Abstract
Meconium aspiration syndrome (MAS), a common cause of respiratory failure in neonates, is associated with high mortality and morbidity. The objectives of this review were to evaluate the effects of administration of (a) surfactant—either as lung lavage (SLL) or bolus surfactant (BS) and (b) antibiotics on mortality and severe morbidities in neonates with MAS. We searched the following databases: MEDLINE via PubMed, Cochrane CENTRAL, WHOLIS and CABI using sensitive search strategies. We included eight studies on use of surfactant and three studies on use of antibiotics. Neither SLL nor BS reduced the risk of mortality in neonates with MAS (relative risk (RR) 0.38, 95% confidence interval (CI) 0.09 to 1.57; and RR 0.80, 95% CI 0.39 to 1.66, respectively). Both SLL and BS reduced the duration of hospital stay (mean difference −2.0, 95% CI −3.66 to −0.34; and RR −4.68, 95% CI −7.11 to −2.24 days, respectively) and duration of mechanical ventilation (mean difference −1.31, 95% CI −1.91 to −0.72; and mean difference 5.4, 95% CI −9.76 to −1.03 days). Neonates who received BS needed extracorporeal membrane oxygenation (ECMO) less often than the controls (RR 0.64, 95% CI 0.46 to 0.91). Use of antibiotics for MAS did not result in significant reduction in the risk of mortality, sepsis or duration of hospital stay. Surfactant administration either as SLL or BS for MAS was found to reduce the duration of mechanical ventilation and hospital stay; BS also reduced the need for ECMO. Administration of antibiotics did not show any significant clinical benefits in neonates with MAS and no evidence of sepsis. Given the limited number of studies and small number of neonates enrolled, there is an urgent need to generate more evidence on the efficacy and cost-effectiveness of these two treatment modalities before recommending them in routine clinical practice.
Similar content being viewed by others
Introduction
Meconium aspiration syndrome (MAS) is defined as respiratory distress in a neonate born through meconium-stained amniotic fluid (MSAF) having characteristic radiological changes whose symptoms cannot be otherwise explained.1, 2 MAS accounts for about 10% of cases of respiratory failure in all neonates, and is associated with significant morbidity and high mortality (up to 39%).2, 3 Although most neonates born through MSAF do not develop MAS, some—particularly those who are non-vigorous and require resuscitation at birth—are more prone to have the condition. Given the lack of evidence for efficacy of most interventions performed before, during or after birth, such as amnioinfusion, intrapartum suctioning and postpartum suctioning of the meconium in non-vigorous neonates, it is difficult to know how to prevent the occurrence of MAS in at-risk neonates.4, 5, 6
Currently, management of neonates with MAS involves only supportive care—oxygen therapy, assisted ventilation, inhaled nitric oxide and if available, extracorporeal membrane oxygenation (ECMO). In the absence of a specific therapy, clinicians often use a diverse range of treatment modalities, most of which are yet to be proven in randomized controlled trials (RCTs). In this article, we systematically reviewed the efficacy of the two commonly used interventions namely, surfactant administration and antibiotics. Surfactant is administered intratracheally as either a bolus dose or in dilute form to lavage the lungs in neonates with MAS. Although bolus administration is thought to replenish the endogenous surfactant inactivated by fatty acids present in the meconium, lung lavage with surfactant is believed to wash the residual meconium from the airways.2, 6 Antibiotics, on the other hand, may reduce the risk of infection in the immediate neonatal period in neonates with MAS.7
Methods
Types of studies
We included all RCTs including quasi-randomized trials that compared the effects of (1) intratracheal surfactant administration with placebo or no therapy and (2) systemic antibiotics with placebo or no antibiotics in late-preterm and term neonates with MAS.
Type of participants
All studies that enrolled late-preterm and term neonates with MAS were eligible for inclusion in this review.
Types of interventions
Intervention 1: surfactant therapy as either bolus surfactant (BS) administration or surfactant lung lavage (SLL).
Intervention 2: administration of systemic antibiotics for any duration.
We excluded studies wherein both SLL and BS were administered.8
Outcome measures and their definitions
Outcomes studied included the following: (i) in-hospital mortality defined as all-cause death during the birth hospitalization; (ii) sepsis defined as clinical features of sepsis with or without isolation of organisms from blood/cerebrospinal fluid/urine and laboratory parameters suggestive of sepsis; (iii) duration of mechanical ventilation defined as number of days—either invasive or noninvasive; (iv) duration of oxygen requirement defined as the number of days of oxygen supplementation required during the initial hospital stay; (v) duration of hospital stay defined as number of days as inpatient; (vi) proportion of neonates who required ECMO therapy; and (vii) the incidence of air leaks, such as pulmonary interstitial emphysema, pneumothorax, pneumomediastinum or pneumopericardium.
Search methods for identification of studies
We searched the following databases: MEDLINE via PubMed; Cochrane CENTRAL; WHOLIS; and CABI Global Health using the following search terms—meconium and (antibiotics or surfactant). No language restrictions were used. We also searched for ongoing/unpublished studies by hand-searching the conference proceedings of the Pediatric Academic Societies for the years 2005 to 2014.
Data extraction
Data extraction was carried out using a form designed and pretested by the authors. Two authors (CKN and KJ) independently extracted the data from the included studies, including year, setting (country, type of population, socioeconomic status, gestation and birth weight of neonates) and outcomes of interest. Disagreements in extracted data were resolved through discussion with the third author (MJS).
Statistical analysis
Data entry and meta-analysis were performed using RevMan version 5.3 (The Nordic Cochrane Centre, Copenhagen, Denmark). We analyzed the data as the proportion of neonates for categorical outcomes and mean (s.d.) or median (inter-quartile range) for continuous outcomes. We calculated relative risks (RRs) or mean differences with 95% confidence intervals (CIs) for the outcomes of interest. Heterogeneity between the studies was quantified by using a measure of the degree of inconsistency in their results (I2 statistic >60%). We evaluated the presence of publication bias by using funnel plots when an adequate number of studies was available for meta-analysis.
Results
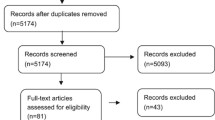
We retrieved 465 citations using the above-mentioned search strategy. After screening the title and abstracts and removal of duplicates, we identified nine and three studies, respectively, on surfactant administration and antibiotics in neonates with MAS (Figure 1). One study on surfactant lavage was presented in abstract form in a conference and could not be included in the final analysis due to lack of complete data; thus, eight studies were finally analyzed for use of surfactant in MAS.
All the studies were randomized trials that used accepted methods of randomization. Studies that used SLL were not blinded, whereas most of those that used BS were blinded. None of the three studies on antibiotic use in MAS attempted to blind the intervention, and none had any major loss to follow-up. About one-half of the studies on surfactant and all of the studies on antibiotic use in MAS were from low- and middle-income countries (LMICs).
Surfactant for MAS
Out of the eight studies on surfactant for MAS, two used SLL9, 10 and the remaining six used BS (Table 1).11, 12, 13, 14, 15, 16 Among studies that used SLL as the intervention, one study used bovine surfactant10 and the other synthetic surfactant9 for lavage. All BS studies used only natural surfactant—either bovine11, 12, 13, 15 or porcine.14, 15, 16
Because of the different nature of interventions including the dosage of surfactant, we pooled the results of studies that used SLL and studies that used BS separately.
Use of surfactant as either SLL (RR 0.38; 95% CI 0.09 to 1.57) or BS (RR 0.80; 95% CI 0.39 to 1.66) did not reduce mortality in neonates with established MAS. Both SLL and BS reduced the duration of hospital stay by 2 days (95% CI −3.7 to −0.3) and 4.7 days (95% CI −7.1 to −2.2), respectively. Similarly, neonates who received surfactant by either SLL or BS had shorter days of mechanical ventilation (Table 2). Neonates who received BS (RR 0.64; 95% CI 0.46 to 0.91) but not SLL (RR 0.26; 95% CI 0.04 to 1.82) needed ECMO less often than those in the control group. Neither method of surfactant administration reduced the duration of oxygen therapy or the incidence of air leaks (Table 3). None of the included studies reported the rates of sepsis in intervention and control groups.
Antibiotics for MAS
Antibiotics used in the studies were either penicillin and aminoglycosides or aminoglycosides alone for 3 to 7 days’ duration (Table 3). None of the outcomes of interest such as neonatal mortality, incidence of sepsis, duration of hospital stay, duration of mechanical ventilation and duration of oxygen therapy were significantly different between neonates who received systemic antibiotics and those who did not (Table 4).
Discussion
In this review we have attempted to summarize the studies on potential adjuvant therapies in the management of MAS such as antibiotics and surfactant administration. Use of intratracheal surfactant either as bolus administration or as lavage therapy in neonates with MAS reduced the duration of mechanical ventilation and duration of hospital stay. There was also a significant reduction in the need for ECMO in neonates who received BS therapy for MAS. Administration of antibiotics in MAS did not result in any beneficial outcomes. Neither intervention reduced in-hospital mortality or sepsis.
Surfactant for MAS
We found two Cochrane reviews on use of surfactant in MAS, one on SLL and the other on BS.17, 18 The Cochrane review on SLL included two studies and reported a significant reduction in the combined outcome of mortality or need for ECMO in the intervention group (RR 0.33; 95% CI 0.11 to 0.96). The risk of mortality was not reduced significantly in neonates who received lung lavage with surfactant (RR: 0.42; 95% CI 0.12 to 1.46). The results of the present review are similar to those from the Cochrane review. The latter review, however, included another study by Gadzinowski et al.,8 which used both surfactant lavage and BS in the intervention arm (this study was analyzed separately). Also, we did not examine the combined outcome of mortality and ECMO in our review.
The Cochrane review on BS included four studies and found the risk of requiring ECMO to be lower in the intervention group (RR 0.64; 95% CI 0.46 to 0.91).18 However, the risk of mortality and air leaks was comparable between the two groups. We included two additional studies from China in our review.15, 16 Inclusion of these did not change the results on neonatal mortality or need for ECMO. However, the duration of mechanical ventilation and hospital stay were found to be significantly shorter in our review compared with the Cochrane review, which reported no difference between the groups.
Lack of benefits in key outcomes such as mortality and air leaks following either mode of surfactant therapy could be related to (a) the degree of illness of the neonates and (b) timing of lavage therapy.19 Neonates included in these trials were moderately ill and received the intervention relatively late when compared with the timing in experimental studies in animals that have shown beneficial effects with surfactant lavage.20, 21
Notwithstanding the lack of benefits in key outcomes, significant benefits observed in the need for ECMO (for BS), and duration of hospital stay and mechanical ventilation (with both SLL and BS) underscore the importance of generating more evidence on surfactant use in neonates with MAS. Although the need for ECMO may not be a critical outcome in LMICs, benefits in duration of hospital stay and ventilation could reduce the cost of neonatal care. This is likely to have huge implications in LMIC settings. It should, however, be balanced against the cost of surfactant, which is often prohibitive in most neonatal units of LMICs. The cost is likely to be a greater concern in MAS than in respiratory distress syndrome (RDS)—the typical indication for surfactant replacement therapy (SRT), because the dose used is likely to be two to three times higher than that used in the latter condition (average weight of neonates with MAS is likely to be two to three times those with RDS). Given that the cost of SRT for RDS exceeds the per-capita gross national product (US$300 to US$500) in some countries,22 the higher costs involved in MAS should be taken into consideration before recommending either of the modes of SRT in neonates with MAS.
Antibiotics for MAS
To our knowledge, our review is the first on the role of systemic antibiotics on MAS. Even though antibiotics are believed to be helpful due to the inherent properties of meconium that support bacterial growth, we did not find any advantage of antibiotic use in the incidence of sepsis in neonates. We also did not find any difference in the risk of mortality. However, only one of the three included studies had enrolled neonates with clinical or culture positive sepsis.7 Despite in vitro evidence of enhanced bacterial growth in the presence of meconium and increased bacterial colonization of neonates born through MSAF,23, 24 incidence of bacteremia is as such less in them.25 Neonates born through MSAF are probably not at increased risk of sepsis and thus antibiotics may not have any role. It would be worth re-examining the rates of sepsis in neonates with MAS and in those admitted to a neonatal intensive care unit (NICU) for other conditions. If antibiotics were really not effective in these neonates, their use is not only going to be unhelpful but will also contribute to the increasing menace of antibiotic resistance in NICUs. There is a definite need for large multicenter studies on the role of antibiotics in neonates with MAS.
To conclude, surfactant administration either as lavage or BS in MAS was found to reduce the duration of mechanical ventilation and hospital stay, while BS reduced the need for ECMO. Administration of antibiotics, on the other hand, did not show any significant benefits in neonates with MAS and no evidence of sepsis. Confidence in these results is likely to be low because of the small number of neonates enrolled in the included studies (a total of around 650 in studies on surfactant and 500 in studies on antibiotics). There is a need to generate more evidence on the efficacy and cost-effectiveness of these two commonly used treatment modalities before recommending them in routine clinical practice.
References
Dargaville PA, Copnell B . Australian and New Zealand Neonatal Network. The epidemiology of meconium aspiration syndrome: incidence, risk factors, therapies, and outcome. Pediatrics 2006; 117 (5):1712–1721.
Swarnam K, Soraisham AS, Sivanandan S . Advances in the management of meconium aspiration syndrome. Int J Pediatr 2012; 2012, 7.
Malik AS, Hillman D . Meconium aspiration syndrome and neonatal outcome in a developing country. Ann Trop Paediatr 1994; 14 (1): 47–51.
Hofmeyr GJ, Xu H, Eke AC . Amnioinfusion for meconium-stained liquor in labour. Cochrane Database Syst Rev 2014; 1: CD000014.
Vain NE, Szyld EG, Prudent LM, Wiswell TE, Aguilar AM, Vivas NI . Oropharyngeal and nasopharyngeal suctioning of meconium-stained neonates before delivery of their shoulders: multicentre, randomised controlled trial. Lancet 2004; 364 (9434): 597–602.
Wiswell TE, Gannon CM, Jacob J, Goldsmith L, Szyld E, Weiss K et al. Delivery room management of the apparently vigorous meconium-stained neonate: results of the multicenter, international collaborative trial. Pediatrics 2000; 105 (1 Pt 1): 1–7.
Basu S, Kumar A, Bhatia BD . Role of antibiotics in meconium aspiration syndrome. Ann Trop Paediatr 2007; 27 (2): 107–113.
Gadzinowski J, Kowalska K, Vidyasagar D . Treatment of MAS with PPHN using combined therapy: SLL, bolus surfactant and iNO. J Perinatol 2008; 28 (Suppl 3): S56–S66.
Wiswell TE, Knight GR, Finer NN, Donn SM, Desai H, Walsh WF et al. A multicenter, randomized, controlled trial comparing Surfaxin (Lucinactant) lavage with standard care for treatment of meconium aspiration syndrome. Pediatrics 2002; 109 (6): 1081–1087.
Dargaville PA, Copnell B, Mills JF, Haron I, Lee JKF, Tingay DG et al. Randomized controlled trial of lung lavage with dilute surfactant for meconium aspiration syndrome. J Pediatr 2011; 158 (3): 383–389.e2.
Findlay RD, Taeusch HW, Walther FJ . Surfactant replacement therapy for meconium aspiration syndrome. Pediatrics 1996; 97 (1): 48–52.
Lotze A, Mitchell BR, Bulas DI, Zola EM, Shalwitz RA, Gunkel JH . Multicenter study of surfactant (beractant) use in the treatment of term infants with severe respiratory failure. Survanta in Term Infants Study Group. J Pediatr 1998; 132 (1): 40–47.
Maturana A, Torres-Pereyra J, Salinas R, Astudillo P, Moya FR . The Chile Surf Group. A randomized trial of natural surfactant for moderate to severe meconium aspiration syndrome. Pediatr Acad Soc 2005; 57: 1545.
Chinese Collaborative Study Group for Neonatal Respiratory Diseases. Treatment of severe meconium aspiration syndrome with porcine surfactant: a multicentre, randomized, controlled trial. Acta Paediatr 2005; 94 (7): 896–902.
Zhixia C, Cuiqing Liu, Haiyan MA . The infuence of exogenous pulmonary surfactant on pulmonary surfactant 2 associated proteins in infants with meconium aspiration syndrome. Chin J Neonatol 2009; 24 (5): 273–276.
Wanying H . Early application of pulmonary surfactant in the neonatal meconium aspiration syndrome. Guangdong Med J 2009; 30 (7):1151–1153.
Hahn S, Choi HJ, Soll R, Dargaville PA . Lung lavage for meconium aspiration syndrome in newborn infants. Cochrane Database Syst Rev 2013; 4: CD003486.
El Shahed AI, Dargaville PA, Ohlsson A, Soll R . Surfactant for meconium aspiration syndrome in term and late preterm infants. Cochrane Database Syst Rev 2014; 12: CD002054.
Choi HJ, Hahn S, Lee J, Park B-J, Lee SM, Kim H-S et al. Surfactant lavage therapy for meconium aspiration syndrome: a systematic review and meta-analysis. Neonatology 2012; 101 (3): 183–191.
Cleary GM, Antunes MJ, Ciesielka DA, Higgins ST, Spitzer AR, Chander A . Exudative lung injury is associated with decreased levels of surfactant proteins in a rat model of meconium aspiration. Pediatrics 1997; 100 (6): 998–1003.
Davey AM, Becker JD, Davis JM . Meconium aspiration syndrome: physiological and inflammatory changes in a newborn piglet model. Pediatr Pulmonol 1993; 16 (2): 101–108.
Vidyasagar D, Velaphi S, Bhat VB . Surfactant replacement therapy in developing countries. Neonatology 2011; 99 (4): 355–366.
Florman AL, Teubner D . Enhancement of bacterial growth in amniotic fluid by meconium. J Pediatr 1969; 74 (1): 111–114.
Ostfeld E, Segal J, Segal A, Bogokovski B . Bacterial colonization of the nose and external ear canal in newborn infants. Isr J Med Sci 1983; 19 (12): 1046–1049.
Wiswell TE, Henley MA . Intratracheal suctioning, systemic infection, and the meconium aspiration syndrome. Pediatrics 1992; 89 (2): 203–206.
Shankar V, Paul VK, Deorari AK, Singh M . Do neonates with meconium aspiration syndrome require antibiotics? Indian J Pediatr 1995; 62 (3): 327–331.
Lin H-C, Su B-H, Tsai C-H, Lin T-W, Yeh T-F . Role of antibiotics in management of non-ventilated cases of meconium aspiration syndrome without risk factors for infection. Biol Neonate 2005; 87 (1): 51–55.
Acknowledgements
The Department of Maternal, Newborn, Child and Adolescent Health and Development, World Health Organization, Geneva, Switzerland, funded this review.
Author contributions
CKN prepared the protocol, applied the search strategy, retrieved the articles, extracted data, made the initial tables and wrote the initial draft of the manuscript. MJS guided development of the study protocol, searched the databases, also extracted data, carried out the statistical analysis and modified the manuscript. RA and VKP modified the study protocol, supervised data extraction and modified the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Natarajan, C., Sankar, M., Jain, K. et al. Surfactant therapy and antibiotics in neonates with meconium aspiration syndrome: a systematic review and meta-analysis. J Perinatol 36 (Suppl 1), S49–S54 (2016). https://doi.org/10.1038/jp.2016.32
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2016.32
- Springer Nature America, Inc.
This article is cited by
-
Meconium aspiration syndrome: a comprehensive review
Journal of Perinatology (2023)
-
Care of the critically ill neonate with hypoxemic respiratory failure and acute pulmonary hypertension: framework for practice based on consensus opinion of neonatal hemodynamics working group
Journal of Perinatology (2022)
-
Noninfectious influencers of early-onset sepsis biomarkers
Pediatric Research (2022)
-
Surfactant use in late preterm infants: a survey among Belgian neonatologists
European Journal of Pediatrics (2021)
-
Steroids for the Management of Neonates With Meconium Aspiration Syndrome: A Systematic Review and Meta-analysis
Indian Pediatrics (2021)