Abstract
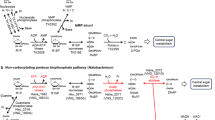
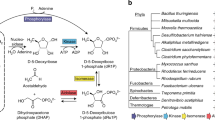
Owing to the absence of the pentose phosphate pathway, the degradation pathway for the ribose moieties of nucleosides is unknown in Archaea. Here, in the archaeon Thermococcus kodakarensis, we identified a metabolic network that links the pentose moieties of nucleosides or nucleotides to central carbon metabolism. The network consists of three nucleoside phosphorylases, an ADP-dependent ribose-1-phosphate kinase and two enzymes of a previously identified NMP degradation pathway, ribose-1,5-bisphosphate isomerase and type III ribulose-1,5-bisphosphate carboxylase/oxygenase. Ribose 1,5-bisphosphate and ribulose 1,5-bisphosphate are intermediates of this pathway, which is thus designated the pentose bisphosphate pathway.

Similar content being viewed by others
References
Wamelink, M.M., Struys, E.A. & Jakobs, C. The biochemistry, metabolism and inherited defects of the pentose phosphate pathway: a review. J. Inherit. Metab. Dis. 31, 703–717 (2008).
Riganti, C., Gazzano, E., Polimeni, M., Aldieri, E. & Ghigo, D. The pentose phosphate pathway: an antioxidant defense and a crossroad in tumor cell fate. Free Radic. Biol. Med. 53, 421–436 (2012).
Soderberg, T. Biosynthesis of ribose-5-phosphate and erythrose-4-phosphate in archaea: a phylogenetic analysis of archaeal genomes. Archaea 1, 347–352 (2005).
Orita, I. et al. The ribulose monophosphate pathway substitutes for the missing pentose phosphate pathway in the archaeon. Thermococcus kodakaraensis . J. Bacteriol. 188, 4698–4704 (2006).
Ezaki, S., Maeda, N., Kishimoto, T., Atomi, H. & Imanaka, T. Presence of a structurally novel type ribulose-bisphosphate carboxylase/oxygenase in the hyperthermophilic archaeon, Pyrococcus kodakaraensis KOD1. J. Biol. Chem. 274, 5078–5082 (1999).
Finn, M.W. & Tabita, F.R. Synthesis of catalytically active form III ribulose 1,5-bisphosphate carboxylase/oxygenase in archaea. J. Bacteriol. 185, 3049–3059 (2003).
Watson, G.M., Yu, J.P. & Tabita, F.R. Unusual ribulose 1,5-bisphosphate carboxylase/oxygenase of anoxic Archaea. J. Bacteriol. 181, 1569–1575 (1999).
Kitano, K. et al. Crystal structure of a novel-type archaeal rubisco with pentagonal symmetry. Structure 9, 473–481 (2001).
Alonso, H., Blayney, M.J., Beck, J.L. & Whitney, S.M. Substrate-induced assembly of Methanococcoides burtonii D-ribulose-1,5-bisphosphate carboxylase/oxygenase dimers into decamers. J. Biol. Chem. 284, 33876–33882 (2009).
Sato, T., Atomi, H. & Imanaka, T. Archaeal type III RuBisCOs function in a pathway for AMP metabolism. Science 315, 1003–1006 (2007).
Nakamura, A. et al. Dynamic, ligand-dependent conformational change triggers reaction of ribose-1,5-bisphosphate isomerase from Thermococcus kodakarensis KOD1. J. Biol. Chem. 287, 20784–20796 (2012).
Nishitani, Y. et al. Structure analysis of archaeal AMP phosphorylase reveals two unique modes of dimerization. J. Mol. Biol. 425, 2709–2721 (2013).
Aono, R. et al. Enzymatic characterization of AMP phosphorylase and ribose-1,5-bisphosphate isomerase functioning in an archaeal AMP metabolic pathway. J. Bacteriol. 194, 6847–6855 (2012).
Hansen, T., Arnfors, L., Ladenstein, R. & Schönheit, P. The phosphofructokinase-B (MJ0406) from Methanocaldococcus jannaschii represents a nucleoside kinase with a broad substrate specificity. Extremophiles 11, 105–114 (2007).
Elkin, S.R., Kumar, A., Price, C.W. & Columbus, L. A broad specificity nucleoside kinase from Thermoplasma acidophilum. Proteins 81, 568–582 (2013).
Park, J. & Gupta, R.S. Adenosine kinase and ribokinase—the RK family of proteins. Cell. Mol. Life Sci. 65, 2875–2896 (2008).
Kengen, S.W. et al. Evidence for the operation of a novel Embden-Meyerhof pathway that involves ADP-dependent kinases during sugar fermentation by Pyrococcus furiosus. J. Biol. Chem. 269, 17537–17541 (1994).
Kengen, S.W., Tuininga, J.E., de Bok, F.A., Stams, A.J. & de Vos, W.M. Purification and characterization of a novel ADP-dependent glucokinase from the hyperthermophilic archaeon Pyrococcus furiosus. J. Biol. Chem. 270, 30453–30457 (1995).
Tuininga, J.E. et al. Molecular and biochemical characterization of the ADP-dependent phosphofructokinase from the hyperthermophilic archaeon Pyrococcus furiosus. J. Biol. Chem. 274, 21023–21028 (1999).
Sakuraba, H. et al. ADP-dependent glucokinase/phosphofructokinase, a novel bifunctional enzyme from the hyperthermophilic archaeon Methanococcus jannaschii. J. Biol. Chem. 277, 12495–12498 (2002).
Guixé, V. & Merino, F. The ADP-dependent sugar kinase family: kinetic and evolutionary aspects. IUBMB Life 61, 753–761 (2009).
Merino, F., Rivas-Pardo, J.A., Caniuguir, A., García, I. & Guixé, V. Catalytic and regulatory roles of divalent metal cations on the phosphoryl-transfer mechanism of ADP-dependent sugar kinases from hyperthermophilic archaea. Biochimie 94, 516–524 (2012).
Castro-Fernandez, V., Bravo-Moraga, F., Herrera-Morande, A. & Guixe, V. Bifunctional ADP-dependent phosphofructokinase/glucokinase activity in the order Methanococcales—biochemical characterization of the mesophilic enzyme from Methanococcus maripaludis. FEBS J. 281, 2017–2029 (2014).
Ronimus, R.S. & Morgan, H.W. Cloning and biochemical characterization of a novel mouse ADP-dependent glucokinase. Biochem. Biophys. Res. Commun. 315, 652–658 (2004).
Marbaix, A.Y. et al. Extremely conserved ATP- or ADP-dependent enzymatic system for nicotinamide nucleotide repair. J. Biol. Chem. 286, 41246–41252 (2011).
Kengen, S.W. et al. ADP-dependent glucokinase and phosphofructokinase from Pyrococcus furiosus. Methods Enzymol. 331, 41–53 (2001).
Boyer, P.D. & Robbins, E.A. Determination of the equilibrium of the hexokinase reaction and the free energy of hydrolysis of adenosine triphosphate. J. Biol. Chem. 224, 121–135 (1957).
Minakami, S. & Yoshikawa, H. Thermodynamic considerations on erythrocyte glycolysis. Biochem. Biophys. Res. Commun. 18, 345–349 (1965).
Cacciapuoti, G., Bertoldo, C., Brio, A., Zappia, V. & Porcelli, M. Purification and characterization of 5′-methylthioadenosine phosphorylase from the hyperthermophilic archaeon Pyrococcus furiosus: substrate specificity and primary structure analysis. Extremophiles 7, 159–168 (2003).
Cacciapuoti, G. et al. Biochemical and structural characterization of mammalian-like purine nucleoside phosphorylase from the Archaeon Pyrococcus furiosus. FEBS J. 274, 2482–2495 (2007).
Cacciapuoti, G., Porcelli, M., Bertoldo, C., De Rosa, M. & Zappia, V. Purification and characterization of extremely thermophilic and thermostable 5-methylthioadenosi 5(-methylthioadenosine phosphorylase from the archaeon Sulfolobus solfataricus. Purine nucleoside phosphorylase activity and evidence for intersubunit disulfide bonds. J. Biol. Chem. 269, 24762–24769 (1994).
Cacciapuoti, G. et al. A novel hyperthermostable 5-deoxy-5-methylthioadenosi phosphorylase from the archaeon Sulfolobus solfataricus. FEBS J. 272, 1886–1899 (2005).
Fukui, T. et al. Complete genome sequence of the hyperthermophilic archaeon Thermococcus kodakaraensis KOD1 and comparison with Pyrococcus genomes. Genome Res. 15, 352–363 (2005).
Ipata, P.L., Camici, M., Micheli, V. & Tozz, M.G. Metabolic network of nucleosides in the brain. Curr. Top. Med. Chem. 11, 909–922 (2011).
Tozzi, M.G., Camici, M., Mascia, L., Sgarrella, F. & Ipata, P.L. Pentose phosphates in nucleoside interconversion and catabolism. FEBS J. 273, 1089–1101 (2006).
Camici, M., Tozzi, M.G. & Ipata, P.L. Methods for the determination of intracellular levels of ribose phosphates. J. Biochem. Biophys. Methods 68, 145–154 (2006).
Walther, T. et al. The PGM3 gene encodes the major phosphoribomutase in the yeast Saccharomyces cerevisiae. FEBS Lett. 586, 4114–4118 (2012).
Sambrook, J. & Russel, D. Molecular Cloning: a Laboratory Manual (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 2001).
Atomi, H., Fukui, T., Kanai, T., Morikawa, M. & Imanaka, T. Description of Thermococcus kodakaraensis sp. nov., a well studied hyperthermophilic archaeon previously reported as Pyrococcus sp. KOD1. Archaea 1, 263–267 (2004).
Robb, F.T. & Place, A.R. Archaea: a Laboratory Manual (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 1995).
Kanai, T. et al. A global transcriptional regulator in Thermococcus kodakaraensis controls the expression levels of both glycolytic and gluconeogenic enzyme–encoding genes. J. Biol. Chem. 282, 33659–33670 (2007).
Kanai, T., Takedomi, S., Fujiwara, S., Atomi, H. & Imanaka, T. Identification of the Phr-dependent heat shock regulon in the hyperthermophilic archaeon, Thermococcus kodakaraensis. J. Biochem. 147, 361–370 (2010).
Sato, T. et al. Genetic evidence identifying the true gluconeogenic fructose-1,6-bisphosphatase in Thermococcus kodakaraensis and other hyperthermophiles. J. Bacteriol. 186, 5799–5807 (2004).
Acknowledgements
This study was funded by the Core Research for Evolutional Science and Technology program of the Japan Science and Technology Agency to H.A. within the research area 'Creation of Basic Technology for Improved Bioenergy Production through Functional Analysis and Regulation of Algae and Other Aquatic Microorganisms'. R.A. is a Research Fellow of the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Contributions
H.A., T.I., T.S. and R.A. designed the work; R.A. carried out the experiments; R.A., T.S. and H.A. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Tables 1–7 and Supplementary Figures 1–16. (PDF 7128 kb)
Rights and permissions
About this article
Cite this article
Aono, R., Sato, T., Imanaka, T. et al. A pentose bisphosphate pathway for nucleoside degradation in Archaea. Nat Chem Biol 11, 355–360 (2015). https://doi.org/10.1038/nchembio.1786
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1786
- Springer Nature America, Inc.
This article is cited by
-
Microbial life in 25-m-deep boreholes in ancient permafrost illuminated by metagenomics
Environmental Microbiome (2023)
-
The Fate of Duplicated Enzymes in Prokaryotes: The Case of Isomerases
Journal of Molecular Evolution (2023)
-
Functional differentiation determines the molecular basis of the symbiotic lifestyle of Ca. Nanohaloarchaeota
Microbiome (2022)
-
A non-carboxylating pentose bisphosphate pathway in halophilic archaea
Communications Biology (2022)
-
Diverse ecophysiological adaptations of subsurface Thaumarchaeota in floodplain sediments revealed through genome-resolved metagenomics
The ISME Journal (2022)