Englert, M. Engineering the elongation factor Tu for efficient selenoprotein synthesis. Nucleic Acids Res. 42, 9976–9983 (2014).
Xu, J., Croitoru, V., Rutishauser, D., Cheng, Q. & Arner, E. S. Wobble decoding by the Escherichia coli selenocysteine insertion machinery. Nucleic Acids Res. 41, 9800–9811 (2013).
Zhang, Y., Romero, H., Salinas, G. & Gladyshev, V. N. Dynamic evolution of selenocysteine utilization in bacteria: a balance between selenoprotein loss and evolution of selenocysteine from redox active cysteine residues. Genome Biol. 7, R94 (2006). Although many new sequences are now available, this is still the definitive resource that documents the evolutionary replacement of Cys with Sec residues in bacterial proteins.
Zhang, Y. & Gladyshev, V. N. Trends in selenium utilization in marine microbial world revealed through the analysis of the global ocean sampling (GOS) project. PLoS Genet. 4, e1000095 (2008).
Stadtman, T. C. Selenocysteine. Annu. Rev. Biochem. 65, 83–100 (1996).
Jormakka, M., Byrne, B. & Iwata, S. Formate dehydrogenase — a versatile enzyme in changing environments. Curr. Opin. Struct. Biol. 13, 418–423 (2003).
Axley, M. J., Böck, A. & Stadtman, T. C. Catalytic properties of an Escherichia coli formate dehydrogenase mutant in which sulfur replaces selenium. Proc. Natl Acad. Sci. USA 88, 8450–8454 (1991).
Stock, T. & Rother, M. Selenoproteins in Archaea and Gram-positive bacteria. Biochim. Biophys. Acta 1790, 1520–1532 (2009).
Stadtman, T. C., Davis, J. N., Zehelein, E. & Böck, A. Biochemical and genetic analysis of Salmonella typhimurium and Escherichia coli mutants defective in specific incorporation of selenium into formate dehydrogenase and tRNAs. Biofactors 2, 35–44 (1989).
Tetteh, A. Y. et al. Transcriptional response of selenopolypeptide genes and selenocysteine biosynthesis machinery genes in Escherichia coli during selenite reduction. Int. J. Microbiol. 2014, 394835 (2014).
Lu, J. & Holmgren, A. The thioredoxin antioxidant system. Free Radic. Biol. Med. 66, 75–87 (2014).
Rother, M. & Krzycki, J. A. Selenocysteine, pyrrolysine, and the unique energy metabolism of methanogenic archaea. Archaea 2010, 453642 (2010).
Stock, T., Selzer, M. & Rother, M. In vivo requirement of selenophosphate for selenoprotein synthesis in archaea. Mol. Microbiol. 75, 149–160 (2010).
Kryukov, G. V. & Gladyshev, V. N. The prokaryotic selenoproteome. EMBO Rep. 5, 538–543 (2004).
Cobucci-Ponzano, B., Rossi, M. & Moracci, M. Translational recoding in archaea. Extremophiles 16, 793–803 (2012).
Rother, M., Mathes, I., Lottspeich, F. & Böck, A. Inactivation of the selB gene in Methanococcus maripaludis: effect on synthesis of selenoproteins and their sulfur-containing homologs. J. Bacteriol. 185, 107–114 (2003). A seminal study on the phenotypic impact of removing Sec from the genetic code of the model archaeaon M. maripaludis.
Hohn, M. J., Palioura, S., Su, D., Yuan, J. & Söll, D. Genetic analysis of selenocysteine biosynthesis in the archaeon Methanococcus maripaludis. Mol. Microbiol. 81, 249–258 (2011).
Hao, B. et al. A new UAG-encoded residue in the structure of a methanogen methyltransferase. Science 296, 1462–1466 (2002). Discovery of the twenty-second genetically encoded amino acid, Pyl.
Srinivasan, G., James, C. M. & Krzycki, J. A. Pyrrolysine encoded by UAG in Archaea: charging of a UAG-decoding specialized tRNA. Science 296, 1459–1462 (2002).
Borrel, G. et al. Genome sequence of 'Candidatus Methanomethylophilus alvus' Mx1201, a methanogenic archaeon from the human gut belonging to a seventh order of methanogens. J. Bacteriol. 194, 6944–6945 (2012).
Borrel, G. et al. Comparative genomics highlights the unique biology of Methanomassiliicoccales, a Thermoplasmatales-related seventh order of methanogenic archaea that encodes pyrrolysine. BMC Genomics 15, 679 (2014).
Gaston, M. A., Zhang, L., Green-Church, K. B. & Krzycki, J. A. The complete biosynthesis of the genetically encoded amino acid pyrrolysine from lysine. Nature 471, 647–650 (2011). Elucidated activities of the biosynthetic route to Pyl.
Krzycki, J. A. The path of lysine to pyrrolysine. Curr. Opin. Chem. Biol. 17, 619–625 (2013).
Blight, S. K. et al. Direct charging of tRNACUA with pyrrolysine in vitro and in vivo. Nature 431, 333–335 (2004). Elucidated the mechanism of Pyl decoding.
Polycarpo, C. et al. An aminoacyl-tRNA synthetase that specifically activates pyrrolysine. Proc. Natl Acad. Sci. USA 101, 12450–12454 (2004). Elucidated the mechanism of Pyl decoding.
Ticak, T., Kountz, D. J., Girosky, K. E., Krzycki, J. A. & Ferguson, D. J. Jr. A nonpyrrolysine member of the widely distributed trimethylamine methyltransferase family is a glycine betaine methyltransferase. Proc. Natl Acad. Sci. USA 111, e4668–e4676 (2014).
Heinemann, I. U. et al. The appearance of pyrrolysine in tRNAHis guanylyltransferase by neutral evolution. Proc. Natl Acad. Sci. USA 106, 21103–21108 (2009).
O'Donoghue, P. et al. Reducing the genetic code induces massive rearrangement of the proteome. Proc. Natl Acad. Sci. USA 111, 17206–17211 (2014). Provided proteome-level view of the phenotypic impact of removing Pyl from the genetic code of M. acetivorans.
Quitterer, F., List, A., Eisenreich, W., Bacher, A. & Groll, M. Crystal structure of methylornithine synthase (PylB): insights into the pyrrolysine biosynthesis. Angew. Chem. Int. Ed. Engl. 51, 1339–1342 (2012).
Alkalaeva, E. et al. Translation termination in pyrrolysine-utilizing archaea. FEBS Lett. 583, 3455–3460 (2009).
Longstaff, D. G., Blight, S. K., Zhang, L., Green-Church, K. B. & Krzycki, J. A. In vivo contextual requirements for UAG translation as pyrrolysine. Mol. Microbiol. 63, 229–241 (2007).
Freistroffer, D. V., Kwiatkowski, M., Buckingham, R. H. & Ehrenberg, M. The accuracy of codon recognition by polypeptide release factors. Proc. Natl Acad. Sci. USA 97, 2046–2051 (2000).
Mansell, J. B., Guevremont, D., Poole, E. S. & Tate, W. P. A dynamic competition between release factor 2 and the tRNASec decoding UGA at the recoding site of Escherichia coli formate dehydrogenase H. EMBO J. 20, 7284–7293 (2001).
Mahapatra, A. et al. Characterization of a Methanosarcina acetivorans mutant unable to translate UAG as pyrrolysine. Mol. Microbiol. 59, 56–66 (2006).
Krzycki, J. A. Function of genetically encoded pyrrolysine in corrinoid-dependent methylamine methyltransferases. Curr. Opin. Chem. Biol. 8, 484–491 (2004).
Oelgeschlager, E. & Rother, M. In vivo role of three fused corrinoid/methyl transfer proteins in Methanosarcina acetivorans. Mol. Microbiol. 72, 1260–1272 (2009).
Polycarpo, C. R. et al. Pyrrolysine analogues as substrates for pyrrolysyl-tRNA synthetase. FEBS Lett. 580, 6695–6700 (2006).
Prat, L. et al. Carbon source-dependent expansion of the genetic code in bacteria. Proc. Natl Acad. Sci. USA 109, 21070–21075 (2012). Demonstrated natural Pyl decoding in bacteria and revealed first example of dynamic genetic code expansion.
Jiang, R. & Krzycki, J. A. PylSn and the homologous N-terminal domain of pyrrolysyl-tRNA synthetase bind the tRNA that is essential for the genetic encoding of pyrrolysine. J. Biol. Chem. 287, 32738–32746 (2012).
Katayama, H., Nozawa, K., Nureki, O., Nakahara, Y. & Hojo, H. Pyrrolysine analogs as substrates for bacterial pyrrolysyl-tRNA synthetase in vitro and in vivo. Biosci. Biotechnol. Biochem. 76, 205–208 (2012).
Nozawa, K. et al. Pyrrolysyl-tRNA synthetase-tRNAPyl structure reveals the molecular basis of orthogonality. Nature 457, 1163–1167 (2009).
O'Donoghue, P., Ling, J., Wang, Y. S. & Söll, D. Upgrading protein synthesis for synthetic biology. Nat. Chem. Biol. 9, 594–598 (2013).
Liu, C. C. & Schultz, P. G. Adding new chemistries to the genetic code. Annu. Rev. Biochem. 79, 413–444 (2010).
Chin, J. W. Reprogramming the genetic code. EMBO J. 30, 2312–2324 (2011).
Johnson, J. A., Lu, Y. Y., Van Deventer, J. A. & Tirrell, D. A. Residue-specific incorporation of non-canonical amino acids into proteins: recent developments and applications. Curr. Opin. Chem. Biol. 14, 774–780 (2010).
Chin, J. W. Expanding and reprogramming the genetic code of cells and animals. Annu. Rev. Biochem. 83, 379–408 (2014). An excellent review on engineering protein synthesis for genetic code expansion in diverse expression systems.
Rovner, A. J. et al. Recoded organisms engineered to depend on synthetic amino acids. Nature 518, 89–93 (2015).
Mandell, D. J. et al. Biocontainment of genetically modified organisms by synthetic protein design. Nature 518, 55–60 (2015).
Campbell, J. H. et al. UGA is an additional glycine codon in uncultured SR1 bacteria from the human microbiota. Proc. Natl Acad. Sci. USA 110, 5540–5545 (2013).
Wang, H. H. et al. Programming cells by multiplex genome engineering and accelerated evolution. Nature 460, 894–898 (2009).
Isaacs, F. J. et al. Precise manipulation of chromosomes in vivo enables genome-wide codon replacement. Science 333, 348–353 (2011). First example of a genome engineered with 62 codons by mutation of all TAGs to TAA.
Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013).
Wiedenheft, B., Sternberg, S. H. & Doudna, J. A. RNA-guided genetic silencing systems in bacteria and archaea. Nature 482, 331–338 (2012).
Gibson, D. G. et al. Creation of a bacterial cell controlled by a chemically synthesized genome. Science 329, 52–56 (2010). First demonstration of genome transplantation with a synthetic genome.
Annaluru, N. et al. Total synthesis of a functional designer eukaryotic chromosome. Science 344, 55–58 (2014).
Budiman, M. E. et al. Eukaryotic initiation factor 4a3 is a selenium-regulated RNA-binding protein that selectively inhibits selenocysteine incorporation. Mol. Cell 35, 479–489 (2009).
Hohn, M. J., Park, H. S., O'Donoghue, P., Schnitzbauer, M. & Söll, D. Emergence of the universal genetic code imprinted in an RNA record. Proc. Natl Acad. Sci. USA 103, 18095–18100 (2006).
Bilokapic, S. et al. Structure of the unusual seryl-tRNA synthetase reveals a distinct zinc-dependent mode of substrate recognition. EMBO J. 25, 2498–2509 (2006).
Itoh, Y. et al. Decameric SelA•tRNASec ring structure reveals mechanism of bacterial selenocysteine formation. Science 340, 75–78 (2013).
Sherrer, R. L., O'Donoghue, P. & Söll, D. Characterization and evolutionary history of an archaeal kinase involved in selenocysteinyl-tRNA formation. Nucleic Acids Res. 36, 1247–1259 (2008).
Carlson, B. A. et al. Identification and characterization of phosphoseryl-tRNA[Ser]Sec kinase. Proc. Natl Acad. Sci. USA 101, 12848–12853 (2004).
Palioura, S., Sherrer, R. L., Steitz, T. A., Söll, D. & Simonovic, M. The human SepSecS–tRNASec complex reveals the mechanism of selenocysteine formation. Science 325, 321–325 (2009).
Copeland, P. R., Fletcher, J. E., Carlson, B. A., Hatfield, D. L. & Driscoll, D. M. A novel RNA binding protein, SBP2, is required for the translation of mammalian selenoprotein mRNAs. EMBO J. 19, 306–314 (2000).
Bifano, A. L., Atassi, T., Ferrara, T. & Driscoll, D. M. Identification of nucleotides and amino acids that mediate the interaction between ribosomal protein L30 and the SECIS element. BMC Mol. Biol. 14, 12 (2013).
Allmang, C., Wurth, L. & Krol, A. The selenium to selenoprotein pathway in eukaryotes: more molecular partners than anticipated. Biochim. Biophys. Acta 1790, 1415–1423 (2009).
Yoshizawa, S. et al. Structural basis for mRNA recognition by elongation factor SelB. Nat. Struct. Mol. Biol. 12, 198–203 (2005).
Yuan, J. et al. Distinct genetic code expansion strategies for selenocysteine and pyrrolysine are reflected in different aminoacyl-tRNA formation systems. FEBS Lett. 584, 342–349 (2010).
Eggertsson, G. & Söll, D. Transfer ribonucleic acid-mediated suppression of termination codons in Escherichia coli. Microbiol. Rev. 52, 354–374 (1988).
Ambrogelly, A. et al. Pyrrolysine is not hardwired for cotranslational insertion at UAG codons. Proc. Natl Acad. Sci. USA 104, 3141–3146 (2007).
Kavran, J. M. et al. Structure of pyrrolysyl-tRNA synthetase, an archaeal enzyme for genetic code innovation. Proc. Natl Acad. Sci. USA 104, 11268–11273 (2007).
Wan, W. et al. A facile system for genetic incorporation of two different noncanonical amino acids into one protein in Escherichia coli. Angew. Chem. Int. Ed. Engl. 49, 3211–3214 (2010).
Neumann, H., Peak-Chew, S. Y. & Chin, J. W. Genetically encoding Nɛ-acetyllysine in recombinant proteins. Nat. Chem. Biol. 4, 232–234 (2008).
Umehara, T. et al. N-acetyl lysyl-tRNA synthetases evolved by a CcdB-based selection possess N-acetyl lysine specificity in vitro and in vivo. FEBS Lett. 586, 729–733 (2012).
Yanagisawa, T., Umehara, T., Sakamoto, K. & Yokoyama, S. Expanded genetic code technologies for incorporating modified lysine at multiple sites. Chembiochem 15, 2181–2187 (2014).
Guo, L. T. et al. Polyspecific pyrrolysyl-tRNA synthetases from directed evolution. Proc. Natl Acad. Sci. USA 111, 16724–16729 (2014).
Lobanov, A. V. et al. Evolutionary dynamics of eukaryotic selenoproteomes: large selenoproteomes may associate with aquatic life and small with terrestrial life. Genome Biol. 8, r198 (2007).
Grobe, T., Reuter, M., Gursinsky, T., Sohling, B. & Andreesen, J. R. Peroxidase activity of selenoprotein GrdB of glycine reductase and stabilisation of its integrity by components of proprotein GrdE from Eubacterium acidaminophilum. Arch. Microbiol. 187, 29–43 (2007).
Hurley, J. M. & Dunlap, J. C. Cell biology: a fable of too much too fast. Nature 495, 57–58 (2013).
Acknowledgements
Work in the authors' laboratories was supported by grants from the US National Institute of General Medical Sciences (GM022854 to D.S.; and GM115431 to J.L.), from the Natural Sciences and Engineering Research Council of Canada (RGPIN 04282–2014 to P.O.), from the Canadian Institutes of Health Research Tier 2 Canada Research Chair (950-229917 to P.O.), and from The University of Texas Health Science Center at Houston start-up fund (to J.L.). The authors are grateful to I. Heinemann for discussions and a critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information S1 (table)
Genetic code flexibility in microorganisms (PDF 215 kb)
Glossary
- Aminoacyl-tRNA
-
(aa-tRNA). A tRNA molecule with an amino acid attached to the 3′ end. It is used as a substrate by the ribosome to synthesize proteins.
- Codon–anticodon pairing
-
During translation, the bases of the mRNA codon and the tRNA anticodon need to match each other. Watson–Crick pairing (A–U and G–C) in the first and second positions of the codon is required for efficient decoding, whereas the third position allows more flexible pairing, for example, between G and U or using modified bases.
- Synonymous codons
-
Different triplet nucleotide sequences that decode the same amino acid.
- tRNA isoacceptors
-
Different tRNA species recognized by the same aminoacyl-tRNA synthetase and ligated with the same amino acid.
- Misacylation
-
Incorrect pairing of an amino acid and tRNA by an aminoacyl-tRNA synthetase. Errors resulting from misacylation, if left uncorrected, reduce the overall translational fidelity.
- Frameshifting
-
Change in the reading frame during translation due to mutations in the DNA, errors during transcription or translation or specific mRNA structures, leading to new protein sequences.
- Shine–Dalgarno-like sequences
-
mRNA sequences that share high similarity with the Shine–Dalgarno sequence, which pairs with the anti-Shine–Dalgarno sequence of the ribosomal RNA.
- Codon adaptation index
-
A method for analysing usage bias of synonymous codons using a set of highly expressed genes from a species as a reference to assign a score to each gene.
- Wobble position
-
The third position of a codon, which is more flexibly recognized by the tRNA compared with other positions.
- Phages
-
(Also called bacteriophages). Viruses that infect and propagate within bacteria. Phages contain their own genome but hijack the translational machinery of the bacterial host for protein synthesis.
- RpoB
-
The β-subunit of the bacterial RNA polymerase and target of the antibiotic rifampicin.
- Nucleophilicity
-
The property to donate an electron in chemical reactions.
- Elongation factor Tu
-
(EF-Tu). A bacterial elongation factor that delivers aminoacyl-tRNAs to the ribosome during peptide synthesis. The counterpart of EF-Tu in archaea and eukaryotes is EF-1A.
Rights and permissions
About this article
Cite this article
Ling, J., O'Donoghue, P. & Söll, D. Genetic code flexibility in microorganisms: novel mechanisms and impact on physiology. Nat Rev Microbiol 13, 707–721 (2015). https://doi.org/10.1038/nrmicro3568
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro3568
- Springer Nature Limited
This article is cited by
-
Escherichia coli methionine-tRNAi/methionyl tRNA synthetase pairs induced protein initiation of interest (PII) expression
Applied Biological Chemistry (2022)
-
Re-reading the genetic code: The evolutionary potential of frameshifting in time
Journal of Biosciences (2022)
-
The Boggarts of biology: how non-genetic changes influence the genotype
Current Genetics (2021)
-
Unorthodox features in two venerid bivalves with doubly uniparental inheritance of mitochondria
Scientific Reports (2020)
-
Tryptophan usage by Helicobacter pylori differs among strains
Scientific Reports (2019)