Key Points
-
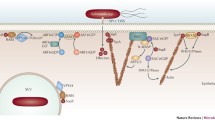
Several distinct pattern recognition receptors cooperate during Salmonella-induced bacteraemia to coordinate responses against lipopolysaccharide through canonical and non-canonical pathways.
-
Different non-canonical pathways are important for Salmonella-induced gastroenteritis, and this differential activation of innate immune responses results from the differential expression of pattern recognition receptors in epithelial cells and mucosal phagocytes.
-
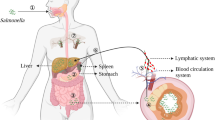
The rapid repression of virulence genes during invasion of the intestinal mucosa enables the causative agent of typhoid fever to evade recognition by pattern recognition receptors.
-
The virulence-associated capsular polysaccharide inhibits activation of complement, a pattern recognition receptor that cooperates with Toll-like receptors and nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) to orchestrate antibacterial responses.
Abstract
Salmonella enterica serovars are associated with an estimated 1 million deaths annually and are also useful model organisms for investigating the mechanisms of host–bacterium interactions. The insights gained from studies on non-typhoidal Salmonella (NTS) serovars have provided a fascinating overview of the mechanisms by which the innate immune system detects and responds to bacterial pathogens. However, specific virulence factors and changes in virulence gene regulation in S. enterica subsp. enterica serovar Typhi alter the innate immune responses to this pathogen. In this Review, we compare and contrast the interactions of S. Typhi and NTS serovars with host innate immune receptors and discuss why the disease manifestations associated with S. Typhi infection differ considerably from those associated with the closely related NTS serovars.



Similar content being viewed by others
References
Tsolis, R. M., Xavier, M. N., Santos, R. L. & Bäumler, A. J. How to become a top model: impact of animal experimentation on human Salmonella disease research. Infect. Immun. 79, 1806–1814 (2011).
Khan, S. A. et al. A lethal role for lipid A in Salmonella infections. Mol. Microbiol. 29, 571–579 (1998).
Zhang, S. et al. Molecular pathogenesis of Salmonella enterica serotype Typhimurium-induced diarrhea. Infect. Immun. 71, 1–12 (2003).
Rabsch, W., Tschape, H. & Bäumler, A. J. Non-typhoidal salmonellosis: emerging problems. Microbes Infect. 3, 237–247 (2001).
Reddy, E. A., Shaw, A. V. & Crump, J. A. Community-acquired bloodstream infections in Africa: a systematic review and meta-analysis. Lancet Infect. Dis. 10, 417–432 (2010).
Feasey, N. A., Dougan, G., Kingsley, R. A., Heyderman, R. S. & Gordon, M. A. Invasive non-typhoidal salmonella disease: an emerging and neglected tropical disease in Africa. Lancet 379, 2489–2499 (2012).
Gordon, M. A. et al. Non-typhoidal salmonella bacteraemia among HIV-infected Malawian adults: high mortality and frequent recrudescence. AIDS 16, 1633–1641 (2002).
De Wit, S., Taelman, H., Van de Perre, P., Rouvroy, D. & Clumeck, N. Salmonella bacteremia in African patients with human immunodeficiency virus infection. Eur. J. Clin. Microbiol. Infect. Dis. 7, 45–47 (1988).
Brown, D. E., McCoy, M. W., Pilonieta, M. C., Nix, R. N. & Detweiler, C. S. Chronic murine typhoid fever is a natural model of secondary hemophagocytic lymphohistiocytosis. PloS ONE 5, e9441 (2010).
O'Brien, A. D., Rosenstreich, D. L. & Taylor, B. A. Control of natural resistance to Salmonella typhimurium and Leishmania donovani in mice by closely linked but distinct genetic loci. Nature 287, 440–442 (1980).
Poltorak, A. et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282, 2085–2088 (1998).
Arpaia, N. et al. TLR signaling is required for Salmonella typhimurium virulence. Cell 144, 675–688 (2011). This article provides rare insights into how the opposing forces of virulence gene expression and host antimicrobial responses shape bacterial survival in tissue.
Lai, M. A. et al. Innate immune detection of flagellin positively and negatively regulates salmonella infection. PLoS ONE 8, e72047 (2013).
Cummings, L. A., Wilkerson, W. D., Bergsbaken, T. & Cookson, B. T. In vivo, fliC expression by Salmonella enterica serovar Typhimurium is heterogeneous, regulated by ClpX, and anatomically restricted. Mol. Microbiol. 61, 795–809 (2006).
Bernheiden, M. et al. LBP, CD14, TLR4 and the murine innate immune response to a peritoneal Salmonella infection. J. Endotoxin Res. 7, 447–450 (2001).
Vazquez-Torres, A. et al. Toll-like receptor 4 dependence of innate and adaptive immunity to Salmonella: importance of the Kupffer cell network. J. Immunol. 172, 6202–6208 (2004).
Weiss, D. S., Raupach, B., Takeda, K., Akira, S. & Zychlinsky, A. Toll-like receptors are temporally involved in host defense. J. Immunol. 172, 4463–4469 (2004).
Talbot, S. et al. Toll-like receptor 4 signalling through MyD88 is essential to control Salmonella enterica serovar Typhimurium infection, but not for the initiation of bacterial clearance. Immunology 128, 472–483 (2009).
Joiner, K. A., Hammer, C. H., Brown, E. J. & Frank, M. M. Studies on the mechanism of bacterial resistance to complement-mediated killing. II. C8 and C9 release C5b67 from the surface of Salmonella minnesota S218 because the terminal complex does not insert into the bacterial outer membrane. J. Exp. Med. 155, 809–819 (1982).
Murray, G. L., Attridge, S. R. & Morona, R. Regulation of Salmonella typhimurium lipopolysaccharide O antigen chain length is required for virulence; identification of FepE as a second Wzz. Mol. Microbiol. 47, 1395–1406 (2003).
Holzer, S. U., Schlumberger, M. C., Jackel, D. & Hensel, M. Effect of the O-antigen length of lipopolysaccharide on the functions of type III secretion systems in Salmonella enterica. Infect. Immun. 77, 5458–5470 (2009).
Wangdi, T. et al. The Vi capsular polysaccharide enables Salmonella enterica serovar Typhi to evade microbe-guided neutrophil chemotaxis. PLoS Pathog. 10, e1004306 (2014).
Smedegard, G., Cui, L. X. & Hugli, T. E. Endotoxin-induced shock in the rat. A role for C5a. Am. J. Pathol. 135, 489–497 (1989).
Riedemann, N. C. et al. Expression and function of the C5a receptor in rat alveolar epithelial cells. J. Immunol. 168, 1919–1925 (2002).
Montz, H., Koch, K. C., Zierz, R. & Gotze, O. The role of C5a in interleukin-6 production induced by lipopolysaccharide or interleukin-1. Immunology 74, 373–379 (1991).
Vidal, S. M., Malo, D., Vogan, K., Skamene, E. & Gros, P. Natural resistance to infection with intracellular parasites: isolation of a candidate for Bcg. Cell 73, 469–485 (1993).
Seok, J. et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl Acad. Sci. USA 110, 3507–3512 (2013).
Broz, P. et al. Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella. J. Exp. Med. 207, 1745–1755 (2010). This report establishes redundant roles for NLRP3 and NLRC4 during host defence against NTS bacteraemia.
Man, S. M. et al. Inflammasome activation causes dual recruitment of NLRC4 and NLRP3 to the same macromolecular complex. Proc. Natl Acad. Sci. USA 111, 7403–7408 (2014).
Franchi, L. et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1β in salmonella-infected macrophages. Nature Immunol. 7, 576–582 (2006).
Miao, E. A. et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1β via Ipaf. Nature Immunol. 7, 569–575 (2006).
Sun, Y. H., Rolan, H. G. & Tsolis, R. M. Injection of flagellin into the host cell cytosol by Salmonella enterica serotype Typhimurium. J. Biol. Chem. 282, 33897–33901 (2007).
Miao, E. A. et al. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proc. Natl Acad. Sci. USA 107, 3076–3080 (2010).
Yang, J., Zhao, Y., Shi, J. & Shao, F. Human NAIP and mouse NAIP1 recognize bacterial type III secretion needle protein for inflammasome activation. Proc. Natl Acad. Sci. USA 110, 14408–14413 (2013).
Rayamajhi, M., Zak, D. E., Chavarria-Smith, J., Vance, R. E. & Miao, E. A. Cutting edge: mouse NAIP1 detects the type III secretion system needle protein. J. Immunol. 191, 3986–3989 (2013).
Kofoed, E. M. & Vance, R. E. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature 477, 592–595 (2011).
Zhao, Y. et al. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 477, 596–600 (2011).
Sander, L. E. et al. Detection of prokaryotic mRNA signifies microbial viability and promotes immunity. Nature 474, 385–389 (2011). This study introduces the concept of 'vita-PAMPs': signals that enable the host to distinguish live from dead bacteria.
Rathinam, V. A. et al. TRIF licenses caspase-11- dependent NLRP3 inflammasome activation by Gram-negative bacteria. Cell 150, 606–619 (2012). The authors demonstrate a connection between TLR4 signalling and the inflammasome by showing that the adaptor protein TRIF induces type I IFN signalling, which is required for caspase 11 activation.
Meunier, E. et al. Caspase-11 activation requires lysis of pathogen-containing vacuoles by IFN-induced GTPases. Nature 509, 366–370 (2014). This report provides an important link between IFN signalling and caspase 11 activation by showing that type I IFNs induce production of GBPs, which function in lysis of the SCV.
Pilla, D. M. et al. Guanylate binding proteins promote caspase-11-dependent pyroptosis in response to cytoplasmic LPS. Proc. Natl Acad. Sci. USA 111, 6046–6051 (2014).
Hagar, J. A., Powell, D. A., Aachoui, Y., Ernst, R. K. & Miao, E. A. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science 341, 1250–1253 (2013). The first of two reports establishing that caspase 11 is a cytosolic LPS sensor that detects the presence of bacteria in the cytosol.
Kayagaki, N. et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341, 1246–1249 (2013). The second of two reports establishing that caspase 11 is a cytosolic LPS sensor that detects the presence of bacteria in the cytosol.
Broz, P. et al. Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature 490, 288–291 (2012).
Shi, J. et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514, 187–192 (2014).
Miao, E. A. et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nature Immunol. 11, 1136–1142 (2010). This article introduces the concept that pyroptosis is a host defence mechanism able to expose intracellular bacteria to neutrophil attack.
Tsolis, R. M. et al. Of mice, calves, and men. Comparison of the mouse typhoid model with other Salmonella infections. Adv. Exp. Med. Biol. 473, 261–274 (1999).
Barthel, M. et al. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect. Immun. 71, 2839–2858 (2003).
Hapfelmeier, S. et al. Microbe sampling by mucosal dendritic cells is a discrete, MyD88-independent step in DeltainvG S. Typhimurium colitis. J. Exp. Med. 205, 437–450 (2008).
Franchi, L. et al. NLRC4-driven production of IL-1β discriminates between pathogenic and commensal bacteria and promotes host intestinal defense. Nature Immunol. 13, 449–456 (2012).
Smith, P. D. et al. Intestinal macrophages lack CD14 and CD89 and consequently are down-regulated for LPS- and IgA-mediated activities. J. Immunol. 167, 2651–2656 (2001).
Cookson, B. T. & Bevan, M. J. Identification of a natural T cell epitope presented by Salmonella-infected macrophages and recognized by T cells from orally immunized mice. J. Immunol. 158, 4310–4319 (1997).
McSorley, S. J., Cookson, B. T. & Jenkins, M. K. Characterization of CD4+ T cell responses during natural infection with Salmonella typhimurium. J. Immunol. 164, 986–993 (2000).
Atif, S. M., Uematsu, S., Akira, S. & McSorley, S. J. CD103–CD11b+ dendritic cells regulate the sensitivity of CD4 T-cell responses to bacterial flagellin. Mucosal Immunol. 7, 68–77 (2014).
Tukel, C. et al. CsgA is a pathogen-associated molecular pattern of Salmonella enterica serotype Typhimurium that is recognized by Toll-like receptor 2. Mol. Microbiol. 58, 289–304 (2005).
Nishimori, J. H. et al. Microbial amyloids induce interleukin 17A (IL-17A) and IL-22 responses via Toll-like receptor 2 activation in the intestinal mucosa. Infect. Immun. 80, 4398–4408 (2012).
Tukel, C. et al. Responses to amyloids of microbial and host origin are mediated through Toll-like receptor 2. Cell Host Microbe 6, 45–53 (2009). This report introduces the concept that amyloid fibrils are a PAMP that is detected by TLR2.
Romling, U. et al. Occurrence and regulation of the multicellular morphotype in Salmonella serovars important in human disease. Int. J. Med. Microbiol. 293, 273–285 (2003).
Tukel, C. et al. Toll-like receptors 1 and 2 cooperatively mediate immune responses to curli, a common amyloid from enterobacterial biofilms. Cell. Microbiol. 12, 1495–1505 (2010).
Hapfelmeier, S. et al. The Salmonella pathogenicity island (SPI)-2 and SPI-1 type III secretion systems allow Salmonella serovar typhimurium to trigger colitis via MyD88-dependent and MyD88-independent mechanisms. J. Immunol. 174, 1675–1685 (2005).
Keestra, A. M. et al. Early MyD88-dependent induction of interleukin-17A expression during Salmonella colitis. Infect. Immun. 79, 3131–3140 (2011).
Muzio, M., Ni, J., Feng, P. & Dixit, V. M. IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling. Science 278, 1612–1615 (1997).
Wesche, H., Henzel, W. J., Shillinglaw, W., Li, S. & Cao, Z. MyD88: an adapter that recruits IRAK to the IL-1 receptor complex. Immunity 7, 837–847 (1997).
Awoniyi, M., Miller, S. I., Wilson, C. B., Hajjar, A. M. & Smith, K. D. Homeostatic regulation of Salmonella-induced mucosal inflammation and injury by IL-23. PloS ONE 7, e37311 (2012).
Rhee, S. J., Walker, W. A. & Cherayil, B. J. Developmentally regulated intestinal expression of IFN-γ and its target genes and the age-specific response to enteric Salmonella infection. J. Immunol. 175, 1127–1136 (2005).
Müller, A. J. et al. The S. typhimurium effector SopE induces caspase-1 activation in stromal cells to initiate gut inflammation. Cell Host Microbe 6, 125–136 (2009).
Knodler, L. A. et al. Dissemination of invasive Salmonella via bacterial-induced extrusion of mucosal epithelia. Proc. Natl Acad. Sci. USA 107, 17733–17738 (2010). This is the first report on a new pathway of inflammasome activation in intestinal epithelial cells, which requires lysis of the SCV.
Knodler, L. A. et al. Noncanonical inflammasome activation of caspase-4/caspase-11 mediates epithelial defenses against enteric bacterial pathogens. Cell Host Microbe 16, 249–256 (2014).
Sellin, M. E. et al. Epithelium-intrinsic NAIP/NLRC4 inflammasome drives infected enterocyte expulsion to restrict Salmonella replication in the intestinal mucosa. Cell Host Microbe 16, 237–248 (2014).
Laughlin, R. C. et al. Spatial segregation of virulence gene expression during acute enteric infection with Salmonella enterica serovar Typhimurium. mBio 5, e00946-13 (2014).
Zhang, K. et al. Age-dependent enterocyte invasion and microcolony formation by Salmonella. PLoS Pathog. 10, e1004385 (2014).
Winter, S. E. et al. Contribution of flagellin pattern recognition to intestinal inflammation during Salmonella enterica serotype Typhimurium infection. Infect. Immun. 77, 1904–1916 (2009).
Zhang, S. et al. SipA, SopA, SopB, SopD and SopE2 act in concert to induce diarrhea in calves infected with Salmonella enterica serotype Typhimurium. Infect. Immun. 70, 3843–3855 (2002).
Zhang, S. et al. Phage-mediated horizontal transfer of the sopE1 gene increases enteropathogenicity of Salmonella enterica serotype Typhimurium for calves. FEMS Microbiol. Lett. 217, 243–247 (2002).
Hapfelmeier, S. et al. Role of the Salmonella pathogenicity island 1 effector proteins SipA, SopB, SopE, and SopE2 in Salmonella enterica subspecies 1 serovar Typhimurium colitis in streptomycin-pretreated mice. Infect. Immun. 72, 795–809 (2004).
Hardt, W. D., Chen, L. M., Schuebel, K. E., Bustelo, X. R. & Galan, J. E. S. typhimurium encodes an activator of Rho GTPases that induces membrane ruffling and nuclear responses in host cells. Cell 93, 815–826 (1998).
Figueiredo, J. F. et al. Salmonella enterica Typhimurium SipA induces CXC-chemokine expression through p38MAPK and JUN pathways. Microbes Infect. 11, 302–310 (2009).
Keestra, A. M. et al. A Salmonella virulence factor activates the NOD1/NOD2 signaling pathway. mBio 2, e00266-11 (2011).
Keestra, A. M. & Bäumler, A. J. Detection of enteric pathogens by the nodosome. Trends Immunol. 35, 123–130 (2014).
Keestra, A. M. et al. Manipulation of small Rho GTPases is a pathogen-induced process detected by NOD1. Nature 496, 233–237 (2013). This study demonstrates that the S. Typhimurium effector protein SopE activates NF-κB through the NOD1–RIP2 signalling pathway, thereby inducing intestinal inflammation.
Yoo, N. J. et al. Nod1, a CARD protein, enhances pro-interleukin-1β processing through the interaction with pro-caspase-1. Biochem. Biophys. Res. Commun. 299, 652–658 (2002).
Nasrallah, S. M. & Nassar, V. H. Enteric fever: a clinicopathologic study of 104 cases. Am. J. Gastroenterol. 69, 63–69 (1978).
Raffatellu, M., Wilson, R. P., Winter, S. E. & Bäumler, A. J. Clinical pathogenesis of typhoid fever. J. Infect. Dev. Ctries 2, 260–266 (2008).
Raffatellu, M. et al. Capsule-mediated immune evasion: a new hypothesis explaining aspects of typhoid fever pathogenesis. Infect. Immun. 74, 19–27 (2006).
Tsolis, R. M., Young, G. M., Solnick, J. V. & Bäumler, A. J. From bench to bedside: stealth of enteroinvasive pathogens. Nature Rev. Microbiol. 6, 883–892 (2008).
Parkhill, J. et al. Complete genome sequence of a multiple drug resistant Salmonella enterica serovar Typhi CT18. Nature 413, 848–852 (2001).
Virlogeux, I., Waxin, H., Ecobichon, C. & Popoff, M. Y. Role of the viaB locus in synthesis, transport and expression of Salmonella typhi Vi antigen. Microbiology 141, 3039–3047 (1995).
Wetter, M. et al. Molecular characterization of the viaB locus encoding the biosynthetic machinery for Vi capsule formation in Salmonella Typhi. PLoS ONE 7, e45609 (2012).
Heyns, K. & Kiessling, G. Strukturaufklarung des Vi-antigens aus Citrobacter freundii (E. coli) 5396/38. Carbohydr. Res. 3, 340–353 (in German) (1967).
Looney, R. J. & Steigbigel, R. T. Role of the Vi antigen of Salmonella typhi in resistance to host defense in vitro. J. Lab. Clin. Med. 108, 506–516 (1986).
Wilson, R. P. et al. The Vi capsular polysaccharide prevents complement receptor 3-mediated clearance of Salmonella enterica serotype Typhi. Infect. Immun. 79, 830–837 (2011).
Miller, R. M., Garbus, J. & Hornick, R. B. Lack of enhanced oxygen consumption by polymorphonuclear leukocytes on phagocytosis of virulent Salmonella typhi. Science 175, 1010–1011 (1972).
Mollnes, T. E. et al. Essential role of the C5a receptor in E coli-induced oxidative burst and phagocytosis revealed by a novel lepirudin-based human whole blood model of inflammation. Blood 100, 1869–1877 (2002).
Wilson, R. P. et al. The Vi-capsule prevents Toll-like receptor 4 recognition of Salmonella. Cell. Microbiol. 10, 876–890 (2008).
Raffatellu, M. et al. The Vi capsular antigen of Salmonella enterica serotype Typhi reduces Toll-like receptor-dependent interleukin-8 expression in the intestinal mucosa. Infect. Immun. 73, 3367–3374 (2005).
Hirose, K. et al. Survival of Vi-capsulated and Vi-deleted Salmonella typhi strains in cultured macrophage expressing different levels of CD14 antigen. FEMS Microbiol. Lett. 147, 259–265 (1997).
Jansen, A. M. et al. A Salmonella Typhimurium-Typhi genomic chimera: a model to study Vi polysaccharide capsule function in vivo. PLoS Pathog. 7, e1002131 (2011).
Noriega, L. M., Van der Auwera, P., Daneau, D., Meunier, F. & Aoun, M. Salmonella infections in a cancer center. Support. Care Cancer 2, 116–122 (1994).
Tumbarello, M., Tacconelli, E., Caponera, S., Cauda, R. & Ortona, L. The impact of bacteraemia on HIV infection. Nine years' experience in a large Italian university hospital. J. Infect. 31, 123–131 (1995).
Cunnington, A. J. et al. Prolonged neutrophil dysfunction after Plasmodium falciparum malaria is related to hemolysis and heme oxygenase-1 induction. J. Immunol. 189, 5336–5346 (2012).
Janis, C. et al. In vivo regulation of the Vi antigen in Salmonella and induction of immune responses with an in vivo-inducible promoter. Infect. Immun. 79, 2481–2488 (2011).
Chitkara, Y. K. & Urquhart, A. E. Fluorescent Vi antibody test in the screening of typhoid carriers. Am. J. Clin. Pathol. 72, 87–89 (1979).
Nolan, C. M. et al. Evaluation of a new assay for Vi antibody in chronic carriers of Salmonella typhi. J. Clin. Microbiol. 12, 22–26 (1980).
Losonsky, G. A. et al. Development and evaluation of an enzyme-linked immunosorbent assay for serum Vi antibodies for detection of chronic Salmonella typhi carriers. J. Clin. Microbiol. 25, 2266–2269 (1987).
Crawford, R. W. et al. Loss of very-long O-antigen chains optimizes capsule-mediated immune evasion by Salmonella enterica serovar Typhi. mBio 4, e00232-13 (2013).
Bravo, D. et al. Growth-phase regulation of lipopolysaccharide O-antigen chain length influences serum resistance in serovars of Salmonella. J. Med. Microbiol. 57, 938–946 (2008).
Haneda, T. et al. The capsule-encoding viaB locus reduces intestinal inflammation by a Salmonella pathogenicity island 1-independent mechanism. Infect. Immun. 77, 2932–2942 (2009).
Winter, S. E. et al. The TviA auxiliary protein renders the Salmonella enterica serotype Typhi RcsB regulon responsive to changes in osmolarity. Mol. Microbiol. 74, 175–193 (2009).
Winter, S. E. et al. A rapid change in virulence gene expression during the transition from the intestinal lumen into tissue promotes systemic dissemination of Salmonella. PLoS Pathog. 6, e1001060 (2010).
Tran, Q. T. et al. The Salmonella enterica serotype Typhi Vi capsular antigen is expressed after the bacterium enters the ileal mucosa. Infect. Immun. 78, 527–535 (2010).
Winter, S. E., Raffatellu, M., Wilson, R. P., Russmann, H. & Bäumler, A. J. The Salmonella enterica serotype Typhi regulator TviA reduces interleukin-8 production in intestinal epithelial cells by repressing flagellin secretion. Cell. Microbiol. 10, 247–261 (2008).
Atif, S. M., Winter, S. E., Winter, M. G., McSorley, S. J. & Bäumler, A. J. Salmonella enterica serovar Typhi impairs CD4 T cell responses by reducing antigen availability. Infect. Immun. 82, 2247–2254 (2014).
Winter, S. E. et al. Salmonella enterica serovar Typhi conceals the invasion-associated type three secretion system from the innate immune system by gene regulation. PLoS Pathog. 10, e1004207 (2014). This report shows that TviA-mediated changes in gene regulation can blunt intestinal inflammation. The underlying mechanism is repression of SopE expression, which prevents RIP2-dependent NF-κB activation.
Raffatellu, M. et al. The capsule encoding the viaB locus reduces interleukin-17 expression and mucosal innate responses in the bovine intestinal mucosa during infection with Salmonella enterica serotype Typhi. Infect. Immun. 75, 4342–4350 (2007).
Olsen, S. J. et al. Outbreaks of typhoid fever in the United States, 1960–1999. Epidemiol. Infect. 130, 13–21 (2003).
Glynn, J. R. & Palmer, S. R. Incubation period, severity of disease, and infecting dose: evidence from a Salmonella outbreak. Am. J. Epidemiol. 136, 1369–1377 (1992).
Crump, J. A., Luby, S. P. & Mintz, E. D. The global burden of typhoid fever. Bull. World Health Organ. 82, 346–353 (2004).
Crump, J. A., Ram, P. K., Gupta, S. K., Miller, M. A. & Mintz, E. D. Part I. Analysis of data gaps pertaining to Salmonella enterica serotype Typhi infections in low and medium human development index countries, 1984–2005. Epidemiol. Infect. 136, 436–448 (2008).
Majowicz, S. E. et al. The global burden of nontyphoidal Salmonella gastroenteritis. Clin. Infect. Dis. 50, 882–889 (2010).
Gilks, C. F. Acute bacterial infections and HIV disease. Br. Med. Bull. 54, 383–393 (1998).
Watera, C. et al. 23-valent pneumococcal polysaccharide vaccine in HIV-infected Ugandan adults: 6-year follow-up of a clinical trial cohort. AIDS 18, 1210–1213 (2004).
van Oosterhout, J. J. et al. A community-based study of the incidence of trimethoprim-sulfamethoxazole-preventable infections in Malawian adults living with HIV. J. Acquired Immune Defic. Syndr. 39, 626–631 (2005).
Gordon, M. A. Salmonella infections in immunocompromised adults. J. Infect. 56, 413–422 (2008).
Berkley, J. A. et al. Bacteremia among children admitted to a rural hospital in Kenya. N. Engl. J. Med. 352, 39–47 (2005).
Enwere, G. et al. Epidemiologic and clinical characteristics of community-acquired invasive bacterial infections in children aged 2–29 months in The Gambia. Pediatr. Infect. Dis. J. 25, 700–705 (2006).
Sigauque, B. et al. Community-acquired bacteremia among children admitted to a rural hospital in Mozambique. Pediatr. Infect. Dis. J. 28, 108–113 (2009).
MacLennan, C. A. et al. The neglected role of antibody in protection against bacteremia caused by nontyphoidal strains of Salmonella in African children. J. Clin. Invest. 118, 1553–1562 (2008).
O'Reilly, C. E. et al. Risk factors for death among children less than 5 years old hospitalized with diarrhea in rural western Kenya, 2005–2007: a cohort study. PLoS Med. 9, e1001256 (2012).
Hsieh, M. M., Everhart, J. E., Byrd-Holt, D. D., Tisdale, J. F. & Rodgers, G. P. Prevalence of neutropenia in the U. S. population: age, sex, smoking status, and ethnic differences. Ann. Intern. Med. 146, 486–492 (2007).
Andres, E. & Maloisel, F. Idiosyncratic drug-induced agranulocytosis or acute neutropenia. Curr. Opin. Hematol. 15, 15–21 (2008).
Grubb, S. C., Bult, C. J. & Bogue, M. A. Mouse phenome database. Nucleic Acids Res. 42, D825–D834 (2014).
Maddatu, T. P., Grubb, S. C., Bult, C. J. & Bogue, M. A. Mouse phenome database (MPD). Nucleic Acids Res. 40, D887–D894 (2012).
Eisenhauer, P. B. & Lehrer, R. I. Mouse neutrophils lack defensins. Infect. Immun. 60, 3446–3447 (1992).
Ganz, T. et al. Defensins. Natural peptide antibiotics of human neutrophils. J. Clin. Invest. 76, 1427–1435 (1985).
Selsted, M. E., Harwig, S. S., Ganz, T., Schilling, J. W. & Lehrer, R. I. Primary structures of three human neutrophil defensins. J. Clin. Invest. 76, 1436–1439 (1985).
Modi, W. S. & Yoshimura, T. Isolation of novel GRO genes and a phylogenetic analysis of the CXC chemokine subfamily in mammals. Mol. Biol. Evol. 16, 180–193 (1999).
van den Berg, J. M. et al. Chronic granulomatous disease: the European experience. PLoS ONE 4, e5234 (2009).
Gotze, O. & Müller-Eberhard, H. J. The C3-activator system: an alternate pathway of complement activation. J. Exp. Med. 134, 90–108 (1971).
Law, S. K. & Levine, R. P. Interaction between the third complement protein and cell surface macromolecules. Proc. Natl Acad. Sci. USA 74, 2701–2705 (1977).
Tack, B. F., Harrison, R. A., Janatova, J., Thomas, M. L. & Prahl, J. W. Evidence for presence of an internal thiolester bond in third component of human complement. Proc. Natl Acad. Sci. USA 77, 5764–5768 (1980).
Densen, P., MacKeen, L. A. & Clark, R. A. Dissemination of gonococcal infection is associated with delayed stimulation of complement-dependent neutrophil chemotaxis in vitro. Infect. Immun. 38, 563–572 (1982).
Musher, D. M., Hague-Park, M., Gyorkey, F., Anderson, D. C. & Baughn, R. E. The interaction between Treponema pallidum and human polymorphonuclear leukocytes. J. Infect. Dis. 147, 77–86 (1983).
Podack, E. R., Kolb, W. P. & Muller-Eberhard, H. J. The C5b-9 complex: subunit composition of the classical and alternative pathway-generated complex. J. Immunol. 116, 1431–1434 (1976).
Acknowledgements
A.M.K.-G. is supported by the American Heart Association Grant 12SDG12220022. Work in R.M.T.'s laboratory is supported by Public Health Service grants AI050553 and AI090387 from the US National Institutes of Health. Work in A.J.B.'s laboratory is supported by Public Health Service grants AI044170, AI096528 and AI107393.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Serovars
-
Distinct variants of bacterial species. Each of the more than 2,500 serovars that can be distinguished in the genus Salmonella is defined by an antigen formula, which lists O factors (antigenic epitopes in the O-antigen), H factors (antigenic epitopes in flagellin) and fermentative characteristics.
- Mesenteric lymph nodes
-
Lymph nodes in the mesentery that drain the afferent lymphatic vessels from the small and large intestine.
- Lipopolysaccharide
-
(LPS). Decorates the surface of Salmonella enterica subsp. enterica serovar Typhimurium. A lipid A moiety anchors the LPS molecule in the outer membrane. The lipid A moiety is linked to the oligosaccharide core, which connects to the O-antigen repeat units that extend from the bacterial surface.
- Pattern recognition receptor
-
A protein of the innate immune system that can sense an infection by identifying pathogen-associated molecular patterns or pathogen- associated processes.
- Zoonotic
-
Describes a pathogenic microorganism that spreads from an animal reservoir to cause disease in humans.
- Lipid A
-
A phosphorylated glucosamine disaccharide carrying multiple fatty acid chains that anchor lipopolysaccharide (LPS) in the outer membrane of most Gram-negative bacteria. The lipid A moiety is responsible for the toxicity of LPS, which is also known as endotoxin.
- Pathogen-associated molecular pattern
-
(PAMP). A conserved microbial product that allows the innate immune system to distinguish microorganisms from the host. Although the name implies an association with pathogens, PAMPs are present in all microorganisms, regardless of their pathogenic potential.
- Lipoproteins
-
Proteins that, in Gram-negative bacteria, possess an amino-terminal cysteine residue that is modified with three fatty acids (N-acyl-S-diacylglyceryl cysteine). This triacyl-modified cysteine residue allows lipoproteins to anchor onto bacterial cell membranes.
- CpG dinucleotides
-
Dinucleotides composed of a cytosine triphosphate deoxynucleotide and guanine triphosphate deoxynucleotide. The 'p' refers to the phosphodiester link between the nucleotides. This dinucleotide is often methylated in the DNA of host cells, thereby preventing its recognition by the innate immune system.
- Flagella
-
Proteinaceous surface appendages that function in motility of the bacterial cell. Each flagellum is composed of a globular protein, termed flagellin, which arranges itself into a hollow cylinder to form the flagellar filament.
- Type III secretion system
-
(T3SS). A multiprotein complex found in Gram-negative bacteria that functions in the secretion of proteins, termed effectors, from the bacterial cytosol across three biological membranes into the cytosol of the host cell. T3SS1 and T3SS2 are the two T3SSs present in Salmonella enterica, and are encoded by Salmonella pathogenicity islands (SPIs) SPI-1 and SPI-2, respectively.
- Salmonella pathogenicity island
-
(SPI). A DNA region that encodes virulence factors, and that has a limited phylogenetic distribution owing to its acquisition through horizontal gene transfer. For example, SPI-1 and SPI-2 are present in Salmonella enterica but absent in the closely related species Escherichia coli. SPI-7 is present only in S. enterica subsp. enterica serovars Typhi, Paratyphi C and Dublin.
- O-antigen
-
In Salmonella enterica subsp. enterica serovar Typhimurium, the O-antigen is composed of a trisaccharide backbone, consisting of α-D-mannose–1,4-α-L-rhamnose–1,3-α-D-galactose, and a branching sugar (abequose) that is α-1,3-linked to D-mannose in the backbone. The length of O-antigen chains in S. Typhimurium lipopolysaccharide (LPS) has a trimodal distribution; LPS species can contain short (1–15 repeat units), long (16–35 repeat units) or very long O-antigen chains (more than 100 repeat units).
- Opsonophagocytosis
-
Phagocytosis of a microorganism that is initiated by opsonization to mark it for ingestion. Opsonization involves the binding of an opsonin, such as complement component 3 fragment b (C3b), to the microbial surface.
- Anaphylatoxin
-
A complement component fragment (C3a, C4a or C5a) that is generated during complement activation. Anaphylatoxins have various pro-inflammatory activities and contribute to the generation of septic shock (also known as anaphylactic shock).
- Pyroptosis
-
A form of programmed cell death associated with the generation of pro-inflammatory responses.
- Inflammasome
-
A multiprotein complex involved in the induction of pyroptosis and the proteolytic activation of the cytokines interleukin-1β (IL-1β) and IL-18.
- Antigen-presenting cells
-
(APCs). Cells that display foreign antigens bound to a major histocompatibility complex class II protein on their surface. APCs include dendritic cells, macrophages and B cells.
- Curli amyloid fibrils
-
Functional amyloids that are major proteinaceous components of the extracellular biofilm matrix produced by many Enterobacteriaceae.
- Effector proteins
-
Bacterial proteins that are injected into the host cell cytosol by a type III secretion system.
- Relative bradycardia
-
A heart rate that is considered to be abnormally slow for the individual's current medical condition.
- Capsular polysaccharide
-
A component of a capsule (that is, a polysaccharide layer) that covers the bacterial cell surface. Each polysaccharide chain is attached to the outer membrane of the bacterial cell by a lipid anchor.
Rights and permissions
About this article
Cite this article
Keestra-Gounder, A., Tsolis, R. & Bäumler, A. Now you see me, now you don't: the interaction of Salmonella with innate immune receptors. Nat Rev Microbiol 13, 206–216 (2015). https://doi.org/10.1038/nrmicro3428
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro3428
- Springer Nature Limited
This article is cited by
-
Salmonella spvC gene suppresses macrophage/neutrophil antibacterial defense mediated by gasdermin D
Inflammation Research (2024)
-
Co-existence of extended-spectrum β-lactamases blaCTX-M-9 and blaCTX-M-15 genes in Salmonella species isolated from febrile and diarrhoeagenic patients in Lagos, Nigeria: a cross-sectional study
European Journal of Medical Research (2023)
-
Salmonella Typhimurium O-antigen and VisP play an important role in swarming and osmotic stress response during intracellular conditions
Brazilian Journal of Microbiology (2022)
-
Essential role of Salmonella Enteritidis DNA adenine methylase in modulating inflammasome activation
BMC Microbiology (2020)
-
Genome analysis of Salmonella enterica subsp. diarizonae isolates from invasive human infections reveals enrichment of virulence-related functions in lineage ST1256
BMC Genomics (2019)