Abstract
Alcohol dependence is a complex disorder that initiates with episodes of excessive alcohol drinking known as binge drinking. It has a 50–60% risk contribution from inherited susceptibility genes; however, their exact identity and function are still poorly understood. We report that alcohol-preferring P rats have innately elevated levels of Toll-like receptor 4 (TLR4) and monocyte chemotactic protein-1 (MCP-1) that colocalize in neurons from the central nucleus of the amygdala (CeA) and ventral tegmental area (VTA). To examine the potential role of a TLR4/MCP-1 signal, we used Herpes Simplex Virus (HSV) vectors (amplicons) that retain in vivo neurotropism. Infusion of amplicons for TLR4 or MCP-1 siRNA into the CeA or VTA from the P rats inhibited target gene expression and blunted binge drinking. A similarly delivered amplicon for scrambled siRNA did not inhibit TLR4 or MCP-1 expression nor reduce binge drinking, identifying a neuronal TLR4/MCP-1 signal that regulates the initiation of voluntary alcohol self-administration. The signal was sustained during alcohol drinking by increased expression of corticotropin-releasing factor and its feedback regulation of TLR4 expression, likely contributing to the transition to alcohol dependence.
Similar content being viewed by others
INTRODUCTION
Alcoholism is a complex disorder that has a 50–60% risk contribution from inherited susceptibility genes (Goldman et al, 2005). It initiates with episodes of excessive alcohol drinking known as binge drinking (blood alcohol level ≥0.08 g% in a 2-h period (National Institute on Alcohol Abuse and Alcoholism, 2004), the most devastating societal cost of which is death in the form of vehicle crashes (Naimi et al, 2003). Neuronal functions that mediate pleasurable effects set the conditions for reward craving and the recruitment of mechanisms, which favor the transition to a relapsing course of sustained heavy drinking (alcohol dependence; Jennison, 2004). This is associated with signaling by activated corticotropin-releasing factor (CRF) and its contribution to the withdrawal/negative affect stage of the addiction cycle (Heilig and Koob, 2007; Lowery-Gionta et al, 2012). Novel therapies that target genes, which predispose to initiate binge drinking and transit to alcohol dependence, may offer promising alternatives to current psychosocial and pharmacotherapeutic interventions; however, gene identification is a clinical challenge.
The γ-aminobutyric acid A (GABAA) receptors contribute to excessive alcohol intake (Harris et al, 2008); however, the involved subunits and their mechanism of action are still controversial (Edenberg, 2012) and may reflect different contribution at distinct brain sites. In the ventral pallidum (VP), which is implicated in drug abuse decisions, voluntary excessive drinking is regulated by the α1 subunits (Liu et al, 2011; Yang et al, 2011), but human clinical linkage studies support a role for the α2 subunits in alcohol dependence (Edenberg, 2012). A large body of evidence indicates that neuroimmune (including Toll-like receptor 4 (TLR)) signaling contributes to reward-system activity and is associated with alcohol dependence and the effects of alcohol, and postmortem human studies correlate neuroimmune gene expression with lifetime alcohol consumption (Crews et al, 2011; 2013; Pascual et al, 2011; Blednov et al, 2012; Crews and Vetreno, 2014). However, the relationship of the neuroimmune signals to the GABAA subunits and the initiation of binge drinking by previously naive individuals is still unclear. We showed that the α2 subunits regulate the initiation of excessive alcohol consumption (binge drinking) in the central nucleus of the amygdala (CeA) by signaling through the innate immune receptor TLR4 (Liu et al, 2011). However, the identity of the TLR4 signal, the cell type in which it operates, and its function at other brain sites that may regulate the susceptibility to initiate excessive alcohol drinking are still unknown.
Our studies were designed to address these questions. They follow on the findings that: (i) TLR4 induces the expression of chemokines/cytokines, at least in inflammatory cells (Bosman et al, 2012; Liu et al, 2013) and (ii) the TLR4-induced chemokine monocyte chemotactic protein-1 (MCP-1; also known as CCL2) has neurotransmitter activity, apparently involving dopamine release (Banisadr et al, 2005; Guyon et al, 2009; Apartis et al, 2010). We report that an innate neuronal TLR4/MCP-1 signal in the CeA and the ventral tegmental area (VTA) controls the predisposition of P rats to initiate binge drinking. The signal is sustained during alcohol drinking through increased expression of corticotropin-releasing factor (CRF) and its feedback regulation of TLR4, potentially contributing to the transition to alcohol dependence.
MATERIALS AND METHODS
Cells, Antibodies, and Reagents
TLR4-expressing RAW264.7 cells were obtained from the American Type Culture Collection and cultured in DMEM with 4 mM L-glutamine (Invitrogen, Carlsbad, CA, USA) and 10% fetal bovine serum (Gemini, West Sacramento, CA, USA). The GABAA α1 (aa: 1–9) and α2 (aa: 322–357) antibodies were obtained from Dr W. Sieghart, University of Vienna, Austria. Their generation and specificity were previously described (Pirker et al, 2000; Liu et al, 2011). The TLR4 antibody was from Novus Biologicals (Littleton, CO); its specificity is confirmed by immunoblotting (Supplementary Data; Supplementary Figure S1). Other commercially obtained antibodies are as follows: goat anti-CRF (SC-21675), actin, and GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA) mouse anti-MCP-1/CCL2, and NeuN (MAb A60; Millipore, Billerica, MA), and rabbit anti-MCP-1 (IBL International, Toronto, ON, Canada). All were used according to the manufacturer’s instructions. Alexa Fluor 488 goat anti-mouse IgG (H+L) and Alexa Fluor 546 goat anti-rabbit IgG (H+L) were from Life Technologies (Grand Island, NY); the TLR4 ligand bacterial lipopolysaccharide (LPS) was from Sigma-Aldrich (St Louis, MO); the MCP-1 ELISA kits were from RayBiotech (Norcross, GA); and antalarmin hydrochloride was from R&D Systems (Minneapolis, MN).
Amplicon Construction
Amplicons are bacterial plasmids that are packaged into HSV-1 particles. They do not contain viral DNA or express viral proteins and are not toxic. They do not cause loss of body weight or alter general activity levels in the P rats, and they do not induce cell death/apoptosis in intrastriatally infused animals (SD; Liu et al, 2011). The amplicon vector for TLR4 siRNA (pHSVsiTLR4) was previously described (Liu et al, 2011). The construction and specificity of the amplicon vectors for MCP-1 siRNA pHSVsiMCP1-A is described in SD, Supplementary Table S1, Supplementary Figures S5 and S6. pHSVsiMCP-1A and a scrambled siRNA vector (pHSVsiNCC) were used in these studies.
Animals
Alcohol-preferring (P) and non-alcohol-preferring (NP) rats (3–4 months old; 250–550 g) were obtained from the Alcohol Research Center, Indiana University School of Medicine. P rats perform an operant response for access to ethanol that is not performed by the NP rats. Animals were individually housed, maintained at an ambient temperature of 21 °C and a reverse 12-h light/dark cycle, and provided with food and water ad libitum. Training and experimental sessions were between 0830 and 1730 hours.
Binge Drinking Paradigm
To initiate binge drinking, we used the drinking-in-the dark-multiple-scheduled-access (DIDMSA) protocol (Bell et al, 2006). Rat cohorts given sucrose (1% (w/v)) served as control. Animals received three daily 30-min access periods, each spaced 1 h apart, across the dark cycle. These were conducted on a 5-d binge and 2-d withdrawal schedule that emulates human binge drinking (Naimi et al, 2003). Using this protocol, the P rats produced consistent BACs ≥80 mg%/dl.
Stereotaxic Procedures
Anesthetized rats were CNS-infused as previously described (Liu et al, 2011; Yang et al, 2011). Microinjection sites in the CeA extended from −1.60 mm posterior to the bregma to −3.3 mm posterior to the bregma, 3.0–4.5 mm lateral to the midline in both hemispheres, and from −7.8 to −8.6 mm into the brain from the surface of the skull. Microinjection sites in the VTA extended from −5.3 mm posterior to the bregma to −6.3 mm posterior to the bregma, 1.6 mm lateral to the midline in both hemispheres, and −9.2 mm into the brain from the surface of the skull (Paxinos and Watson, 1998). Because amplicons do not diffuse over long distances, we gave 9 or 13 injections in each hemisphere spaced across the entire CeA or VTA. Each locus received 200 nl of PBS or amplicon (2.5 × 105 TU) delivered by calibrated glass micropipettes (≈20-μm tip) connected to a pneumatic pressure injector (Science Products GmbH). Antalarmin (4 μg in PBS) was bilaterally infused into the CeA or VTA (0.1 μl/min) for 5 min using a Harvard infusion pump (0.5 μl/hemisphere). It was given for three consecutive days, at which time CeA and VTA micropunches were collected and examined for CRF and TLR4 expression. The Institutional Animal Care and Use Committee at the Howard University (IACUC) approved all the procedures.
Immunoblotting
Tissue micropunches (300-μm thick) were lysed with CelLytic MT (dialyzable mild detergent, bicine, and 150 mM NaCl; Sigma-Aldrich) according to the manufacturer’s instructions. Total protein was determined by the bicinchoninic assay (Pierce, Rockford, IL). Proteins were resolved with SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. Blots were exposed to primary antibody overnight at 4 °C followed by horseradish peroxidase (Cell Signaling)-labeled secondary antibody for 1 h at room temperature (RT). Detection was carried out with the ECL kit reagents (Amersham Life Science) and quantitation was carried out by densitometric scanning with a Bio-Rad GS-700 imaging densitometer (Liu et al, 2011).
Immunofluorescence
Free-floating (30-μm thick) frozen sections were collected as described in SD (Materials and Methods (M&M)), rinsed in PBS, treated (95 °C, 10 min) with Retrievagen A (BD Pharmingen), cooled (20 min, RT), and blocked with 5% goat serum (90 min, RT). They were stained (overnight, 4 °C) in double immunofluorescence with antibodies to TLR4 and NeuN, MCP-1 and NeuN, or TLR4 and MCP-1 and exposed (1 h, RT) to the appropriate Alexa Fluor-labeled secondary antibodies diluted (1 : 500) in PBS with 0.4% Triton X-100. Sections were coverslipped with Prolong Gold Antifade mounting media with DAPI (Life Technologies). Z-stack images (1 μm optical steps) were collected on an Olympus FluoView 500 confocal microscope fitted with standard excitation and emission filters.
MCP-1 Assay
Tissue micropunches were lysed with celLyte MT as per the manufacturer’s instructions and the extracts were assayed for protein content by the BCA procedure (Pierce) and for MCP-1 using the rat MCP-1 ELISA kit.
Statistics
Data were analyzed by appropriate ANOVAs. Significant ANOVAs were followed by Newman–Keuls post hoc tests. Analyses were performed using the SigmaPlot 11.2 software program (Systat Software, San Jose, CA).
RESULTS
TLR4 and α2 are Upregulated in the VTA from P Rats and Control Binge Drinking
Immunoblotting assays comparing P and NP rats indicated that P rats have elevated levels of α2 and TLR4 (not α1) in the VTA (Figure 1a), but not the in the VP (SD, Supplementary Figure S2). To examine whether the TLR4 elevation predisposes the P rats to binge drinking, cohorts were randomly given pHSVsiTLR4 (n=9) or pHSVsiNCC (n=8) in the VTA by bilateral stereotaxic infusion, allowed to recover from the stress of surgery for 3 days, and examined for alcohol drinking daily for 16 days. Animals given pHSVsiNCC evidenced minimally reduced stress-related alcohol drinking on day 3 after surgery, but displayed baseline (pre-surgery) levels of alcohol drinking during the next 13 days of follow-up. By contrast, alcohol drinking was significantly reduced by treatment with pHSVsiTLR4 (F(29 225)=16.119, p<0.001 vs pHSVsiNCC). Post hoc tests confirmed significant reduction of responding for alcohol on days 3 through 13 (p≤0.05). Maximal reduction of binge responding was seen on days 3–7 post infusion and it returned to the pre-surgery levels on day 14 after infusion (Figure 1b). Reflecting its effect on binge drinking, pHSVsiTLR4 inhibited TLR4 expression on day 3 (p≤0.05), but not on day 16 post infusion (p>0.05), and TLR4 expression was not inhibited by pHSVsiNCC (Figure 1d). Responding for sucrose was not affected by pHSVsiTLR4 microinjection (Figure 1c, p>0.05), confirming the specificity of its activity for alcohol drinking. The duration of the amplicon inhibitory effect is consistent with that previously reported and likely reflects the duration of siRNA integrity/availability and the resulting post-transcriptional gene silencing (Saydam et al, 2005; Liu et al, 2011).
Toll-like receptor 4 (TLR4) regulates alcohol self-administration in the ventral tegmental area (VTA). (a) Protein extracts of micropunches collected from the VTA of non-alcohol-preferring (NP) and –preferring (P) rats were immunoblotted with antibody to γ-aminobutyric acid A (GABAA) α1, and the blots were sequentially stripped and immunoblotted with antibodies to GABAA α2, TLR4 and GAPDH as a loading control. Results are densitometric units±SEM. Each lane is a distinct animal. The levels of α2 and TLR4 are significantly higher in P than in NP rats (p≤0.05). (b) pHSVsiTLR4 microinjection in the VTA from naive P rats reduces responding for alcohol on days 3–13 post surgery, but responding returns to baseline levels on day 14 post surgery. pHSVsiNCC does not alter responding for alcohol (*p≤0.05 by ANOVA). (c) Microinjection of pHSVsiTLR4 and pHSVsiNCC into the VTA does not alter responding for sucrose. (d) pHSVsiTLR4, but not pHSVsiNCC, inhibits TLR4 expression in the VTA on day 3 post infusion (p≤0.05), but TLR4 expression is restored to its innate levels on day 16 after pHSVsiTLR4 infusion (p>0.05).
Because α2 regulates the TLR4 activity in the CeA (Liu et al, 2011), duplicate sets of animals were given pHSVsiLA2 (n=6) or pHSVsiNCC (n=8) in the VTA and similarly examined for alcohol or sucrose drinking. Alcohol drinking was severely reduced in animals that were given pHSVsiLA2, but not pHSVsiNCC beginning on day 3 and continuing for the next 13 days (p<0.001 vs pHSVsiNCC), and this was associated with the inhibition of α2 expression at 3 but not 15 days post treatment. Sucrose drinking was not affected (SD, Supplementary Figure S4). Collectively, the data confirm that the α2/TLR4 signal also operates in the VTA.
MCP-1 Levels are Elevated in the CeA and VTA, but not in the VP, from P as Compared with NP Rats
Having seen that the levels of TLR4 are elevated in the CeA (Liu et al, 2011) and VTA (Figure 1a) from P as compared with NP rats, we wanted to know whether MCP-1 is also elevated at these sites. CeA and VTA micropunches were collected from naive (not alcohol exposed) P (n=4) and NP (n=4) rats and assayed for MCP-1 using ELISA. VP micropunches were studied in parallel and served as control. The levels of MCP-1 were significantly elevated in the CeA (p≤0.05) and VTA (p≤0.05) from P, as compared with NP rats, but elevation was not seen in the VP (p>0.05; Figure 2a).
Monocyte chemotactic protein-1 (MCP-1) innately elevated in the ventral tegmental area (VTA) and central nucleus of the amygdala (CeA) from alcohol-preferring (P) rats and siRNA amplicon vectors localize in neurons at the injection site. (a) MCP-1 levels are elevated in the CeA and VTA, but not in the ventral pallidum (VP) from P as compared with non-alcohol-preferring (NP) rats. (*p<0.05 by ANOVA). (b–e). Neurons and neuronal spines and processes located near one of the pHSVsiTLR4 (b), pHSVsiNCC (d), or pHSVsiMCP-1 (e) infusion sites show EGFP staining (green). Transduction is effective, with 25–40 neurons at the injection site showing EGFP staining as shown for pHSVsiTLR4 (c). Glial and other cells stained with DAPI (blue) do not stain for EGFP (e). Scale bars are 20 μm.
CNS-Delivered Amplicons Infect Neurons
HSV has a natural in vivo tropism for neurons, a property that is reflected in its ability to cause encephalitis and establish life-long infection of peripheral ganglia sensory neurons known as latency, and HSV vectors are also neurotropic (Perkins et al, 2003; Taylor et al, 2005; Saeki, 2006; Berges et al, 2007; Suzuki et al, 2008; Manservigi et al, 2010; Cohen et al, 2011; de Silva and Bowers, 2011; Liu et al, 2011; Yang et al, 2011; Fiandaca et al, 2012; Aurelian, 2014). Consistent with these findings, EGF imaging confirmed that the CNS-delivered amplicons pHSVsiTLR4 (Figure 2b), pHSVsiNCC (Figure 2d), and pHSVsiMCP-1 (Figure 2e) also localize in neurons with clusters of 20–45 transduced neurons seen near the injection site. This is shown in Figure 2c, for one of the 9–13 sites given pHSVsiTLR4 and there was no traffic to distant brain areas.
Neurons Co-Express TLR4 and MCP-1
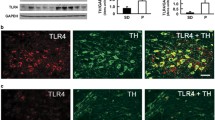
Having seen that CNS-delivered pHSVsiTLR4 and pHSVsiMCP-1 infect neurons, we wanted to know whether neurons innately co-express TLR4 and MCP-1, thereby identifying the presence of a likely TLR4/MCP-1 signal that is the amplicons’ target. Double immunofluorescent staining indicated that in both the CeA and VTA staining with antibody to the NeuN neuronal marker (Figure 3b and f) was predominantly nuclear, but also seen in the cytoplasm (Lavezzi et al, 2013). TLR4 (Figure 3a) and MCP-1 (Figure 3e) staining was strictly non-nuclear; it colocalized with NeuN (Figure 3c, g, d and h); and TLR4/MCP-1 colocalization was seen at both brain sites (Figure 3i). TLR4 and MCP-1 staining was also seen in NeuN− cells, and double immunofluorescence with antibodies to GFAP (astrocytes) or Iba-1 (microglia) confirmed that TLR4 and MCP-1 are expressed in astrocytes and microglia (SD, Supplementary Figure S3), as previously reported (Tang et al, 2007; Okun et al, 2011; Pascual et al, 2012). However, whereas glial cells express TLR4 and MCP-1 and we cannot exclude the possibility that they can be infected by the CNS-delivered amplicons, this latter interpretation is not supported by our and independently obtained data (Perkins et al, 2003; Taylor et al, 2005; Saeki, 2006; Berges et al, 2007; Suzuki et al, 2008; Manservigi et al, 2010; Cohen et al, 2011; de Silva and Bowers, 2011; Liu et al, 2011; Yang et al, 2011; Fiandaca et al, 2012; Zhang et al, 2015).
Toll-like receptor 4 (TLR4) and monocyte chemotactic protein-1 (MCP-1) innately colocalize in neurons from the ventral tegmental area (VTA) and central nucleus of the amygdala (CeA) of P rats. Confocal microscopy and Z-stack imaging of double immunofluorescence staining for TLR4/NeuN (a–d), MCP-1/NeuN (e–h), and TLR4/MCP-1 (i) are shown for the CeA and VTA. Merged images for TLR4 (a), NeuN (b), and DAPI (blue) reveal TLR4 expression in neurons (c, d). A TLR4+ neuron (NeuN+) in a–c is identified (*) and a magnified image is shown in d. Panel (d) also shows a magnification of a TLR4+ non-neuronal cell (NeuN−) identified in c (arrow). Merged images for MCP-1 (e), NeuN (f), and DAPI (blue) reveal MCP-1 expression in neurons (g, h). A MCP-1+ neuron (NeuN+) in panels (e–g) is identified (**) and a magnified image is shown in h. Panel (h) also shows a magnification of a MCP-1+ non-neuronal cell (NeuN−; g; arrow). Merged images for TLR4 (red) and MCP-1 (green) reveal co-expression of TLR4 and MCP-1 (i). Scale bars are (c, g): 30 μm, (d, h): 5 μm. Paraffin-embedded (5-μm-thick) sections prepared as described in SD (Materials and Methods) were also examined with similar results.
MCP-1 Regulates Binge Drinking in the VTA and CeA
Two series of experiments were carried out in order to examine whether MCP-1 is also involved in predisposing to alcohol, likely as a downstream component of a TLR4 signal. First, TLR4-expressing RAW264.7 cells were transduced with pHSVsiTLR4 or pHSVsiNCC (3 TU/cell) in the absence or presence of the TLR4 ligand LPS (2 μg/ml; 48 h), and the culture supernatants were assayed for MCP-1 using ELISA. MCP-1 expression was inhibited by pHSVsiTLR4, but not by pHSVsiNCC, confirming that TLR4 induces MCP-1 expression (Figure 4a). Second, cohorts of P rats were randomly given pHSVsiMCP-1A (n=9) or pHSVsiNCC (n=9) in the VTA, allowed to recover from the stress of surgery for 3 days, and examined for alcohol drinking daily for 14 days. Drinking was reduced by pHSVsiMCP-1A, but not by pHSVsiNCC infusion (Figure 4c) with significant main effects of Treatment (F(1210)=321.81, p<0.001), Day (F(14 210)=22.815, p<0.001), and Treatment × Day interaction (F(14 210)=15.676, p<0.001). Post hoc tests confirmed significant reduction of responding for alcohol on days 3–13 post infusion of pHSVsiMCP-1A, and responding returned to the pre-infusion levels on day 14 post infusion. Responding for sucrose was not affected by microinjection of pHSVsiMCP-1A (Figure 4d; p>0.05), confirming that the pHSVsiMCP-1A effect is specific for alcohol drinking. Reduction in responding for alcohol was associated with MCP-1 inhibition on day 3 (p≤0.05), but not day 16 post infusion (p>0.05; Figure 4b). pHSVsiNCC did not inhibit MCP-1 expression (p>0.05) or binge sucrose-motivated responding (p>0.05; Figure 4b and d). Collectively, the data indicate that MCP-1 regulates binge drinking in the VTA, likely as a component of the TLR4 signal.
pHSVsiMCP-1 infusion in the ventral tegmental area (VTA) or central nucleus of the amygdala (CeA) inhibits monocyte chemotactic protein-1 (MCP-1) expression and binge drinking. (a) Toll-like receptor 4 (TLR4)-expressing RAW264.7 cells were transduced with pHSVsiTLR4 (3 TU/cell) and treated or not with the TLR4 ligand lipopolysaccharide (LPS) (2 μg/ml). Culture supernatants were collected 48 h later and assayed for MCP-1 by ELISA. pHSVsiTLR4 inhibited MCP-1 expression. Expression was not affected in parallel experiments using pHSVsiNCC (data not shown). (b–d) pHSVsiMCP-1 infusion in the VTA inhibits MCP-1 expression at 3 days after injection, but the levels are returned to baseline on day 16 post injection and expression is not inhibited by pHSVsiNCC (b). pHSVsiMCP-1 reduces P rat responding for alcohol on days 3–13 post surgery compared with pHSVsiNCC; responding returns to baseline levels on day 14 post surgery and pHSVsiNCC does not alter responding for alcohol (c); pHSVsiMCP-1 and pHSVsiNCC do not alter responding for sucrose (d). (*p≤0.05 by ANOVA). (e–g) pHSVsiMCP-1 infusion in the CeA inhibits MCP-1 expression at 3 days after injection, but the levels are returned to baseline on day 14 post injection and expression is not inhibited by pHSVsiNCC (e). pHSVsiMCP-1 reduces P rat responding for alcohol on days 3–10 post surgery compared with pHSVsiNCC, but responding returns to baseline levels on day 11 post surgery. pHSVsiNCC does not alter responding for alcohol (f). pHSVsiMCP-1 or pHSVsiNCC do not alter responding for sucrose (g). (*p≤0.05 by ANOVA).
To examine whether MCP-1 is also a component of the TLR4 signal in the CeA, P rats (n=6/group) were randomly given by cohort pHSVsiMCP-1A or pHSVsiNCC into the CeA, allowed to recover for 3 days, and examined for alcohol or sucrose drinking daily. Alcohol intake was reduced in the pHSVsiMCP-1A-infused rats on days 3–10 after surgery (Figure 4f; p≤0.05) and increased thereafter, returning to the pre-surgery levels on day 11. These effects are because of MCP-1 regulation, as evidenced by a dramatic decrease in MCP-1 expression at 3 (p≤0.05) but not 14 (p>0.05) days after pHSVsiMCP-1A infusion (Figure 4e). pHSVsiMCP-1A did not decrease sucrose consumption (Figure 4f, p>0.05) and it failed to alter alcohol responding when given into the VP (data not shown). In addition, rats given pHSVsiNCC displayed baseline (pre-surgery) levels of alcohol drinking during the follow-up (Figure 4f; p>0.05) and pHSVsiNCC did not inhibit MCP-1 expression (Figure 4e).
Feedback TLR4 Regulation by Alcohol-Induced CRF
Having seen that a TLR4/MCP-1 signal in the CeA and VTA predisposes to alcohol drinking in naive animals, we wanted to know whether the signal is altered by alcohol consumption. We focused on CRF because previous studies had shown that it is induced by alcohol (Lowery-Gionta et al, 2012) and contributes to the transition to alcohol dependence, apparently through CRFR1 activation (Sommer et al, 2008; Roberto et al, 2010; Huang et al, 2010). P rats were allowed to drink alcohol for 20 days, infused with the CRFR1 inhibitor antalarmin in the CeA or VTA as described in M&M and micropunches collected before and after 3 days of exposure to antalarmin were examined for CRF expression by immunoblotting. The blots were stripped and re-probed with antibodies to TLR4 followed by GAPDH (loading control) and the results were analyzed by densitometric scanning. As shown in Figure 5 for the CeA, the levels of CRF were significantly higher in the alcohol-drinking than naive P rats (p≤0.05), an increase that was blocked by the infusion of antalarmin (p≤0.05; Figure 5a). Significantly, antalarmin infusion also reduced the levels of TLR4 (p≤0.05; Figure 5b) and similar results were obtained for the VTA. The data identify a CRF-mediated feedback regulatory control (autocrine and/or paracrine) that likely contributes to sustaining the TLR4/MCP-1 signal during alcohol consumption and may contribute to alcohol dependence. This is schematically shown in Figure 5c.
Antalarmin inhibits Toll-like receptor 4 (TLR4) expression in alcohol-drinking P rats. (a) Alcohol drinking increases the levels of corticotropin-releasing factor (CRF) in the central nucleus of the amygdala (CeA) from P rats, an increase that is inhibited by antalarmin. (b) Antalarmin inhibits the levels of TLR4 in alcohol-consuming P rats. (*p≤0.05 by ANOVA). Similar results were obtained in the ventral tegmental area (VTA). (c) Schematic representation defines the initiation of alcohol self-administration through a neuronal TLR4/monocyte chemotactic protein-1 (MCP-1) signal that modulates neurotransmission and is sustained during alcohol drinking through CRF upregulation and its feedback control of TLR4. Dotted line represents potential involvement of CRF-mediated α2 regulation (Roberto et al, 2010).
DISCUSSION
The salient feature of the data presented in this report is the finding that an innate neuronal TLR4/MCP-1 signal that is elevated in the CeA and VTA from alcohol-preferring P rats predisposes them to alcohol self-administration (binge drinking). Alcohol consumption sustains this signal through CRF-mediated TLR4 feedback regulation. The following comments seem pertinent with respect to these findings.
A large body of evidence supports the role of GABAergic transmission and its interaction with neuroimmune mechanisms that include TLR4, in alcohol dependence and its effects (Alfonso-Loeches et al, 2010; Pascual et al, 2011; Blednov et al, 2012; Crews and Vetreno, 2014; Bajo et al, 2014). Alcohol releases endogenous ligands for TLR4 in the brain (Crews et al, 2013) and knockout of the TLR4 accessory protein CD14 interferes with the ability of LPS to increase alcohol drinking in wild-type mice (Blednov et al, 2011). In humans, lifetime alcohol consumption correlates with neuroimmune gene expression (Crews and Vetreno, 2014) and LPS leaks from the gut of human alcoholics were shown to activate pro-inflammatory signaling that may contribute to neuroinflammation and neurodegeneration (Qin et al, 2007). The levels of microglia-associated MCP-1 are increased in the VTA, CeA, substantia nigra, and hippocampus from human alcoholics compared with controls (He and Crews, 2008) and activation of dopamine neurons, which enhance reward-system activity via the MCP-1/CCR2 axis, was shown to have a crucial role in drug addiction (Wakida et al, 2014). However, the identity and role of innate TLR4 signals in the predisposition to initiate excessive alcohol drinking (binge drinking), the cell type in which such a signal might function, and its brain site(s) are still poorly understood. Our studies were designed to address these questions. They follow on previous findings that P rats have innately increased levels of GABAA α2-regulated TLR4 in the CeA, a brain region, which is critically involved in anxiety and fear-conditioning and participates in the learning of stimulus-reward responses and mediation of the motivational effects of alcohol and self-administration (Koob, 2009; Koob and Volkow, 2010). These elevations were not seen in the VP and their knockdown blunted binge drinking, as measured by the operant response performance (Liu et al, 2011). We focused on the VTA because it also regulates emotionality (Kalivas, 2002; Gifkins et al, 2002; Kauffman et al, 2003; Koob and Le Moal, 2005), and on MCP-1, because it is TLR4-regulated and has neurotransmitter activity (Banisadr et al, 2005; Guyon et al, 2009; Apartis et al, 2010; Bosman et al, 2012; Liu et al, 2013). Relative to NP rats, P rats had innately elevated levels of α2 and TLR4 also in the VTA, and the levels of MCP-1 were elevated in both the VTA and CeA.
Owing to the ubiquitous receptors that it employs, HSV tends to be relatively nonselective in infection of a variety of cells in culture, including astrocytes and microglia. However, in vivo, particularly after CNS administration, it shows a naturally discriminative neurotropism. This is reflected by the ability of the virus to: (i) establish life-long infection of the peripheral ganglia sensory neurons, known as latency, and (ii) cause encephalitis, which is the most common viral encephalitis and is associated with virus-induced neuronal apoptosis. HSV vectors, including amplicons, retain neurotropism and are therefore recognized as promising vectors for gene therapy of the nervous system and tools to differentially label CNS neurons (Perkins et al, 2003; Taylor et al, 2005; Saeki, 2006; Berges et al, 2007; Suzuki et al, 2008; Manservigi et al, 2010; Cohen et al, 2011; de Silva and Bowers, 2011; Liu et al, 2011; Yang et al, 2011; Fiandaca et al, 2012; Aurelian, 2014; Zhang et al, 2015). The molecular mechanism of this in vivo neuronal lineage-restricted infection/transduction is still unclear. Recent studies have implicated hyaluronic acid, a major component of the extracellular matrix, in HSV-1 infection of brain neurons (Cohen et al, 2011), and TLR3 was reported to function in astrocytes as a sensor of HSV-2 infection immediately after entry into the CNS, possibly preventing virus spread beyond the neurons (Reinert et al, 2012). Whereas final conclusions about the molecular mechanism of neurotropism require additional investigation, our findings confirm that: (i) the CNS-delivered pHSVsiTLR4, pHSVsiMCP-1, and pHSVsiNCC amplicons infect neurons and (ii) neurons express an innate TLR4/MCP-1 signal, which is the natural target for these amplicons. We recognize that knockout controls are needed in order to fully establish antibody specificity. However, our data are consistent with current findings for MCP-1 brain regional distribution (He and Crews, 2008) and with the observation that, in addition to microglia (Pascual et al, 2012), astrocytes and neurons also express TLR4 where it was implicated in neural plasticity and disease (Tang et al, 2007; Okun et al, 2011).
We conclude that MCP-1 contributes to the ability of TLR4 to regulate binge drinking because: (i) pHSVsiTLR4 controls MCP-1 expression and (ii) pHSVsiMCP-1 infusion into the VTA or CeA blunted binge drinking, associated with MCP-1 inhibition. The data are not an artifact of the experimental procedures. The HSV-based amplicon vectors are not toxic, as documented both at the gross and histologic levels, they do not cause apoptosis, and they deliver siRNA that specifically inhibits target gene expression. As previously reported (Liu et al, 2011, Yang et al, 2011), transduction was effective with clusters of 20–45 transduced neurons localized in neurons at the injection sites and failing to traffic to distant brain areas. Importantly, amplicons with identical properties that deliver scrambled siRNA were studied in parallel with pHSVsiTLR4 and pHSVsiMCP-1, and they did not affect gene expression or alcohol drinking. The amplicons specifically inhibited alcohol, but not sucrose self-administration, and neither pHSVsiTLR4 nor pHSVsiMCP-1 affected binge drinking when infused into the VP, which does not have innately elevated levels of TLR4 or MCP-1. We note that the duration of the pHSVsiMCP-1 inhibitory effect was shorter in the CeA than in the VTA (10 vs 14 days). Together with our similar previous findings for the CeA (Liu et al, 2011), this reveals a site-specific difference for the duration of the pHSVsiTLR4 effect that potentially identifies the VTA as a dominant regulatory site for binge drinking, at least in the P rats.
The neuropeptide CRF has a key role in excessive, dependence-like ethanol drinking through activation of the CRF1 receptor in the CeA (Sommer et al, 2008; Roberto et al, 2010; Lowery-Gionta et al, 2012). Its role in alcohol dependence is believed to develop over an extended course of excessive drinking (Koob, 2009; Heilig et al, 2010) but CRF does not modulate ethanol consumption in non-dependent rats (Chu et al, 2007). Having seen that the TLR4/MCP-1 signal controls the initiation of alcohol drinking, we wanted to know whether and how this relates to CRF. Examination of P rats that had engaged in binge drinking for 20 days revealed a significant increase in the levels of CRF relative to those seen before drinking initiation, and this increase was blocked by the CRF1 inhibitor antalarmin. Significantly, antalarmin simultaneously decreased the levels of TLR4, identifying a previously unrecognized neuronal CRF–TLR4 interaction that may contribute to the transition to alcohol dependence. This is schematically represented in Figure 5c, and it carries the implicit conclusion that both the TLR4 and MCP-1 levels may be further increased in animals with long-term drinking dependence. However, alcohol dependence involves the transition from such a strictly neuronal signal to a glial-centric inflammatory response (Fernandez-Lizarbe et al, 2009; Alfonso-Loeches et al, 2010; Crews et al, 2013; Pascual et al, 2011), by a mechanism that is still unclear. We confirmed that TLR4 and MCP-1 are expressed in glial cells (SD, Supplementary Figure S3), and posit that transition involves bidirectional neuron-glia cross-talk through released soluble factors and glial cell cytokine production (Frank et al, 2010; Hines et al, 2013).
In summary, our data identify an innate neuronal TLR4 signal that functions in the CeA and VTA to regulate the initiation of excessive alcohol drinking through MCP-1 neurotransmission likely at the level of dopamine (Banisadr et al, 2005; Guyon et al, 2009; Apartis et al, 2010). However, the MCP-1 neurotransmission mechanism, the role of TLR4-regulated cytokines/chemokines with neuron–neuron, and bidirectional neuron–microglia communications (namely, fractalkine (Laing et al, 2010)) in the transition to alcohol dependence, their relationship to alcohol-induced CRF expression, and the role of the brain site are still unclear. Ongoing studies are designed to address these questions.
FUNDING AND DISCLOSURE
These studies were supported by Grant AA021261 from the National Institute on Alcohol Abuse and Alcoholism to LA and HLJ and Grant AR053512 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases to LA. The authors declare no conflict of interest.
References
Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, Sanchez-Vera I, Guerri C (2010). Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. J Neurosci 30: 8285–8295.
Apartis E, Mélik-Parsadaniantz S, Guyon A, Kitabgi P, Rostène W (2010). Chemokines as new actors in the dopaminergic system. Biol Aujourdhui 204: 295–300.
Aurelian L (2014) Herpes Simplex Viruses: General Features, Encyclopedia of Virology 3rd edn In: Mahy BWJ, van Regenmortel MHV, (eds), Elsevier, ltd pp 383–397.
Bajo M, Madamba SG, Roberto M, Blednov YA, Sagi VN, Roberts E et al (2014). Innate immune factors modulate ethanol interaction with GABAergic transmission in mouse central amygdala. Brain Behav Immun 40: 191–202.
Banisadr G, Gosselin RD, Mechighel P, Kitabgi P, Rostène W, Parsadaniantz SM (2005). Highly regionalized neuronal expression of monocyte chemoattractant protein-1 (MCP-1/CCL2) in rat brain: evidence for its colocalization with neurotransmitters and neuropeptides. J Comp Neurol 489: 275–292.
Bell RL, Lumeng ZA, Murphy L, Mc JM, Bride WJ (2006). The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addict Biol 11: 270–288.
Berges BK, Wolfe JH, Fraser NW (2007). Transduction of brain by herpes simplex virus vectors. Mol Ther 15: 20–29.
Blednov YA, Benavidez JM, Geil C, Perra S, Morikawa H, Harris RA (2011). Activation of inflammatory signaling by lipopolysaccharide produces a prolonged increase of voluntary alcohol intake in mice. Brain Behav Immun 25 (Suppl 1): S92–S105.
Blednov YA, Ponomarev I, Geil C, Bergeson S, Koob GF, Harris RA (2012). Neuroimmune regulation of alcohol consumption: behavioral validation of genes obtained from genomic studies. Addict Biol 17: 108–120.
Bosmann M, Russkamp NF, Ward PA (2012). Fingerprinting of the TLR4-induced acute inflammatory response. Exp Mol Pathol 93: 319–323.
Chu K, Koob GF, Cole M, Zorrilla EP, Roberts AJ (2007). Dependence-induced increases in ethanol self-administration in mice are blocked by the CRF1 receptor antagonist antalarmin and by CRF1 receptor knockout. Pharmacol Biochem Behav 86: 813–821.
Cohen M, Braun E, Tsalenchuck Y, Panet A, Steiner I Restrictions that control herpes simplex virus type 1 infection in mouse brain ex vivo. J Gen Virol, (2011) 92 (Pt 10): 2383–2393.
Crews FT, Zou J, Qin L (2011). Induction of innate immune genes in brain create the neurobiology of addiction. Brain Behav Immun 25 (Suppl. 1): S4–S12.
Crews FT, Qin L, Sheedy D, Vetreno RP, Zou J (2013). High mobility group box 1/Toll-like receptor danger signaling increases brain neuroimmune activation in alcohol dependence. Biol Psychiatry 73: 602–612.
Crews FT, Vetreno RP (2014). Neuroimmune basis of alcoholic brain damage. Int Rev Neurobiol 118: 315–357.
de Silva S, Bowers WJ Targeting the central nervous system with herpes simplex virus/Sleeping Beauty hybrid amplicon vectors. Curr Gene Ther, (2011) 11: 332–340.
Edenberg HJ (2012). Genes contributing to the development of alcoholism: an overview. Alcohol Res 34: 336–338.
Fernandez-Lizarbe S, Pascual M, Guerri C (2009). Critical role of TLR4 response in the activation of microglia induced by ethanol. J Immunol 183: 4733–4744.
Fiandaca MS, Bankiewicz KS, Federoff HJ (2012). Gene therapy for the treatment of Parkinson's disease: the nature of the biologics expands the future indications. Pharmaceuticals (Basel) 5: 553–590.
Frank MG, Miguel ZD, Watkins LR, Maier SF (2010). Prior exposure to glucocorticoids sensitizes the neuroinflammatory and peripheral inflammatory responses to E. coli lipopolysaccharide. Brain Behav Immun 24: 19–30.
Gifkins A, Greba Q, Kokkinidis L (2002). Ventral tegmental area dopamine neurons mediate the shock sensitization of acoustic startle: a potential site of action for benzodiazepine anxiolytics. Behav Neurosci 116: 785–794.
Goldman D, Oroszi G, Ducci F (2005). The genetics of addictions: uncovering the genes. Nat Rev Genet 6: 521–532.
Guyon A, Skrzydelski D, De Giry I, Rovère C, Conductier G, Trocello JM et al (2009). Long term exposure to the chemokine CCL2 activates the nigrostriatal dopamine system: a novel mechanism for the control of dopamine release. Neuroscience 162: 1072–1080.
Harris RA, Trudell JR, Mihic SJ (2008). Ethanol’s molecular targets. Sci Signal 1: re7.
He J, Crews FT (2008). Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp Neurol 210: 349–358.
Heilig M, Egli M, Crabbe JC, Becker HC (2010). Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addict Biol 15: 169–184.
Heilig M, Koob GF (2007). A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci 30: 399–406.
Hines DJ, Choi HB, Hines RM, Phillips AG, MacVicar BA (2013). Prevention of LPS-induced microglia activation, cytokine production and sickness behavior with TLR4 receptor interfering peptides. PLoS ONE 8: e60388.
Huang MM, Overstreet DH, Knapp DJ, Angel R, Wills TA, Navarro M et al (2010). Corticotropin-releasing factor (CRF) sensitization of ethanol withdrawal-induced anxiety-like behavior is brain site specific and mediated by CRF-1 receptors: relation to stress-induced sensitization. J Pharmacol Exp Ther 332: 298–307.
Jennison KM (2004). The short-term effects and unintended long-term consequences of binge drinking in college: a 10-year follow-up study. Am J Drug Alcohol Abuse 30: 659–684.
Kaufmann WA, Humpel C, Alheid GF, Marksteiner J (2003). Compartmentation of alpha 1 and alpha 2 GABA(A) receptor subunits within rat extended amygdala: implications for benzodiazepine action. Brain Res 964: 91–99.
Kalivas P (2002). Neurocircuitry of addiction. Neuropyschopharmacology: 5th Generation of Progress In: Davis K, Charney D, Coyle JT, Nemeroff C, (eds), American College of Neuropsychopharmacology pp: Brentwood, TN, 1357–1366.
Koob GF (2009). Brain stress systems in the amygdala and addiction. Brain Res 1293: 61–75.
Koob GF, Volkow ND (2010). Neurocircuitry of addiction. Neuropsychopharmacology 35: 217–238.
Koob GF, Le Moal M (2005). Plasticity of reward neurocircuitry and the 'dark side' of drug addiction. Nat Neurosci 8: 1442–1444.
Laing JM, Smith CC, Aurelian L (2010). Multi-targeted neuroprotection by the HSV-2 gene ICP10PK includes robust bystander activity through PI3-K/Akt and/or MEK/ERK-dependent neuronal release of vascular endothelial growth factor and fractalkine. J Neurochem 112: 662–676.
Lavezzi AM, Corna MF, Matturri L (2013). Neuronal nuclear antigen (NeuN): a useful marker of neuronal immaturity in sudden unexplained perinatal death. J Neurol Sci 329: 45–50.
Liu J, Yang AR, Kelly T, Puche A, Esoga C, June HL Jr et al (2011). Binge alcohol drinking is associated with GABAA alpha2-regulated Toll-like receptor 4 (TLR4) expression in the central amygdala. Proc Natl Acad Sci USA 108: 4465–4470.
Liu Z, Jiang Y, Li Y, Wang J, Fan L, Scott MJ et al (2013). TLR4 Signaling augments monocyte chemotaxis by regulating G protein-coupled receptor kinase 2 translocation. J Immunol 191: 857–864.
Lowery-Gionta EG, Navarro M, Li C, Pleil KE, Rinker JA, Cox BR et al (2012). Corticotropin releasing factor signaling in the central amygdala is recruited during binge-like ethanol consumption in C57BL/6J mice. J Neurosci 32: 3405–3413.
Manservigi R, Argnani R, Marconi P (2010). HSV recombinant vectors for gene therapy. Open Virol J. 4: 123–156.
Naimi TS, Brewer RD, Mokdad A, Denny C, Serdula MK, Marks JS (2003). Binge drinking among US adults. JAMA 289: 70–75.
National Institute on Alcohol Abuse and Alcoholism (2004). NIAAA Council approves definition of binge drinking. NIAAA Newslett 04–5346.
Okun E, Griffioen KJ, Mattson MP (2011). Toll-like receptor signaling in neural plasticity and disease. Trends Neurosci 34: 269–281.
Pascual M, Baliño P, Alfonso-Loeches S, Aragón CM, Guerri C (2011). Impact of TLR4 on behavioral and cognitive dysfunctions associated with alcohol-induced neuroinflammatory damage. Brain Behav Immun 25 (Suppl 1): S80–S91.
Pascual O, Ben Achour S, Rostaing P, Triller A, Bessis A (2012). Microglia activation triggers astrocyte-mediated modulation of excitatory neurotransmission. Proc Natl Acad Sci USA 109: E197–E205.
Paxinos G, Watson C (1998) The Rat Brain in Stereotaxic Coordinates 4th edn Academic Press: San Diego.
Perkins D, Gyure KA, Pereira EF, Aurelian L (2003). Herpes simplex virus type 1-induced encephalitis has an apoptotic component associated with activation of c-Jun N-terminal kinase. J Neurovirol 9: 101–111.
Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G (2000). GABA(A) receptors: Immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience 101: 815–850.
Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS et al (2007). Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 55: 453–462.
Reinert LS, Harder L, Holm CK, Iversen MB, Horan KA, Dagnæs-Hansen F et al (2012). TLR3 deficiency renders astrocytes permissive to herpes simplex virus infection and facilitates establishment of CNS infection in mice. J Clin Invest 122: 1368–1376.
Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M et al (2010). Corticotropin releasing factor-induced amygdala gamma-aminobutyric acid release plays a key role in alcohol dependence. Biol Psychiatry 67: 831–839.
Saeki Y (2006). Stable CNS gene delivery with Sleeping Beauty armed with a high-capacity HSV virion. Mol Ther 13: 457–458.
Saydam O, Glauser DL, Heid I, Turkeri G, Hilbe M, Jacobs AH et al (2005). Herpes simplex virus 1 amplicon vector-mediated siRNA targeting epidermal growth factor receptor inhibits growth of human glioma cells in vivo. Mol Ther 12: 803–812.
Sommer WH, Rimondini R, Hansson AC, Hipskind PA, Gehlert DR, Barr CS et al (2008). Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala crhr1 expression following a history of dependence. Biol Psychiatry 63: 139–145.
Suzuki M, Antonio Chiocca E, Saeki Y (2008). Stable transgene expression from HSV amplicon vectors in the brain: potential involvement of immunoregulatory signals. Mol Ther 16: 1727–1736.
Tang SC, Arumugam TV, Xu X, Cheng A, Mughal MR, Jo DG et al (2007). Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci USA 104: 13798–13803.
Taylor SW, Smith RM, Pari G, Wobeser W, Rossiter JP, Jackson AC (2005). Herpes simplex encephalitis. Can J Neurol Sci 32: 246–247.
Wakida N, Kiguchi N, Saika F, Nishiue H, Kobayashi Y, Kishioka S (2014). CC-chemokine ligand 2 facilitates conditioned place preference to methamphetamine through the activation of dopamine systems. J Pharmacol Sci 125: 68–73.
Yang AR, Liu J, Yi HS, Warnock KT, Wang M, June HL Jr et al (2011). Binge drinking: in search of its molecular target via the GABA(A) receptor. Front Neurosci 5: 123.
Zhang GR, Zhao H, Abdul-Muneer PM, Cao H, Li X, Geller AI (2015). Neurons can be labeled with unique hues by helper virus-free HSV-1 vectors expressing Brainbow. J Neurosci Methods 240: 77–88.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
June, H., Liu, J., Warnock, K. et al. CRF-Amplified Neuronal TLR4/MCP-1 Signaling Regulates Alcohol Self-Administration. Neuropsychopharmacol 40, 1549–1559 (2015). https://doi.org/10.1038/npp.2015.4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2015.4
- Springer Nature Switzerland AG
This article is cited by
-
Neurosteroid allopregnanolone (3α,5α-THP) inhibits inflammatory signals induced by activated MyD88-dependent toll-like receptors
Translational Psychiatry (2021)
-
Potential of Glial Cell Modulators in the Management of Substance Use Disorders
CNS Drugs (2020)
-
Glial gene networks associated with alcohol dependence
Scientific Reports (2019)
-
Endogenous Neurosteroid (3α,5α)3-Hydroxypregnan-20-one Inhibits Toll-like-4 Receptor Activation and Pro-inflammatory Signaling in Macrophages and Brain
Scientific Reports (2019)
-
GABAAR α2-activated neuroimmune signal controls binge drinking and impulsivity through regulation of the CCL2/CX3CL1 balance
Psychopharmacology (2019)