Abstract
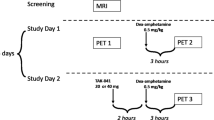
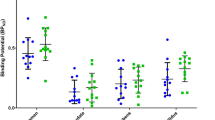
Recent genetic, molecular and post-mortem studies suggest impaired dopamine (DA)-D2 receptor (D2R) trafficking in patients with schizophrenia (SZ). Imaging and preclinical studies have shown agonist-induced D2R internalization can be imaged with positron emission tomography (PET) using D2R radiotracers combined with psychostimulant challenge. This is feasible if radiotracer binding is measured when postchallenge DA levels have returned to baseline, following the initial competition phase between DA and radiotracer for binding to D2R. Here we used ‘late-phase' imaging after challenge to test the hypothesis that impaired D2R internalization in SZ leads to blunted late-phase displacement, or a faster return to baseline, in patients compared with healthy controls (HCs). We imaged 10 patients with SZ and 9 HCs with PET and [11C]raclopride at baseline and two times (3–5 and 6–10 h) following 0.5 mg kg−1 dextroamphetamine. We measured binding potential relative to non-displaceable compartment (BPND) and derived percent reduction from baseline (ΔBPND) for each postamphetamine scan. To test the hypothesis that time course of return of striatal BPND to baseline differed between SZ and HCs, we implemented a linear model with ΔBPND as dependent variable, time after amphetamine as repeated measure and time after amphetamine and diagnostic group as fixed effects. Neither diagnostic group nor interaction of diagnostic group-by-time after amphetamine significantly affected striatal ΔBPND (F=1.38, P=0.26; F=0.51, P=0.61). These results show similar pattern of return of BPND to baseline as a function of time in patients with SZ and HC, suggesting that striatal D2R internalization as measured by our imaging paradigm is normal in patients with SZ.



Similar content being viewed by others
References
Laruelle M, Abi-Dargham A, van Dyck CH, Gil R, D'Souza CD, Erdos J et al. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci USA 1996; 93: 9235–9240.
Abi-Dargham A, Gil R, Krystal J, Baldwin RM, Seibyl JP, Bowers M et al. Increased striatal dopamine transmission in schizophrenia: confirmation in a second cohort. Am J Psychiatry 1998; 155: 761–767.
Abi-Dargham A, van de Giessen E, Slifstein M, Kegeles LS, Laruelle M . Baseline and amphetamine-stimulated dopamine activity are related in drug-naive schizophrenic subjects. Biol Psychiatry 2009; 65: 1091–1093.
Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, de Bartolomeis A et al. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc Natl Acad Sci USA 1997; 94: 2569–2574.
Laruelle M, Abi-Dargham A, Gil R, Kegeles L, Innis R . Increased dopamine transmission in schizophrenia: relationship to illness phases. Biol Psychiatry 1999; 46: 56–72.
Skinbjerg M, Liow JS, Seneca N, Hong J, Lu S, Thorsell A et al. D2 dopamine receptor internalization prolongs the decrease of radioligand binding after amphetamine: a PET study in a receptor internalization-deficient mouse model. Neuroimage 2010; 50: 1402–1407.
Houston GC, Hume SP, Hirani E, Goggi JL, Grasby PM . Temporal characterisation of amphetamine-induced dopamine release assessed with [11C]raclopride in anaesthetised rodents. Synapse 2004; 51: 206–212.
Ginovart N, Wilson AA, Houle S, Kapur S . Amphetamine pretreatment induces a change in both D2-Receptor density and apparent affinity: a [11C]raclopride positron emission tomography study in cats. Biol Psychiatry 2004; 55: 1188–1194.
Narendran R, Slifstein M, Hwang DR, Hwang Y, Scher E, Reeder S et al. Amphetamine-induced dopamine release: duration of action as assessed with the D2/3 receptor agonist radiotracer (−)-N-[(11)C]propyl-norapomorphine ([11C]NPA) in an anesthetized nonhuman primate. Synapse 2007; 61: 106–109.
Narendran R, Hwang DR, Slifstein M, Hwang Y, Huang Y, Ekelund J et al. Measurement of the proportion of D2 receptors configured in state of high affinity for agonists in vivo: a positron emission tomography study using [11C]N-propyl-norapomorphine and [11C]raclopride in baboons. J Pharmacol Exp Ther 2005; 315: 80–90.
Cardenas L, Houle S, Kapur S, Busto UE . Oral d-amphetamine causes prolonged displacement of [11C]raclopride as measured by PET. Synapse 2004; 51: 27–31.
Laruelle M . Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab 2000; 20: 423–451.
Laruelle M . The role of endogenous sensitization in the pathophysiology of schizophrenia: implications from recent brain imaging studies. Brain Res Brain Res Rev 2000; 31: 371–384.
Ginovart N . Imaging the dopamine system with in vivo [11C]raclopride displacement studies: understanding the true mechanism. Mol Imaging Biol 2005; 7: 45–52.
Guo N, Guo W, Kralikova M, Jiang M, Schieren I, Narendran R et al. Impact of D2 receptor internalization on binding affinity of neuroimaging radiotracers. Neuropsychopharmacology 2010; 35: 806–817.
Quelch DR, Withey SL, Nutt DJ, Tyacke RJ, Parker CA . The influence of different cellular environments on PET radioligand binding: an application to D2/3-dopamine receptor imaging. Neuropharmacology 2014; 85: 305–313.
Chugani DC, Ackermann RF, Phelps ME . In vivo [3H]spiperone binding: evidence for accumulation in corpus striatum by agonist-mediated receptor internalization. J Cereb Blood Flow Metab 1988; 8: 291–303.
Skinbjerg M, Ariano MA, Thorsell A, Heilig M, Halldin C, Innis RB et al. Arrestin3 mediates D(2) dopamine receptor internalization. Synapse 2009; 63: 621–624.
Laruelle M, Guo N, Guo W, Jiang M, Schieren I, Abi-Dargham A et al. Impact of dopamine D2 receptor internalization on binding parameters of D2 PET radiotracers. Neuroimage 2008; 41: T36–T36.
Narendran R, Hwang DR, Slifstein M, Talbot PS, Erritzoe D, Huang Y et al. In vivo vulnerability to competition by endogenous dopamine: comparison of the D2 receptor agonist radiotracer (−)-N-[11C]propyl-norapomorphine ([11C]NPA) with the D2 receptor antagonist radiotracer [11C]-raclopride. Synapse 2004; 52: 188–208.
Hwang DR, Narendran R, Laruelle M . Positron-labeled dopamine agonists for probing the high affinity states of dopamine subtype 2 receptors. Bioconjug Chem 2005; 16: 27–31.
Papaleo F, Weinberger DR . Dysbindin and Schizophrenia: it's dopamine and glutamate all over again. Biol Psychiatry 2011; 69: 2–4.
Papaleo F, Yang F, Garcia S, Chen J, Lu B, Crawley JN et al. Dysbindin-1 modulates prefrontal cortical activity and schizophrenia-like behaviors via dopamine/D2 pathways. Mol Psychiatry 2012; 17: 85–98.
Schubert KO, Focking M, Prehn JH, Cotter DR . Hypothesis review: are clathrin-mediated endocytosis and clathrin-dependent membrane and protein trafficking core pathophysiological processes in schizophrenia and bipolar disorder? Mol Psychiatry 2012; 17: 669–681.
Beaulieu JM . A role for Akt and glycogen synthase kinase-3 as integrators of dopamine and serotonin neurotransmission in mental health. J Psychiatry Neurosci 2012; 37: 7–16.
Ji Y, Yang F, Papaleo F, Wang HX, Gao WJ, Weinberger DR et al. Role of dysbindin in dopamine receptor trafficking and cortical GABA function. Proc Natl Acad Sci USA 2009; 106: 19593–19598.
Su P, Li S, Chen S, Lipina TV, Wang M, Lai TK et al. A dopamine D2 receptor–DISC1 protein complex may contribute to antipsychotic-like effects. Neuron 2014; 84: 1302–1316.
Appelbaum PS, Grisso T . The MacArthur Treatment Competence Study. I: Mental illness and competence to consent to treatment. Law Hum Behav 1995; 19: 105–126.
Nurnberger JI Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J et al. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry 1994; 51: 849–859; discussion 844–863.
Hollingshead AB . Four factor index of social status. Working paper published by the author.: New Haven, Connecticut, 1975.
Mason OJ, Morgan CJ, Stefanovic A, Curran HV . The psychotomimetic states inventory (PSI): measuring psychotic-type experiences from ketamine and cannabis. Schizophr Res 2008; 103: 138–142.
Kay SR, Fiszbein A, Opler LA . The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13: 261–276.
Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR et al. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab 2001; 21: 1034–1057.
Lammertsma AA, Hume SP . Simplified reference tissue model for PET receptor studies. Neuroimage 1996; 4 (Part 1): 153–158.
Thompson JL, Urban N, Slifstein M, Xu X, Kegeles LS, Girgis RR et al. Striatal dopamine release in schizophrenia comorbid with substance dependence. Mol Psychiatry 2013; 18: 909–915.
Allen JA, Yost JM, Setola V, Chen X, Sassano MF, Chen M et al. Discovery of beta-arrestin-biased dopamine D2 ligands for probing signal transduction pathways essential for antipsychotic efficacy. Proc Natl Acad Sci USA 2011; 108: 18488–18493.
Tan HY, Chen AG, Chen Q, Browne LB, Verchinski B, Kolachana B et al. Epistatic interactions of AKT1 on human medial temporal lobe biology and pharmacogenetic implications. Mol Psychiatry 2012; 17: 1007–1016.
Kotowski SJ, Hopf FW, Seif T, Bonci A, von Zastrow M . Endocytosis promotes rapid dopaminergic signaling. Neuron 2011; 71: 278–290.
Eichel K, Jullie D, von Zastrow M . Beta-arrestin drives MAP kinase signalling from clathrin-coated structures after GPCR dissociation. Nat Cell Biol 2016; 18: 303–310.
Irannejad R, Kotowski SJ, von Zastrow M . Investigating signaling consequences of GPCR trafficking in the endocytic pathway. Methods Enzymol 2014; 535: 403–418.
Laruelle M, Abi-Dargham A . Dopamine as the wind of the psychotic fire: new evidence from brain imaging studies. J Psychopharmacol 1999; 13: 358–371.
Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci USA 2000; 97: 8104–8109.
Smith CT, Dang LC, Cowan RL, Kessler RM, Zald DH . Variability in paralimbic dopamine signaling correlates with subjective responses to d-amphetamine. Neuropharmacology 2016; 108: 394–402.
Smith CT, Weafer J, Cowan RL, Kessler RM, Palmer AA, de Wit H et al. Individual differences in timing of peak positive subjective responses to d-amphetamine: relationship to pharmacokinetics and physiology. J Psychopharmacol 2016; 30: 330–343.
Angrist B, Corwin J, Bartlik B, Cooper T . Early pharmacokinetics and clinical effects of oral d-amphetamine in normal subjects. Biol Psychiatry 1987; 22: 1357–1368.
Slifstein M, Kegeles LS, Xu X, Thompson JL, Urban N, Castrillon J et al. Striatal and extrastriatal dopamine release measured with PET and [(18)F] fallypride. Synapse 2010; 64: 350–362.
Bray NJ, Preece A, Williams NM, Moskvina V, Buckland PR, Owen MJ et al. Haplotypes at the dystrobrevin binding protein 1 (DTNBP1) gene locus mediate risk for schizophrenia through reduced DTNBP1 expression. Hum Mol Genet 2005; 14: 1947–1954.
Acknowledgements
Funding for this study was provided by Grant R21 MH099509 from the National Institute of Mental Health (NIMH) to Dr Abi-Dargham. Dr Weinstein was supported by a T32 Grant from NIMH (MH018870). Dr van de Giessen was supported by a Rubicon grant from the Netherlands Organization for Scientific Research (825.12.009). We would also like to acknowledge the contributions of Dr Skinbjerg in early stages of project development, Rassil Ghazzoui in participant recruitment, Seth Baker in data collection, Nadia-Tina Dandan in data entry and Dr Cassidy in early stages of data interpretation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
About this article
Cite this article
Weinstein, J., van de Giessen, E., Rosengard, R. et al. PET imaging of dopamine-D2 receptor internalization in schizophrenia. Mol Psychiatry 23, 1506–1511 (2018). https://doi.org/10.1038/mp.2017.107
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2017.107
- Springer Nature Limited
This article is cited by
-
Schizophrenia: from neurochemistry to circuits, symptoms and treatments
Nature Reviews Neurology (2024)
-
Impaired brain glucose metabolism and presynaptic dopaminergic functioning in a mouse model of schizophrenia
EJNMMI Research (2020)
-
The relationship between childhood trauma, dopamine release and dexamphetamine-induced positive psychotic symptoms: a [11C]-(+)-PHNO PET study
Translational Psychiatry (2019)